Introduction
Bladder cancer is the most common urothelial
carcinoma, with its incidence being fourth in men and tenth in
women among all cancers. Despite recent advances in this field, the
death rate remains relatively high. Even 70% of superficial bladder
tumors have a propensity for recurrence or progression within 5
years (1,2). However, patients with bladder cancer
are curable if diagnosed and treated early. Thus, a major concern
for patients with bladder cancer is whether earlier detection is
possible. However, early diagnosis of bladder cancer remains a
clinical challenge (3). Currently,
the reference standard of diagnosis and detection of bladder tumors
is histopathology, but it is an invasive and relatively costly
technique, and, occasionally, inconclusive, while conventional
imaging tests, such as ultrasonography, computed tomography (CT)
and magnetic resonance imaging (MRI) have significant limitations
in determining the stage of bladder cancer, particularly for
superficial lesions (4). Thus, a
real-time, improved tool is urgently required for detecting early
bladder cancer patients.
Optical coherence tomography (OCT) is a type of
biomedical optical imaging technique and optical biopsy that was
first introduced in 1991 (5). Unlike
conventional histopathology, OCT may function as ‘optical biopsy’
and is analogous to ultrasound providing real-time and
cross-sectional images of tissue structure at a resolution of ~10
µm, which is similar to that of histopathology (6). OCT was initially applied for
quantitative assessment of retinal structures in patients with
macular edema (7). Subsequently,
this approach was used in a wide spectrum of clinical applications,
including human coronary arteries, structure of the digestive
system, cervical epithelium and urinary tissues (6,8–10). These studies considered OCT to be a
successful optical imaging modality (11). Recent studies suggest that OCT is
used to help diagnose bladder cancer or to detect recurrence in
patients who have already been treated, and this approach may be
helpful with staging and grading of bladder cancer (12,13). The
present meta-analysis was performed to assess the diagnostic
performance of OCT in patients with bladder cancer and recurrent
lesions using histopathology as the golden standard.
Data collection methods
Search strategy and selection
criteria
The PubMed, EMBASE and Cochrane Library databases
were searched for studies using OCT in patients with bladder cancer
between January 1991 and December 2014. The searches were performed
using the terms ‘optical coherence tomography’, ‘OCT’, ‘optical
biopsy’, ‘bladder cancer’, ‘transitional cell carcinoma’ and
‘urothelial carcinoma’. Studies were included if they compared OCT
with the gold standard (histopathology/cytology) in the diagnosis
of patients with bladder cancer, and reported data such as
sensitivity, specificity, negative predictive value (NPV), positive
predictive value (PPV), true-positive (TP), false-positive (FP),
true-negative (TN) and false-negative (FN). Studies were excluded
if they were reviews, laboratory articles or case reports, if they
were not published in English, if there was duplication of data, or
if they did not provide detailed data to perform a
meta-analysis.
Study selection and data
extraction
The eligible studies were assessed by two
independent reviewers (J Huang and XL Ma). Disagreements on study
selection or data extraction were resolved by consensus or by
discussion with a third reviewer (L Liu). The data were extracted
from eligible studies using a standardized data collection form,
including related items: First author, publication year, number of
patients, patient source, study design, patient age, reference
standard for the diagnosis and other useful information. TP, FP, TN
and FN were eventually acquired/calculated to perform the
meta-analysis.
Quality assessment
Quality assessment was independently performed by
two investigators (J Huang and XL Ma) using the Quality Assessment
Tool for Diagnostic Accuracy Studies (QUADAS) (14). Briefly, this tool assesses diagnostic
trials and contains 14 questions. Each item was assessed as ‘yes’,
‘no’ or ‘unclear’. Any discrepancies were resolved by
consensus.
