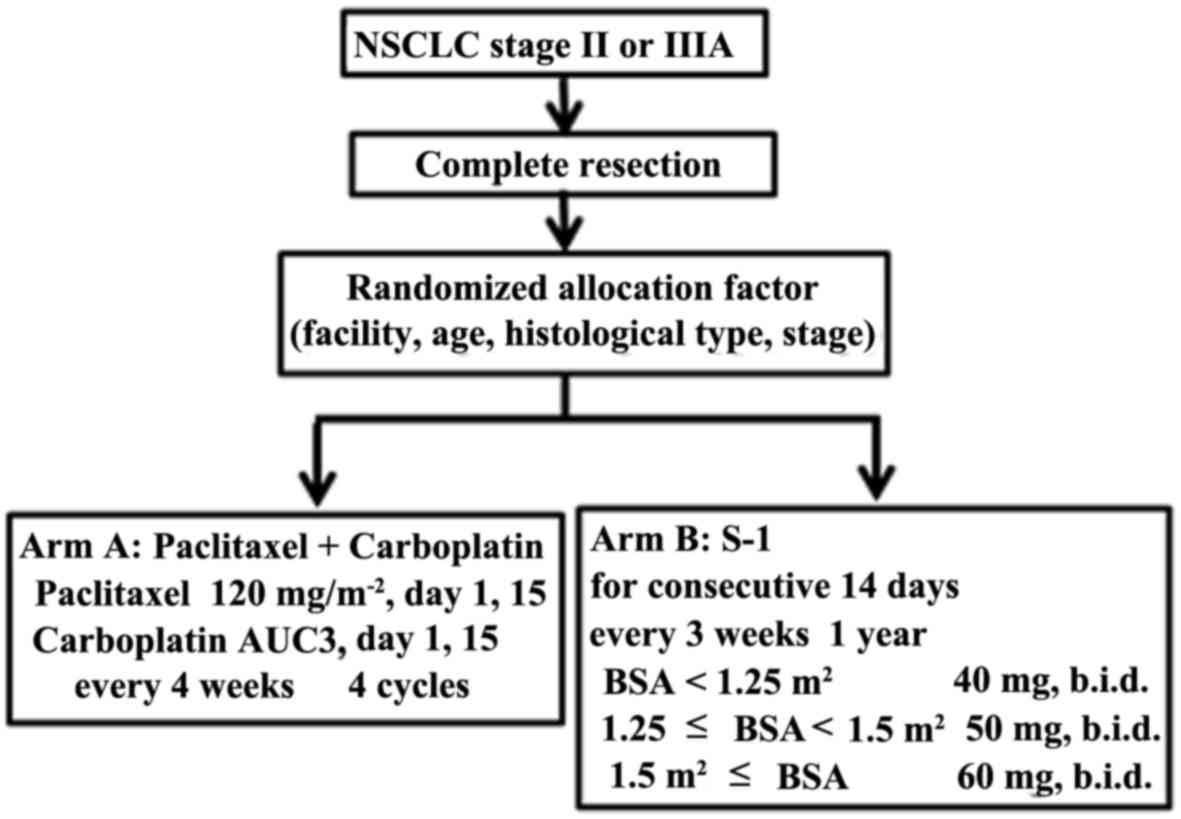
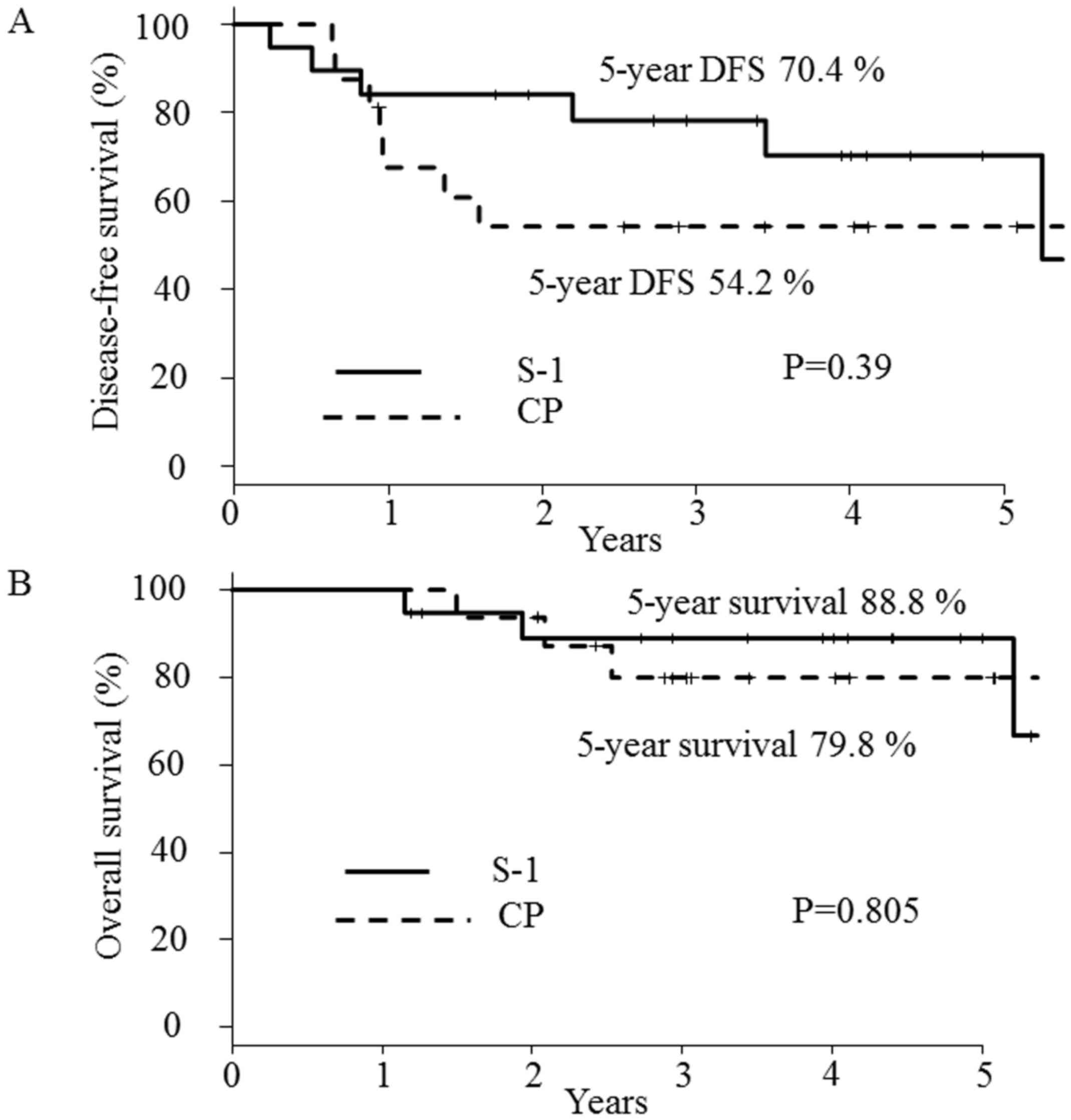
|
1
|
Asamura H, Goya T, Koshiishi Y, Sohara Y,
Eguchi K, Mori M, Nakanishi Y, Tsuchiya R, Shimokata K, Inoue H, et
al: A Japanese lung cancer registry study: Prognosis of 13,010
resected lung cancers. J Thorac Oncol. 3:46–52. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pignon JP, Tribodet H, Scagliotti GV,
Douillard JY, Shepherd FA, Stephens RJ, Dunant A, Torri V, Rosell
R, Seymour L, et al: Lung adjuvant cisplatin evaluation: A pooled
analysis by the LACE collaborative group. J Clin Oncol.
26:3552–3559. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pisters KM, Evans WK, Azzoli CG, Kris MG,
Smith CA, Desch CE, Somefield MR, Brouwers MC, Darling G, Ellis PM,
et al: Cancer care onario and American society of clinical oncology
adjuvant chemotherapy and adjuvant radiation therapy for stages
I–IIIA resectable non-small cell lung cancer guideline. J Clin
Oncol. 25:5506–5518. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crinò L, Weder W, van Meerbeeck J and
Felip E; ESMO Guidelines Working Group, : Early stage and locally
acvanced (non-metastatic) non-small-cell lung cancer: ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 21 Suppl 5:v103–v115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
NSCLC Meta-analyses Collaborative Group, ;
Arriagada R, Auperin A, Burdett S, Higgins JP, Johnson DH, Le
Chevalier T, LePecoux C, Parmar MK, Pignon JP, et al: Adjuvant
chemotherapy, with or without postoperative radiotherapy, in
operable non-small-cell lung cancer: Two meta-analyses of
individual patient data. Lancet. 375:1267–1277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fruh M, Rolland E, Pignon JP, Seymour L,
Ding K, Tribodet H, Winton T, Le Chevalier T, Scagliotti GV,
Douillard JY, et al: Pooled analysis of the effect of age on
adjuvant cisplatin-based chemotherapy for completely resected
non-small-cell lung cancer. J Clin Oncol. 26:3573–3581. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hotta K, Matsuo K, Ueoka H, Kiura K,
Tabata M and Tanimoto M: Role of adjuvant chemotherapy in patients
with resected non-small-cell lung cancer: Reappraisal with
meta-analysis of randomized controlled trials. J Clin Oncol.
