Introduction
Adverse health effects due to exposure to ionizing
radiation (IR) have been reported since the first application of
X-rays. Stochastic effects, primarily the carcinogenic effects of
IR exposure, first became known from the Life Span Study of atomic
bomb survivors in Japan (1). In
contrast to the high-dose or high-dose-rate IR only seen in
Japanese atomic bomb survivors (2)
and nuclear accidents, such as Chernobyl (3), nuclear industry and medical workers are
nominally only exposed to low-dose or low-dose-rate IR. A large,
international cohort study strongly supported that long-term
exposure to low-dose IR (LDIR) increases the risk of leukemia,
although the increase is only minuscule (4–6).
However, recent epidemiological studies highlighted the detrimental
effect of persistent exposure to LDIR, and research on nuclear
industry workers has demonstrated increased cancer mortality risks
following a cumulative dose of <100 mSv and dose rates of <10
mSv per year (7), particularly in
solid cancers, by the linear non-threshold model (8). As the extensive use of IR in the
medical industry, including radiodiagnosis and radiotherapy, is
justified and has been well-studied, the aim of the present study
was to focus on the health effects of occupational and
environmental IR exposure in the nuclear industry.
Mining, historically the primary source of
occupational and environmental health risk exposure, was the only
means of obtaining natural radionuclides of uranium. As is well
known, U-238 comprises >99% of uranium ore, and radioactive
U-235 comprises only 0.71% in nature. Uranium mining constituted an
internal radiation exposure risk in nuclear industrial workers when
they inhaled massive amounts of radon gas and its decay species in
mines (9). While there is little
information on the association between health risks and internal
exposure after inhaling uranium dust (10), the physicochemical properties of
uranium are known to present a hazard (11). An international retrospective cohort
study demonstrated that uranium workers exhibited a higher solid
cancer mortality risk compared with control populations living near
nuclear facilities (12–14). In fact, uranium workers were put at
significant risk, not only by the α-particles of radioactive
uranium decay, but also by γ-ray exposure in the mines.
Radioepidemiology studies in nuclear industry
workers confirmed that external exposure to γ-rays and X-rays in
medical care settings increased the health risks of partial solid
cancers (15,16). The effect on health care workers was
a source of bias in occupational epidemiological studies and was
recorded (17–19). The risk was not statistically
significant in terms of excess relative risk (ERR) and/or standard
mortality ratio (SMR), but there was evidence of increasing cancer
mortality risk from exposure to IR (20,21). A
number of complex factors affect the health of nuclear industry
workers, their external exposure to radiation and death of the
residents (20,22). There are important statistical
limitations in recent epidemiological studies, including the number
of subjects in those cohorts, the follow-up period, the mode of
adjustment and the differences in statistical methods. Therefore,
the challenge was to increase the number of international cohort
studies in order to improve the ability to assess health risk and
to monitor the long-term follow-up evaluations of nuclear industry
workers.
The present study utilized a systematic review of
the literature related to the mortality risk of solid cancers,
including cancers of the lung, brain and central nervous system
(CNS), liver, stomach, colorectum, kidney, bladder and prostate,
affecting nuclear industry workers in uranium mining, refining,
enrichment and gaseous diffusion plants. The primary aims of this
meta-analysis were to determine whether LDIR increases the
mortality risk of solid cancers in nuclear industry workers, to
determine whether there is a standard mortality risk value among
any of the solid cancers from LDIR, and whether the cancer
mortality risks exhibited a trend for variation from the classical
epidemiological studies.
Data collection methods
Search strategy
Two electronic search strategies were performed
through the PubMed and Embase databases using key words for all
fields of ‘solid cancer’ OR ‘lung cancer’ OR ‘brain cancer’ OR
‘central nervous system cancer’ OR ‘liver cancer’ OR ‘stomach
cancer’ OR ‘colorectal cancer’ OR ‘colon cancer’ OR ‘intestinal
cancer’ OR ‘rectum cancer’ OR ‘kidney cancer’ OR ‘bladder cancer’
OR ‘prostate cancer’ AND ‘mortality’ AND ‘nuclear industry’ OR
‘nuclear facility’. The search was limited to journal articles
published between January 1, 2000 and December 31, 2016, and there
were no language restrictions. The bibliographies of all articles
included for data extraction were searched independently for
further eligible articles by two authors (S-GQ and JG).