Data analysis/statistical
analysis
The present meta-analysis was performed to assess
the accuracy of OCT in patients with bladder cancer. The pooled
estimates were determined by the fixed-effects model
(Mantel-Haenszel method) if significant heterogeneity was not
detected, whereas the random-effects model (DerSimonian-Laird
method) was applied if there was heterogeneity between studies. The
χ2 test and I2 statistic were applied to
assess heterogeneity: P<0.05 for the χ2 test and
I2>50% were considered to indicate heterogeneity
between studies. The summary Receiver Operating Characteristic
(sROC) approach was a type of standard method applied in the
evaluation of diagnostic technologies of diagnostic accuracy
studies reporting pairs of sensitivity and specificity. The Q-point
is the point on the sROC curve where sensitivity equals specificity
(15). The area under the curve
(AUC) was applied to assess the quality of the diagnostic tool,
which is defined as perfect when the AUC is 100% (16). The data including TP, FP, TN, FN were
acquired using RevMan 5.1 software (Cochrane Collaboration, Oxford,
UK). The pooled estimates [sensitivity, specificity, positive
likelihood ratio (LR), negative LR and diagnostic odd ratio (OR)]
were calculated using Meta-Disc software, version 1.4 (Unit of
Clinical Biostatistics, Ramon y Cajal Hospital, Madrid, Spain).
Assessment of publication bias
Begg's test and funnel plots were used to determine
potential publication bias using STATA 11.0 software (STATA
Corporation, College Station, TX, USA), and P>0.05 was not
considered as potential publication bias.
Results
Eligible studies
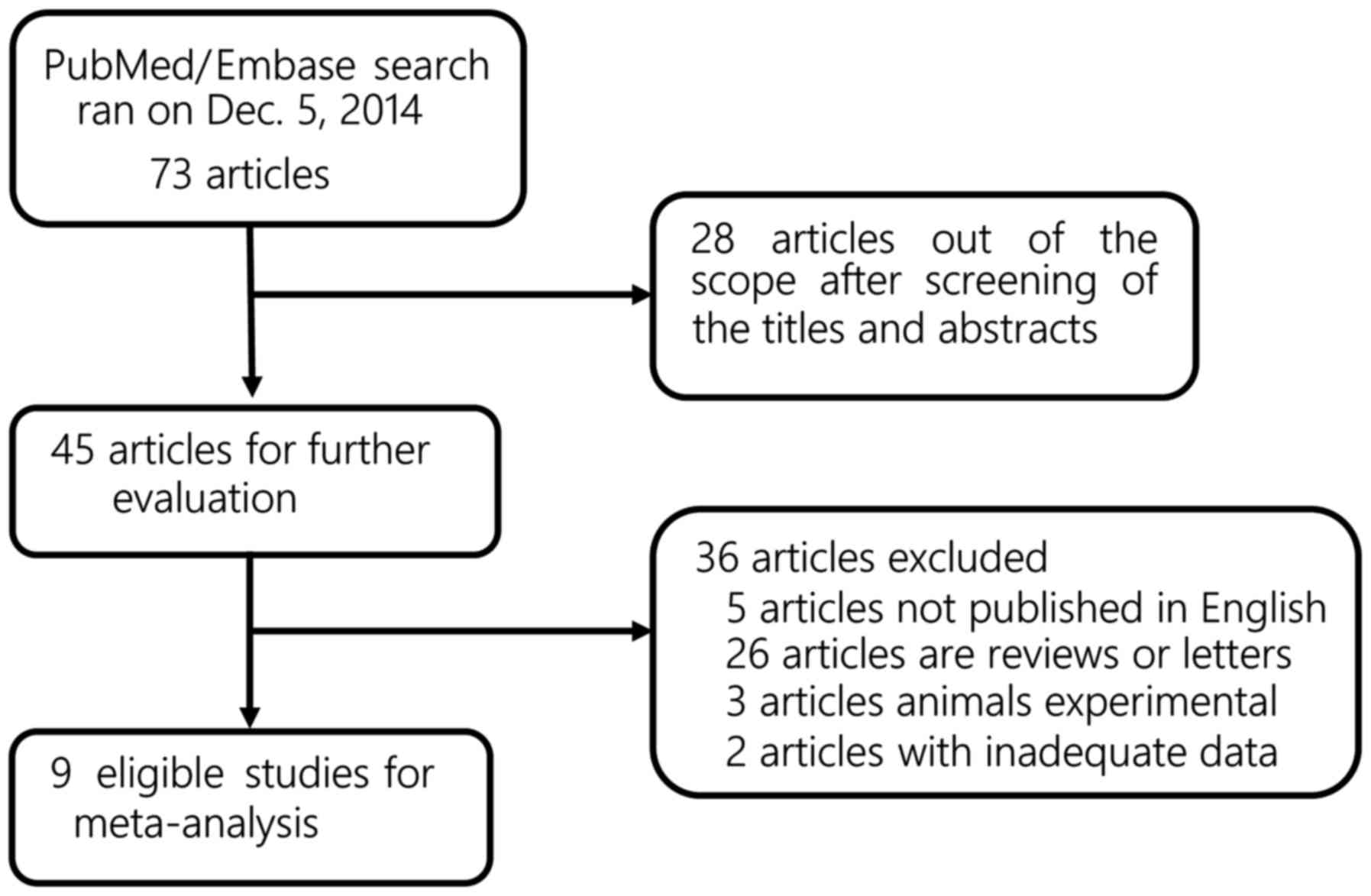
The electronic search through PubMed, EMBASE and the
Cochrane Library identified 73 publications. After screening the
titles and abstracts, 45 studies were considered for further
evaluation. Of the 45 studies, only 9 met the inclusion criteria
and were considered suitable for inclusion in the meta-analysis for
OCT and bladder cancer (Fig. 1)
(4,17–24). All
the eligible studies were published between 2002 and 2014. A total
of 468 patients were included in these studies (range, 20–105
patients). The clinical characteristics of the patients and other
useful information, such as authors, country and tumor stage, are
summarized in Table I.