22:3860–3867. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sedrakyan A, van der Meullen J, O'Byrne K,
Prendiville J, Hill J and Treasure T: Postoperative chemotherapy
for non-small cell lung cancer: A systematic review and
meta-analysis. J Thorac Cardiovasc Surg. 128:414–419. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berghmans T, Paesmans M, Meert AP, Mascaux
C, Lothaire P, Lafitte JJ and Sculier JP: Survival improvement in
resectable non-small cell lung cancer with (neo)adjuvant
chemotherapy: Results of a meta-analysis of the literature. Lung
Cancer. 49:13–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kelly K, Crowley J, Bunn PA Jr, Presant
CA, Grevstad PK, Moinpour CM, Ramsey SD, Wozniak AJ, Weiss GR,
Moore DF, et al: Randomized phase III trial of paclitaxel plus
carboplatin versus vinorelbine plus cisplatin in the treatment of
patients with advanced non-small-cell lung cancer: A southwest
oncology group trial. J Clin Oncol. 19:3210–3218. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ohe Y, Ohashi Y, Kubota K, Tamura T,
Nkagawa K, Negoro S, Nishiwaki Y, Saijo N, Ariyoshi Y and Fukuoka
M: Randomized phase III study of cisplatin plus irinotecan versus
carboplatin plus paclitaxel, cisplatin plus gemcitabine, and
cisplatin plus vinorelbine for advanced non-small-cell lung cancer:
Four-Arm cooperative study in Japan. Ann Oncol. 18:317–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH; Eastern
Cooperative Oncology Group, : Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamashita Y, Kataoka K, Ishida T, Matsuura
M, Seno N, Mukaida H, Miyahara E, Miyata Y, Okita R, Shimizu K, et
al: A feasibility study of postoperative adjuvant therapy of
carboplatin and weekly paclitaxel for completely resected non-small
cell lung cancer. J Thorac Oncol. 3:612–616. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maruyama R, Yoshino I, Tokunaga S, Ohta M,
Kato M, Yoshimine H, Yamazaki K, Nakanishi Y and Ichinose Y:
Feasibility trial of adjuvant chemotherapy with paclitaxel and
carboplatin after surgical resection in Japanese patients with
non-small cell lung cancer: Report of the lung oncology group in
kyushu (LOGIK) protocol 0501. Gen Thorac Cardiovasc Surg. 56:68–73.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ichiki M, Kawasaki M, Takayama K, Ninomiya
K, Kuba M, Iwami F, Miyazaki N, Oishi K, Takeo S, Aizawa H and
Nakanishi Y: A multicenter phase II study of carboplatin and
paclitaxel with a biweekly schedule in patients with advanced
non-small-cell lung cancer: Kyushu thoracic oncology group trial.
Cancer Chemother Pharmacol. 58:368–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Strauss GM, Herndon JE II, Maddaus MA,
Johnstone DW, Johnson EA, Harpole DH, Gilenwater HH, Watson DM,
Sugarbaker DJ, Schilsky RL, et al: Adjuvant paclitaxel plus
carboplatin compared with observation in stage IB non-small-cell
lung cancer: CALGB 9633 with the cancer and leukemia group B,
radiation therapy oncology group and north central cancer treatment
group study groups. J Clin Oncol. 26:5043–5051. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sugaya M, Uramoto H, Uchiyama A, Nagashima
A, Nakanishi R, Sakata H, Nakanishi K, Hanagiri T and Uasumoto K.:
PPhase II trial of adjuvant chemotherapy with bi-weekly carboplatin
plus paclitaxel in patients with completely resected non-small cell
lung cancer. Anticancer Res. 30:3039–3044. 2010.PubMed/NCBI
|
|
18
|
Shirasaka T, Nakano K, Takechi T, Satake
H, Uchida J, Fujioka A, Saito H, Okabe H, Oyama K, Takeda S, et al:
Antitumor activity of 1 M tegafur-0.4 M
5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against
human colon carcinoma orthotopically implanted into nude rats.