Data selection
The present meta-analysis included original research
evaluating subjects working in the nuclear industry with a main
occupation in mining, refining, enrichment, non-destructive testing
and nuclear weapon research, but not in nuclear power plants,
medical facilities, education or nuclear accidents. Atomic bomb
survivors were also excluded. LDIR was limited to whole-body IR
exposure, with a cumulative mean dose of <0.5 Sv per year, or at
a low dose rate (<10 mSv/day) (23). Only studies published in English were
considered for inclusion.
The quality of this systematic review was assessed
by detailed selection of participants and by comparison of the
results. The data in this study included cohort workers, follow-up
period, number of deaths caused by cancers of particular interest
to the present study, SMR and 95% confidence interval (CI). Data
were excluded for all reviews, books and reports where workers were
engaged in their activities for <1 year, and from all articles
containing insufficient/incomplete data. Three articles were
excluded, although they involved uranium workers and nuclear power
(20,24) and uranium gaseous diffusion plants
(25), as the radiation doses were
closely controlled and were within the range considered as safe.
Three articles on the Oak Ridge National Laboratory staff (26,27) and
nuclear test participants (28) were
also excluded. The data selection was confirmed by carefully
reading the full text and supplementary information for each
article. In the identified studies, disease was observed and graded
according to the International Classification of Diseases,
revisions 9/10, and the disease categories were carefully examined.
Small intestinal, colon and rectum cancer cases were combined under
‘colorectal cancer’ in this meta-analysis, as the number of those
cancers was small (29).
Statistical analysis
The SMR and 95% CI were used to evaluate the outcome
of the cohort and as measures of solid cancer mortality. If the SMR
and 95% CI were not available for meta-analysis, cohort outcome and
mortality were calculated by comparing the number of reported
deaths against the expected number of deaths in each group. If the
results were published for a single type of cancer, a combined
value was computed via analysis of the single sample value. The SMR
and 95% CI were unified in analysis, although reports using 90% CI
were also common for meta-analyses of disease outcomes for the
cancers of interest.
Forest plots were used to visually assess the pooled
estimates and corresponding 95% CIs. Homogeneity across studies was
tested using Cochran's Q test at P<0.1, and quantified using the
I2 statistics, which represents the percentage of
heterogeneity that may be attributed to the variation across
studies. In the presence of significant heterogeneity, a
random-effects model was applied. We further performed a
sensitivity analysis to investigate the influence of a single study
on the overall risk estimate by omitting one study in each
iteration. The presence of publication bias was assessed using the
Begg's and Egger's tests and by examining funnel plots. Two-tailed
P<0.05 was considered statistically significant. All the data
were analyzed using STATA software, version 11.0 (Stata Corp LP,
College Station, TX, USA).