 | Table I.Characteristics of the eligible
studies. |
Table I.
Characteristics of the eligible
studies.
| Authors | Year | Origin | Study design | Patient no.
(M/F) | Age (range or mean,
years) | Tumor stage | Analysis method | (Refs.) |
|---|
| Goh and Lerner | 2008 | USA | Retro | 32 (25/7) | 59 (49,84) | Ta, T1, T2 | OCT | (12) |
| Gladkova et
al | 2013 | Russia | NR | 26 (18/8) | 64.7 (3479) | CIS, Ta, T1, T2 | Cross-polarization
OCT | (22) |
| Wang et
al | 2006 | USA | Retro | >20 | NR | T1, T2a | OCT | (23) |
| Schmidbauer et
al | 2009 | Austria | Prosp | 66 (49/17) | 67 (38–84) | CIS, Ta, T1,
T2 | OCT | (20) |
| Ren et
al | 2009 | USA | NR | 56 (46/10) | 70 (25–75) | pTis and
pTa-pT1 | Cystoscopic
OCT | (19) |
| Manyak et
al | 2005 | USA | NR | 24 | NR | Papillary and flat
lesions | OCT | (4) |
|
Lingley-Papadopoulos et al | 2009 | USA | NR | 21 | NR | CIS, papillary
lesion, or invasive tumor | OCT | (24) |
| Karl et
al | 2010 | Germany | NR | 52 | 21–91 | CIS, Ta, T1,
T2 | OCT | (18) |
| Hermes et
al | 2008 | Germany | Retro | 105 | NR | CIS, invasive
carcinoma | Ultrahigh
resolution OCT | (17) |
Quality assessment
Quality assessment was performed in all the included
studies using the QUADAS tool (Table
II). Of the 14 items, at least 10 items were clearly stated in
each eligible study, which indicates high quality.
 | Table II.Quality assessment of included
studies. |
Table II.
Quality assessment of included
studies.