Cancer Res. 56:2602–2606. 1996.PubMed/NCBI
|
|
19
|
Sakuramoto S, Ssako M, Yamaguchi T,
Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi
Y, Imamura H, et al: Adjuvant chemotherapy for gastric cancer with
S-1, an oral fluoropyrimidine. N Engl J Med. 357:1810–1820. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Foukakis T, Lundell L, Gubanski M and Lind
PA: Advances in the treatment of patients with gastric
adenocarcinoma. Acta Oncol. 46:277–285. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sasako M: Surgery and adjuvant
chemotherapy. Int J Clin Oncol. 13:193–195. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iwamoto Y, Mitsudomi T, Sakai K, Ymanaka
T, Yoshioka H, Takahama M, Yoshimura M, Yoshino I, Takeda M,
Sugawara S, et al: Randomized phase II study of adjuvant
chemotherapy with long-term S-1 versus Cisplatin+S-1 in completely
resected stage II–IIIA non-small cell lung cancer. Clin Cancer Res.
21:5245–5252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Okumura S, Sasaki T, Satoh K, Kitada M,
Nagase A, Yatsuyanagi E and Ohsaki Y: Feasibility of adjuvant
chemotherapy with S-1 consisting of a 4-week administration and a
two-week rest period in patients with completely resected non-small
cell lung cancer. Mol Clin Oncol. 1:124–130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Soh J, Okumura N, Nakata M, Nakamura H,
Fukuda M, Kataoka M, Kajiwara S, Sano Y, Aoe M, Kataoka K, et al:
Randomized feasibility study of S-1 for adjuvant chemotherapy in
completely resected Stage IA non-small-cell lung cancer: Results of
the setouchi lng cancer group study 0701. Jpn J Clin Oncol.
46:741–747. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
TNM Classification of Malignant Tumours.
Wiley-Blackwell; Oxford: 2009
|
|
26
|
Calvert AH, Newell DR, Gumbrell LA,
O'Reilly S, Burnell M, Boxall FE, Siddik ZH, Judson IR, Gore ME and
Wiltshaw E: Carboplatin dosage: Prospective evaluation of a simple
formula based on renal function. J Clin Oncol. 7:1748–1756. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jellifie RW and Jelliffe SM: A computer
program for estimation of creatine clearance from unstable serum
creatine levels, age, sex and weight. Math Biosci. 14:17–24. 1972.
View Article : Google Scholar
|
|
28
|
Cancer Therapy Evaluation Program (CTEP),
. Common Terminology Criteria for Adverse Events, version 3.0,
DCTD, NCI, NIH, DHHS. March 31–2003, http://ctep.cancer.govAugust 9–2006
|
|
29
|
Kawamura M, Eguchi K, Izumi Y, Ymato Y,
Koike T, Sakaguchi H, Hada E and Kobayashi K: Phase II trial of
gemcitabine and docetaxel in patients with completely resected
stage IIA-IIIA non-small-cell lung cancer. Cancer Chemother
Pharmacol. 60:495–501. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vallières E, Shepherd FA, Crowley J, Van
Houtte P, Postmus PE, Carney D, Chansky K, Shaikh Z and Goldstraw
P; International Association for the Study of Lung Cancer
International Staging Committee and Participating Institutions, :
The IASLC lung cancer staging project: Proposals regarding the
relevance of TNM in the pathologic staging of small cell lung
cancer in the forthcoming (seventh) edition of the TNM
classification for lung cancer. J Thorac Oncol. 4:1049–1059. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kato H, Ichinose Y, Ohta M, Hata E,
Tsubota N, Tada H, Watanabe Y, Wada H, Tsuboi M, Hamajima N, et al:
A randomized trial of adjuvant chemotherapy with uracil-tegafur for
adenocarcinoma of the lung. N Engl J Med. 350:1713–1721. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsukuda M, Kida A, Fujii M, Kono N,
Yoshihara T, Hasegawa Y and Sugita M; Chemotherapy Study Group of
Head and Neck Cancer, : Randomized scheduling feasibility study of
S-1 for adjuvant chemotherapy in advanced head and neck cancer. Br
J Cancer. 93:884–889. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shigekawa T, Osaki A, Sekine H, Sato N,
Kanbayashi C, Sano H, Takeuchi H, Ueda S, Nakamiya N, Sugitani I,
et al: SSafety and feasibility of adjuvant chemotherapy with S-1 in
Japanese breast cancer patients after primary systemic
chemotherapy: A feasibility study. BMC Cancer. 15:2532015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yano T, Yamazaki K, Maruyama R, Tokunaga
S, Shoji F, Higashi H, Takeo S, Ichinose Y and Maehara Y; Lung
Oncology Group in Kyushu (LOGIK), : Feasibility study of
postoperative adjuvant chemotherapy with S-1 (tegaful, gimeracil,
oteracil potassium) for non-small cell lung cancer-LOGIK 0601
study. Lung Cancer. 67:184–187. 2010. View Article : Google Scholar : PubMed/NCBI
|