Results
Selected articles
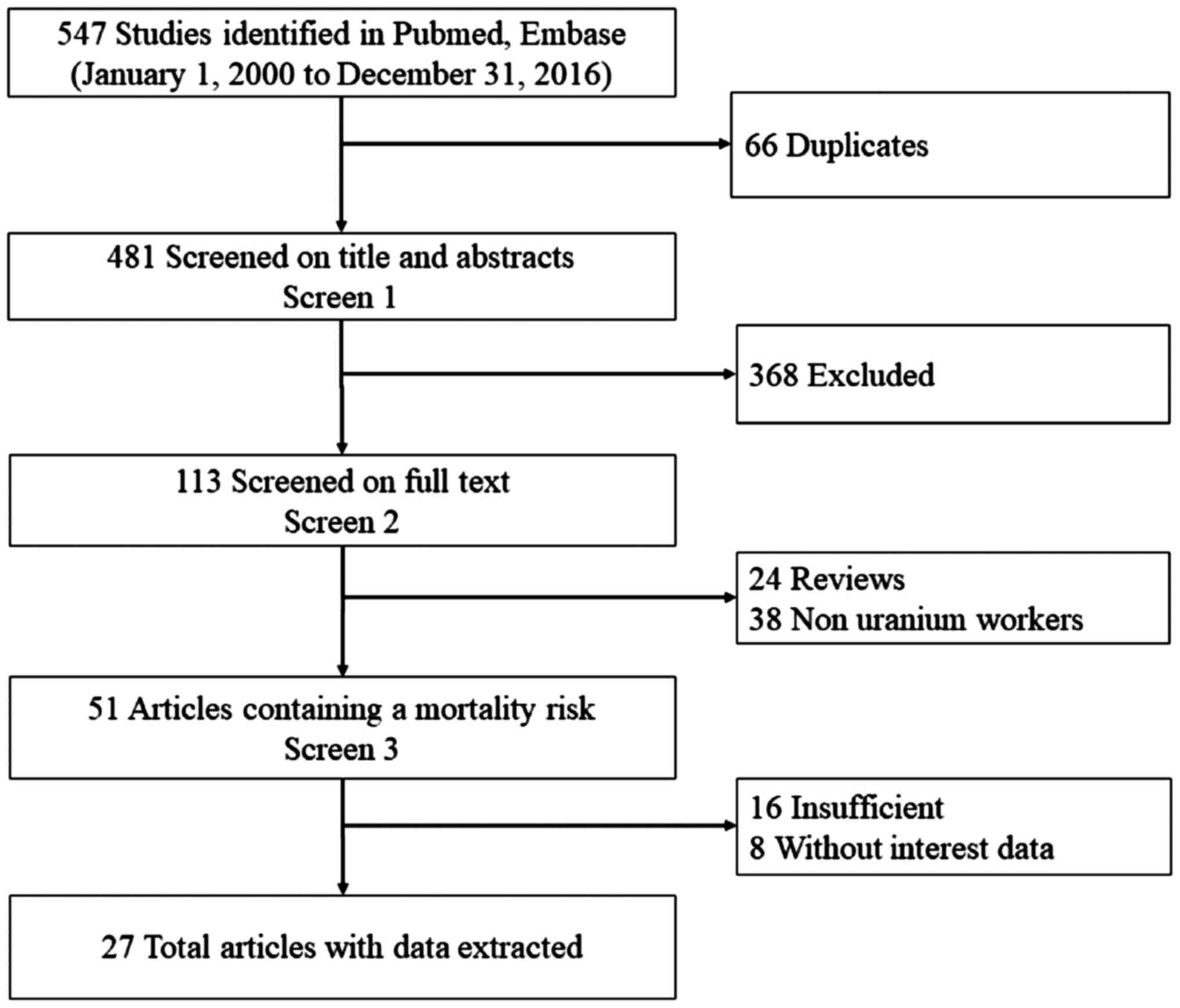
In the initial search, 547 relevant articles were
identified. Of these, 66 were excluded as duplicates, 368 were
excluded after reviewing their titles and abstracts, 24 were
excluded as reviews, and 62 were excluded as they fell outside the
dates of interest of the present study. Following the review, 27
articles (15,17,26–50) were
finally selected for the present meta-analysis. The study selection
process is summarized in Fig. 1.
Description of studies
The characteristics of the 27 articles included in
the present meta-analysis are detailed in Table I. The articles were all retrospective
cohorts and the majority were published after 2010. Of the articles
included in this study, 1 was performed in Asia, 2 in Australia, 10
in North America and 14 in Europe. Not all the studies included
data on all eight types of solid cancers of interest in the present
analysis plus the total cancers. Such was the case for the article
published by Drubay et al (30), which only included information on
kidney cancer and its SMR and 95% CI.
 | Table I.Description of reviewed studies on
the mortality risk of solid cancer following exposure to
uranium. |
Table I.