|
| Studies
(Refs.) |
|---|
|
|
|
|---|
| Item | Goh and Lerner
(12) | Ren et al
(19) | Hermes et al
(17) | Manyak et al
(4) | Gladkova et
al (22) | Karl et al
(18) | Wang et al
(23) | Schmidbauer et
al (20) |
Lingley-Papadopoulos et al
(24) |
|---|
| 1 | Y | N | Y | Y | N | Y | Y | Y | Y |
| 2 | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 3 | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 4 | Y | Y | Y | Y | Y | Y | Y | N | Y |
| 5 | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 6 | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 7 | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 8 | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 9 | Y | Y | Y | N | Y | N | N | Y | Y |
| 10 | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 11 | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 12 | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 13 | Y | U | Y | Y | Y | Y | Y | N | Y |
| 14 | Y | U | Y | Y | Y | Y | U | N | Y |
Diagnostic accuracy of OCT in bladder
cancer
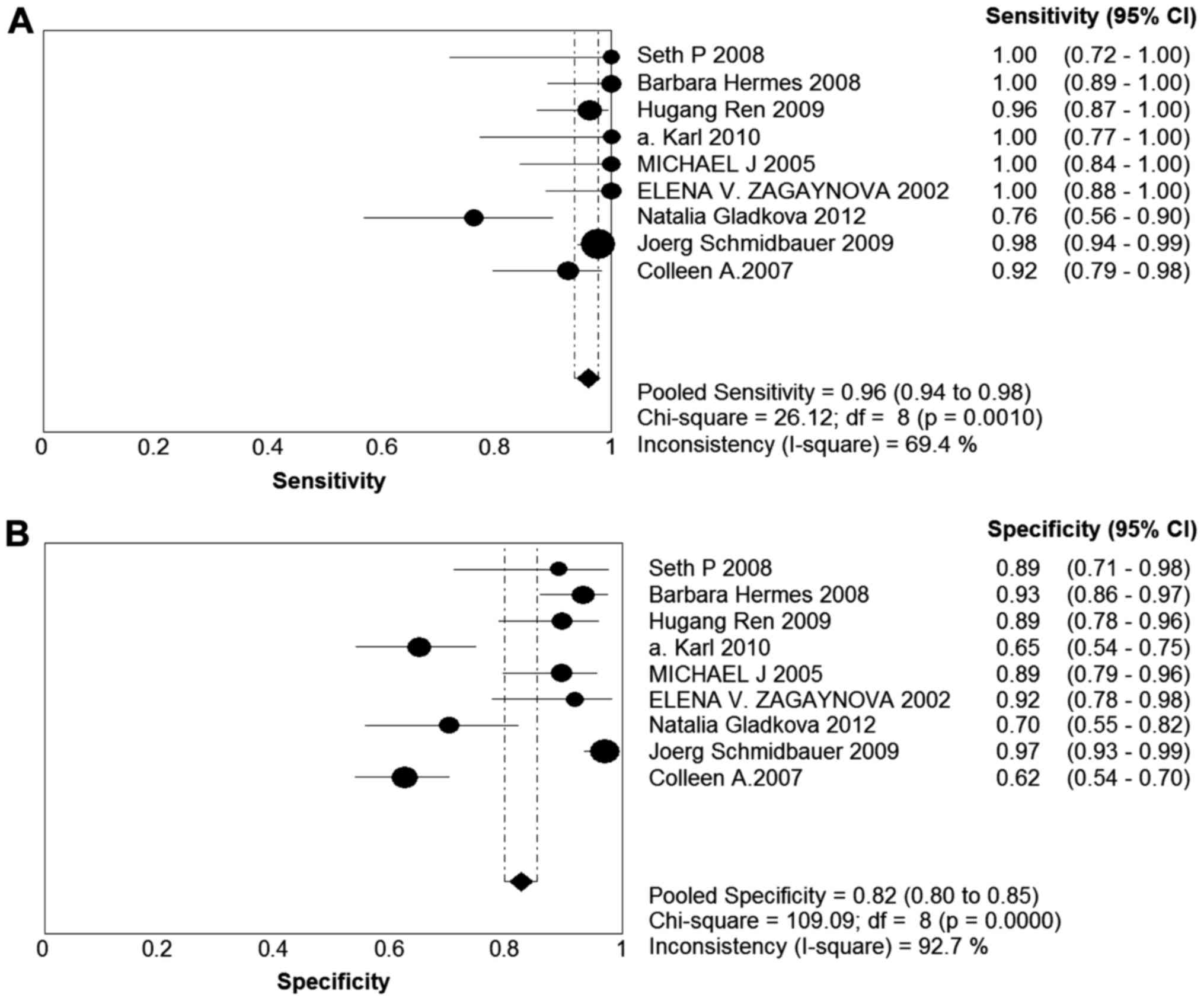
The forest plot of sensitivity and specificity for
diagnostic accuracy of OCT in bladder cancer patients is presented
in Fig. 2. The sensitivity of the
eligible studies ranged from 0.76 to 1.00, and the specificity
ranged from 0.62 to 0.97. The pooled sensitivity and specificity
with 95% confidence interval (95% CI) for OCT were 0.96 (95% CI:
0.94–0.98) and 0.82 (95% CI: 0.80–0.85), respectively. The pooled
PLRs and NLRs were 6.83 (95% CI: 3.24–14.1) and 0.05 (95% CI:
0.02–0.16), respectively. The combined diagnostic odds ratio (DOR)
in the diagnosis of bladder cancer was 138.88 (95% CI:
29.63–650.89). The pooled values (sensitivity, specificity, PLR,
NLR and DOR) are listed in Table
III. The sROC and the Q* index were used to assess diagnostic
accuracy. The AUC and the Q* index were 0.9735 and 0.9257,
respectively.
 | Table III.Diagnostic accuracy of OCT in bladder
cancer in 9 selected studies. |
Table III.
Diagnostic accuracy of OCT in bladder
cancer in 9 selected studies.
| Data analysis | Pooled value | 95% confidence
interval |
|---|
| Sensitivity | 0.96 | 0.94–0.98 |
| Specificity | 0.82 | 0.80–0.85 |
| PLR | 6.83 | 3.24–14.41 |
| NLR | 0.05 | 0.02–0.16 |
| DOR | 138.88 | 29.63–650.89 |
Study heterogeneity and publication
bias
Heterogeneity was assessed on the basis of the
χ2 test and the I2 statistic. There was
statistically significant heterogeneity in sensitivity
(χ2=26.12, P=0.0010; I2=69.4%), specificity
(χ2=109.09, P=0.0000; I2=92.7%), positive LR
(χ2=154.93, P=0.0000; I2=94.8%), negative LR
(χ2=35.40, P=0.0000; I2=77.4%) and DOR
(χ2=49.94, P=0.0000; I2=84.0%). Publication
bias was analyzed by Begg's test and funnel plots. No significant
publication bias was found for DOR in the present meta-analysis
(P=0.83).
Discussion
Bladder cancer is the sixth most common type of
cancer worldwide. Approximately 75–85% of patients have superficial
bladder cancer when first diagnosed, confined to the mucosa or
lamina propria. However, a significant proportion of patients with
superficial bladder cancer are at risk for recurrence and
progression. The risk factors for tumor recurrence and/or
progression may be summarized as follows: i) New tumor occurrence
and progression; ii) tumor implantation during transurethral
resection (TUR); iii) residual tumor following incomplete resection
and/or iv) overlooking of neoplastic lesions such as dysplasia and
carcinoma in situ (CIS) (25). Tumor recurrence and/or progression
are partially attributed to the detection tools. Therefore, new
diagnostic modalities for detection and monitoring are required to
decrease the rate of tumor recurrence and/or progression, which
affect the patient outcome.
Accumulated evidence indicates that current methods
of diagnosing bladder cancer mainly rely on histological and
cytological examination of tissue. White-light cystoscopy in
combination with TUR are currently applied to resect lesions,
followed by histopathological examination to evaluate the level of
bladder wall involvement. Histopathological examination is
currently the gold standard for identifying bladder cancer tissue.
This pathological examination is a time-consuming procedure that
requires removal of suspicious lesions, followed by fixing and
staining prior to diagnosis. Furthermore, histopathological
evaluation in the diagnosis of bladder cancer has certain
limitations in terms of real-time differentiation of grade and
stage of superficial bladder cancer, since relevant early-stage and
precancerous lesions are often missed (26–28).
Cystoscopic evaluation is available for papillary transitional cell
carcinoma (TCC); however, it is of low diagnostic sensitivity and
specificity for differentiating non-papillary TCC, particularly CIS
(29–31). Urine cytology has been proven to have
potential advantages for bladder CIS and high-grade neoplasms, but
is of quite low sensitivity for low-grade lesions and follow-up
investigations of bladder cancer (32). The abovementioned methods are
invasive detection techniques that remain insufficiently validated
in terms of diagnosis and follow-up, particularly for low-grade
bladder cancer. In addition, as regards non-invasive detection
tools, due to the limited resolution, conventional imaging tools,
including intravenous pyelography, CT and MRI, fail to detect
early-stage bladder cancer (3).
Furthermore, real-time grading of bladder cancer is clinically
important, but the previously mentioned approaches for diagnosis
and grading cannot provide this information. Thus, a new,
real-time, promising detection method is needed to enable accurate
diagnosis for superficial bladder cancer and recurrent disease.
OCT as a real-time high-resolution and non-invasive
technology, may provide cross-sectional imaging of biological
tissue to a depth of 1–2 mm (33).