Description of reviewed studies on
the mortality risk of solid cancer following exposure to
uranium.
|
|
|
|
|
|
|
No. of
cancer deaths |
|---|
|
|
|
|
|
|
|
|
|---|
| Study (year of
publication) | Country | Follow-up
period | Average follow-up
period (years) | Work type | Cohort workers | Total | Solid | Lung | Brain and CNS | Liver | Stomach | Colorectal | Kidney | Bladder | Prostate | (Refs.) |
|---|
| Zhivin et al
(2016) | France | 1968–2008 | 30.0 | B | 4,688 | 429 | 406 | 100 | 17 | 17 | 12 | 39 | 13 | 12 | 30 | (46) |
| Samson et al
(2016) | France | 1968–2008 | 27.9 | All | 12,649 | 912 | NA | 217 | 32 | 31 | 29 | 86 | 24 | 26 | 71 | (44) |
| Rage et al
(2015) | France | 1946–2007 | 35.4 | A | 5,086 | 721 | NA | 211 | 28 | 31 | 33 | 63 | 24 | 25 | 51 | (29) |
| Schubauer-Berigan
et al (2015) | USA | 1944–2005 | 33.7 | All | 119,195 | 11,332 | 9,979 | 3,228 | NA | 535 | NA | NA | NA | NA | NA | (27) |
| Sokolnikov et
al (2015) | Russia | 1948–2008 | NA | O | 25,757 | 2,980 | NA | 841 | 66 | 91 | 452 | 302 | NA | NA | NA | (31) |
| Kreuzer et
al (2015) | Germany | 1946–2008 | 39.0 | A | 4,054 | 457 | 434 | 159 | 13 | NA | 49 | 48 | 12 | 20 | 30 | (38) |
| Zablotska et
al (2014) | Canada | 1956–1993 | 13.4 | O | 15,937 | NA | 208 | NA | NA | NA | NA | NA | NA | NA | NA | (32) |
| Drubay et al
(2014) | Germany | 1968–2003 | 34.8 | O | 58,985 | NA | NA | NA | NA | NA | NA | NA | 165 | NA | NA | (30) |
| Silver et al
(2013) | USA | 1951–2004 | 37.0 | O | 5,551 | 786 | NA | 285 | 23 | 20 | 30 | 77 | 15 | 21 | 71 | (42) |
| Richardson et
al (2013) | USA | 1943–2008 | 39.0 | O | 5,919 | 467 | NA | 110 | 6 | 7 | 7 | 46 | 6 | 14 | NA | (26) |
| Zablotska et
al (2013) | Canada | 1950–1999 | 6.4 | A | 2,645 | 266 | 225 | 99 | 5 | NA | 14 | 37 | 6 | 10 | 21 | (39) |
| Gilbert et
al (2013) | Russia | 1953–2008 | NA | O | 14,621 | NA | NA | 486 | NA | NA | NA | NA | NA | NA | NA | (33) |
| Dufey et al
(2013) | Germany | 1946–2003 | 34.0 | A | 58,987 | NA | NA | NA | NA | 159 | NA | NA | NA | NA | NA | (50) |
| Metz-Flamant et
al (2011) | France | 1968–2004 | 27.6 | All | 36,769 | NA | 2,035 | 508 | 102 | 72 | 88 | 206 | 58 | 51 | 137 | (47) |
| Chan et al