This tool may increase accuracy and specificity in differentiating
grade and stage in bladder cancerous lesions in particular,
offering great potential for the detection of precancerous and
low-grade lesions, and may also be available for visualization and
resection. To date, OCT has been widely applied to diagnose
patients suffering from bladder cancer. Hermes et al, as
well as other groups, demonstrated that OCT is a clinically useful
tool for bladder cancer diagnosis with high sensitivity and
specificity (13,17). Johnson et al demonstrated the
feasibility of OCT in the diagnosis of glaucoma by a systematic
review and meta-analysis (34). OCT
diagnostic technologies for bladder cancer have not been compared
with histopathology examination by meta-analysis to date.
To the best of our knowledge, this is the first
meta-analysis investigating the diagnostic accuracy of emerging OCT
in bladder cancer. Histopathology served as the reference standard.
A total of 9 eligible studies (468 patients) were included in our
meta-analysis, and the pooled estimated sensitivity and specificity
of OCT in detecting bladder cancer were 0.96 (95% CI: 0.94–0.98)
and 0.82 (95% CI: 0.80–0.85), respectively. As seen in Table I, the included patients were mainly
low-grade and early-stage (superficial bladder cancer and CIS). The
results of the present meta-analysis suggested that OCT has
excellent diagnostic performance for low-grade and early-stage
disease in bladder cancer patients.
In this study, the OCT signal was assessed per
se, as well as in combination with other imaging modalities,
such as fluorescence spectroscopy. The results revealed that there
was no significant difference in the diagnostic value (data not
shown). The factors limiting the validity of the results are
summarized as follows: i) The eligible studies mainly analyzed the
diagnostic role of OCT in early-stage bladder cancer patients; and
ii) there was not enough evidence for further analysis.
DOR is a single indicator of diagnostic accuracy
that combines the data into a number (9); it ranges from 0 to infinity, and higher
values indicate higher accuracy. Although there is no absolute
cut-off, a good diagnostic test must have a DOR of >100. The
pooled DOR value for OCT was 138.88 (95% CI: 29.63–650.89) in the
present meta-analysis. AUC was also applied to determine the
diagnostic accuracy. The value of AUC was 97.35% in the diagnosis
of bladder cancer. Taken together, these results indicate that OCT,
a real-time high-resolution and non-invasive technique, has a very
high level of accuracy.
There were several limitations in our studies. The
major limitation of OCT are its innate characteristics. OCT
functions as an ‘optical biopsy’ and is equivalent to ultrasound
based on depth-resolved detection of elastic light scattering. The
imaging depth is usually limited to <2 mm due to light
scattering by the sample. Therefore, OCT has the potential to
differentiate grade and stage of early bladder cancer, but is less
useful for advanced tumors. Combining OCT with other imaging
modalities, such as fluorescence spectroscopy or advanced analysis
of the OCT signal itself, may distinguish between benign and
malignant bladder tissue, regardless of disease stage. Another
limitation of OCT is the difficulty in differentiating between
chronic inflammatory tissue and CIS, which is also the case for
edema and scar tissue. In addition, the numbers of the patients in
the eligible studies were small, and the majority had low-grade
(non-invasive) bladder cancer and CIS, which may have introduced a
bias to the results. Therefore, a study including a larger
population is required to assess the accuracy of OCT. Selective
reporting biases are one of common risks with diagnostic studies.
At present, the results appear to be in favor of OCT. In addition,
the exclusion of studies, regardless of the reason, may have also
led to potential reporting bias. It is also noteworthy that this
clinical diagnostic tool has not been widely adopted and there are
no consolidated guidelines regarding imaging for bladder
cancer.
Significant heterogeneity was found in the present
meta-analysis. The heterogeneity in sensitivity, specificity,
positive LR, negative LR and DOR were χ2=26.12,
P=0.0010, I2=69.4%; χ2=109.09, P=0.0000,
I2=92.7%; χ2=154.93, P=0.0000,
I2=94.8%; χ2=35.40, P=0.0000,
I2=77.4%; and χ2=49.94, P=0.0000,
I2=84.0%, respectively. This indicated that there were
significant variations in the studies, such as the examiner's
experience, analysis imaging using the OCT signal per se or
combining OCT with other imaging modalities, number of patients or
detected lesions and study design. In addition, Begg's test is
likely underpowered due to the small number of studies and the high
heterogeneity.