(2010) | USA | 1952–2003 | NA | O | 6,759 | 461 | NA | 146 | 16 | NA | 11 | NA | 5 | 4 | 18 | (48) |
| Guseva et
al(2010) | France | 1968–2005 | 28.0 | B | 2,709 | 193 | NA | 48 | 7 | 5 | 6 | 19 | 14 | NA | NA | (45) |
| Muirhead et
al (2009) | UK | 1976–2001 | NA | All | 174,541 | 7,136 | NA | 2,130 | 261 | 83 | 498 | 823 | 170 | 261 | 605 | (17) |
| Boice et
al(2008) | USA | 1979–2005 | 36.4 | A | 2,745 | 246 | NA | 117 | 5 | 9 | 5 | 12 | 6 | 4 | 13 | (40) |
| Ahn et al
(2008) | Korea | 1992–2004 | NA | O | 30,147 | 72 | NA | 7 | NA | NA | NA | NA | NA | NA | NA | (49) |
| Gun et al
(2008) | Australia | 1982–2001 | NA | O | 10,983 | 1,465 | NA | 433 | NA | 19 | 77 | 209 | NA | 9 | 136 | (28) |
| Telle-Lamberton
et al (2007) | France | 1968–1994 | 17.8 | All | 29,204 | 745 | NA | 159 | 25 | 26 | 30 | NA | 24 | 16 | 32 | (34) |
| Engels et al
(2005) | Belgium | 1969–1994 | 22.0 | O | 7,229 | 79 | NA | 25 | 5 | 6 | 5 | 10 | NA | 7 | NA | (37) |
| Habib et al
(2005) | Australia | 1970–1998 | 17.4 | O | 4,717 | 135 | NA | 26 | 6 | 1 | 6 | 18 | 6 | 6 | 12 | (36) |
| Pinkerton et
al (2004) | USA | 1979–1998 | 37.0 | A | 1,484 | 184 | NA | 78 | NA | 4 | NA | 14 | 4 | NA | 15 | (41) |
| Shilnikova et
al (2003) | Russia | 1948–1997 | NA | All | 21,557 | 1,854 | 1,730 | 569 | NA | 67 | 308 | 142 | NA | 39 | NA | (35) |
| Dupree-Ellis et
al (2000) | USA | 1942–1993 | 34.6 | O | 2,514 | 283 | 257 | 98 | 12 | 2 | 4 | 36 | 8 | 8 | 23 | (43) |
| Ritz et al
(2008) | USA | 1959–1994 | 25.4 | O | 2,297 | 133 | NA | 46 | 6 | NA | 6 | 15 | 5 | 3 | 7 | (51) |
Solid cancer analysis
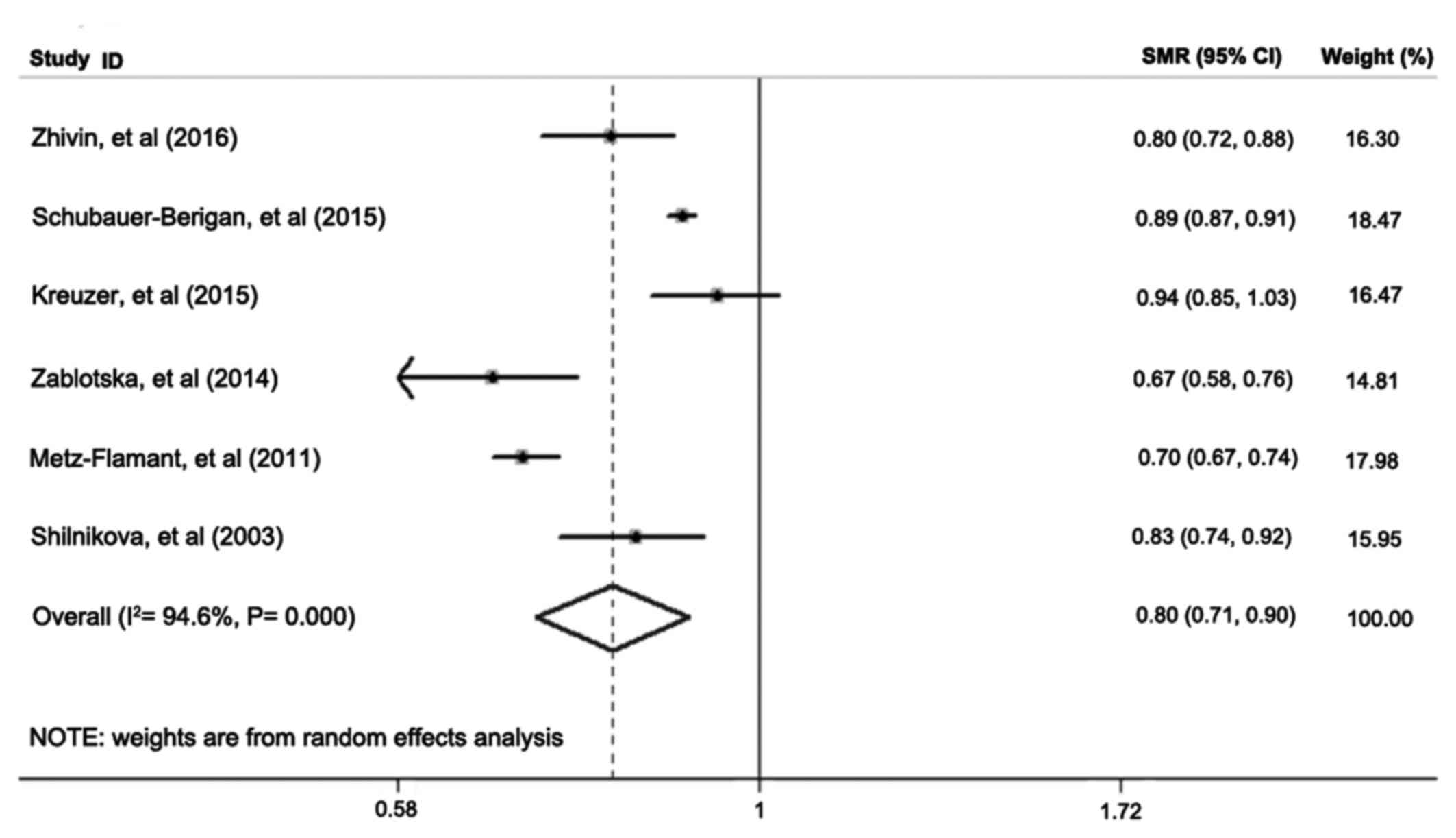
Only 6 of the 27 studies reported SMR for the solid
cancers of interest. The meta-SMR (95% CI) of solid cancers in
nuclear industry workers was 0.80 (0.71–0.90) after a meta-analysis
using the random-effects model (Fig.
2). The fixed-effects model yielded a meta-SMR (95% CI) of 0.85
(0.84–0.87), as shown in Table II.
There was significant heterogeneity across the 6 studies
(I2=94.6%, P=0.00). These results indicated that LDIR
did not significantly increase solid cancer mortality risk.
 | Table II.Meta-analysis results on SMR and
heterogeneity analysis for solid cancers of interest in nuclear
industry workers. |
Table II.
Meta-analysis results on SMR and
heterogeneity analysis for solid cancers of interest in nuclear
industry workers.
|
| SMR (95% CI) | Heterogeneity
analysis |
|---|
|
|
|
|
|---|
| Category | Fixed-effects
model | Random-effects
model | Q-value | P-value | df | I2
(%) |
|---|
| Total cancer | 0.87
(0.860.88) | 0.88
(0.830.94) | 376.35 | 0.00 | 19 | 95.0 |
| Solid cancer | 0.85
(0.840.87) | 0.80
(0.710.90) | 92.45 | 0.00 | 5 | 94.6 |
| Lung cancer | 0.89
(0.800.98) | 0.89
(0.800.98) | 267.25 | 0.00 | 20 | 92.5 |
| Brain and CNS | 1.05
(0.961.14) | 1.09
(0.981.21) | 18.16 | 0.31 | 16 | 11.9 |
| Liver cancer | 0.73
(0.680.78) | 0.75
(0.670.84) | 20.18 | 0.17 | 15 | 25.7 |
| Stomach cancer | 0.85
(0.800.91) | 0.51
(0.750.97) | 33.18 | 0.01 | 17 | 48.8 |
| Colorectal
cancer | 0.91
(0.870.95) | 0.93
(0.841.04) | 45.72 | 0.00 | 16 | 65.0 |
| Kidney | 0.93
(0.851.01) | 0.93
(0.851.01) | 13.86 | 0.68 | 17 | 0.0 |
| Bladder | 0.87
(0.790.95) | 0.96
(0.801.17) | 40.48 | 0.00 | 16 | 60.5 |
| Prostate | 1.00
(0.941.06) | 0.99
(0.911.08) | 23.69 | 0.07 | 15 | 36.7 |
Analysis of other tumors
The meta-analysis results of the SMR and 95% CI for
the 8 solid cancers of interest in this study are shown in Table II. The combined SMR was lower
compared with that for total cancer (0.87), solid cancer (0.85),
lung (0.89), liver (0.73) and stomach cancer (0.85) compared with
the general population, and the homogeneity for colorectal cancer,
bladder cancer and prostate cancer was unsatisfactory using the
random-effects model after the meta-analysis. The heterogeneity
analysis revealed significance of the SMR of total cancer, solid
cancer, lung, stomach, colorectal cancer, bladder and prostate
cancer (P<0.1), and the I2 value was <50% for
stomach and prostate cancers. However, the SMRs of the brain and
CNS, liver and kidney cancers displayed little heterogeneity in
nuclear industry workers (P>0.1). Furthermore, the I2
value for kidney cancer was 0.00%, with the same results for SMR
obtained using the fixed-effects and random-effects models. The
forest plots for the SMR of the 8 solid cancers of interest are not
shown.