The meta-analysis indicated that OCT may be a useful
and promising tool for earlier detection, diagnosis and staging of
superficial low-grade tumors and CIS, as well as detection of
recurrent tumors. Since real-time high-resolution OCT images may be
obtained in a non-invasive manner, it would play an important role
in guided therapies. In particular, this tool may prove useful for
guidance of biopsy procedures and staging of suspected tissue areas
within the bladder. However, multicenter and prospective studies
are required to provide definitive answers and evaluate the
potential diagnostic accuracy of OCT in the detection of early
bladder cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. NSFC 81101729) and
the Soft Science Project of Sichuan Province (grant no.
2010ZR0161).
References
|
1
|
Hall MC, Chang SS, Dalbagni G, Pruthi RS,
Seigne JD, Skinner EC, Wolf JS Jr and Schellhammer PF: Guideline
for the management of nonmuscle invasive bladder cancer (stages Ta,
T1 and Tis): 2007 update. J Urol. 178:2314–2330. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Messing EM and Catalona W: Urothelial
tumors of the urinary tract = Campbell's Urolology. 9th. WB
Saunders; Philadelphia: pp. 2383–2411. 2002
|
|
4
|
Manyak MJ, Gladkova ND, Makari JH,
Schwartz AM, Zagaynova EV, Zolfaghari L, Zara JM, Iksanov R and
Feldchtein FI: Evaluation of superficial bladder transitional-cell
carcinoma by optical coherence tomography. J Endourol. 19:570–574.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang D, Swanson EA, Lin CP, Schuman JS,
Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA, et
al: Optical coherence tomography. Science. 254:1178–1181. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tearney G, Brezinski M, Southern J, Bouma
B, Boppart S and Fujimoto J: Optical biopsy in human urologic
tissue using optical coherence tomography. J Urol. 157:1915–1919.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hee MR, Puliafito CA, Wong C, Duker JS,
Reichel E, Rutledge B, Schuman JS, Swanson EA and Fujimoto JG:
Quantitative assessment of macular edema with optical coherence
tomography. Arch Ophthalmol. 113:1019–1029. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gogas BD, Farooq V, Onuma Y, Magro M, Radu
MD, van Geuns RJ, Regar E and Serruys PW: 3-dimensional optical
frequency domain imaging for the evaluation of primary percutaneous
coronary intervention in ST-segment elevation myocardial
infarction. Int J Cardiol. 151:103–105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sivak MV Jr, Kobayashi K, Izatt JA,
Rollins AM, Ung-Runyawee R, Chak A, Wong RC, Isenberg GA and Willis
J: High-resolution endoscopic imaging of the GI tract using optical
coherence tomography. Gastrointest Endosc. 51:474–479. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gallwas J, Turk L, Friese K and Dannecker
C: Optical coherence tomography as a non-invasive imaging technique
for preinvasive and invasive neoplasia of the uterine cervix.
Ultrasound Obstet Gynecol. 36:624–629. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carignan CS and Yagi Y: Optical
endomicroscopy and the road to real-time, in vivo pathology:
Present and future. Diagn Pathol. 7:982012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goh AC and Lerner SP: Application of new
technology in bladder cancer diagnosis and treatment. World J Urol.
27:301–307. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wessels R, De Bruin DM, Faber DJ, Van
Leeuwen TG, Van Beurden M and Ruers TJ: Optical biopsy of
epithelial cancers by optical coherence tomography (OCT). Lasers
Med Sci. 29:1297–1305. 2014.PubMed/NCBI
|
|
14
|
Whiting P, Rutjes AW, Reitsma JB, Bossuyt
PM and Kleijnen J: The development of QUADAS: A tool for the
quality assessment of studies of diagnostic accuracy included in
systematic reviews. BMC Med Res Methodol. 3:252003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moses LE, Shapiro D and Littenberg B:
Combining independent studies of a diagnostic test into a summary
roc curve: Data-analytic approaches and some additional
considerations. Stat Med. 12:1293–1316. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Swets JA: Measuring the accuracy of
diagnostic systems. Science. 240:1285–1293. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hermes B, Spöler F, Naami A, Bornemann J,
Först M, Grosse J, Jakse G and Knüchel R: Visualization of the
basement membrane zone of the bladder by optical coherence
tomography: Feasibility of noninvasive evaluation of tumor
invasion. Urology. 72:677–681. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Karl A, Stepp H, Willmann E, Buchner A,
Hocaoglu Y, Stief C and Tritschler S: Optical coherence tomography
for bladder cancer-ready as a surrogate for optical biopsy?-Results
of a prospective mono-centre study. Eur J Med Res. 15:131–134.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ren H, Waltzer WC, Bhalla R, Liu J, Yuan
Z, Lee CS, Darras F, Schulsinger D, Adler HL, Kim J, et al:
Diagnosis of bladder cancer with microelectromechanical
systems-based cystoscopic optical coherence tomography. Urology.
74:1351–1357. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schmidbauer J, Remzi M, Klatte T, Waldert
M, Mauermann J, Susani M and Marberger M: Fluorescence cystoscopy
with high-resolution optical coherence tomography imaging as an
adjunct reduces false-positive findings in the diagnosis of
urothelial carcinoma of the bladder. Eur Urol. 56:914–919. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zagaynova EV, Streltsova OS, Gladkova ND,
Snopova LB, Gelikonov GV, Feldchtein FI and Morozov A: In vivo
optical coherence tomography feasibility for bladder disease. J
Urol. 167:1492–1496. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gladkova N, Kiseleva E, Streltsova O,
Prodanets N, Snopova L, Karabut M, Gubarkova E and Zagaynova E:
Combined use of fluorescence cystoscopy and cross-polarization OCT
for diagnosis of bladder cancer and correlation with
immunohistochemical markers. J Biophotonics. 6:687–698. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang ZG, Lee C, Waltzer W, Yuan ZJ, Wu ZL,
Xie HK and Pan YT: Optical coherence tomography for noninvasive
diagnosis of epithelial cancers. Conf Proc IEEE Eng Med Biol Soc.
1:pp. 129–132. 2006; PubMed/NCBI
|
|
24
|
Lingley-Papadopoulos CA, Loew MH, Manyak
MJ and Zara JM: Computer recognition of cancer in the urinary
bladder using optical coherence tomography and texture analysis. J
Biomed Opt. 13:0240032008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zaak D and Hofstetter A: The current
diagnosis of superficial bladder cancer must be reconsidered. Urol
Int. 69:85–90. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shariat SF, Palapattu GS, Karakiewicz PI,
Rogers CG, Vazina A, Bastian PJ, Schoenberg MP, Lerner SP,
Sagalowsky AI and Lotan Y: Discrepancy between clinical and
pathologic stage: Impact on prognosis after radical cystectomy. Eur
Urol. 51:137–151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ficarra V, Dalpiaz O, Alrabi N, Novara G,
Galfano A and Artibani W: Correlation between clinical and
pathological staging in a series of radical cystectomies for
bladder carcinoma. BJU Int. 95:786–790. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stein JP: Indications for early
cystectomy. Urology. 62:1–595. 2003. View Article : Google Scholar
|
|
29
|
Leyh H, Marberger M, Conort P, Sternberg
C, Pansadoro V, Pagano F, Bassi P, Boccon-Gibod L, Ravery V,
Treiber U and Ishak L: Comparison of the BTA stat test with voided
urine cytology and bladder wash cytology in the diagnosis and
monitoring of bladder cancer. Eur Urol. 35:52–56. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kriegmair M, Baumgartner R, Knüchel R,
Stepp H, Hofstädter F and Hofstetter A: Detection of early bladder
cancer by 5-aminolevulinic acid induced porphyrin fluorescence. J
Urol. 155:105–110. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cina SJ, Epstein JI, Endrizzi JM, Harmon
WJ, Seay TM and Schoenberg MP: Correlation of cystoscopic
impression with histologic diagnosis of biopsy specimens of the
bladder. Hum Pathol. 32:630–637. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cajulis RS, Haines GK III, Frias-Hidvegi
D, McVary K and Bacus JW: Cytology, flow cytometry, image analysis,
and interphase cytogenetics by fluorescence in situ hybridization
in the diagnosis of transitional cell carcinoma in bladder washes:
A comparative study. Diagn Cytopathol. 13:214–224. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fujimoto JG, Pitris C, Boppart SA and
Brezinski ME: Optical coherence tomography: An emerging technology
for biomedical imaging and optical biopsy. Neoplasia. 2:9–25. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Johnson ZK, Siddiqui MA and Azuara-Blanco
A: The quality of reporting of diagnostic accuracy studies of
optical coherence tomography in glaucoma. Ophthalmology.
114:1607–1612. 2007. View Article : Google Scholar : PubMed/NCBI
|