Sensitivity analysis
Sensitivity analysis was conducted to explore
potential sources of heterogeneity in the association between solid
cancer mortality risk and LDIR, and to determine the influence of
various exclusion criteria on the overall risk estimates. No
sensitivity analysis was performed for total cancer, solid cancer,
or lung cancer as the heterogeneity (I2) was >90% for
these cases. The analysis of brain and CNS cancer produced a
meta-SMR (95% CI) of 1.16 (1.02–1.31), with the exclusion of the
Muirhead et al study, using the fixed-effects model
(I2=0.00%, P=0.02) (17).
Exclusion of the study by Gun et al (28) decreased the SMR for heterogeneity in
colorectal cancer (I2) to 14.6% (P=0.00) and the
combined SMR was 0.88 (95% CI: 0.82–0.94) using the random-effects
model. However, in kidney cancer, exclusion of the Rage et
al study decreased the P-value to 0.03 with the I2
remaining at 0.00% (29), and the
difference was statistically significant.
Subgroup analysis of the observed SMR was not
performed in the present study, as the reviewed studies did not all
include grouping in their reports.
Publication bias
No sign of publication bias was observed when the
funnel plots were examined, although the heterogeneity of total
cancer, solid cancer and lung cancer was relatively high (Table III). The result of Begg's test
(continuity corrected) and Egger's test did not indicate evidence
of publication bias (P>0.1).
 | Table III.Begg's and Egger's tests of the
reviewed studies in the metaanalysis of solid cancers of interest
from LDIR in the nuclear industry. |
Table III.
Begg's and Egger's tests of the
reviewed studies in the metaanalysis of solid cancers of interest
from LDIR in the nuclear industry.
| Cancer
category | Begg's test | Egger's test |
|---|
| Total cancer | 0.974 | 0.563 |
| Solid | 0.452 | 0.340 |
| Lung | 0.651 | 0.413 |
| Brain and CNS | 0.127 | 0.332 |
| Liver | 0.685 | 0.562 |
| Stomach | 0.820 | 0.657 |
| Colorectal | 0.837 | 0.607 |
| Kidney | 0.596 | 0.446 |
| Bladder | 0.650 | 0.197 |
| Prostate | 0.558 | 0.494 |
Discussion
There has been a rapidly growing interest in the
association between LDIR and stochastic effects in nuclear industry
workers. It has been demonstrated that LDIR may increase the
mortality and morbidity risk of solid cancers, particularly in the
lung, brain and CNS, liver and kidney. However, there have been no
published pooled studies investigating point-estimate risk of
radiation-induced health effects in workers involved in uranium
mining, milling, machining and reprocessing. The present study
reviewed the available relevant literature to investigate whether
exposure to LDIR affects the mortality of solid cancers, and
focused on uranium-processing workers, excluding exposure in the
medical setting, radiation research, nuclear weapons manufacturing
and nuclear power plant industries.
There are several complicating factors of solid
cancer mortality risk in the nuclear industry, such as age at first
exposure, mean length of occupational exposure, follow-up period,
race, type of occupation, socioeconomic status and lifestyle.
Meta-analyses combine multiple articles to highlight the advantages
of SMR, while avoiding the limitations inherent in sporadic, single
reports. Thus, our meta-analysis of the 27 independent
observational studies provided strong evidence that LDIR increases
solid cancer mortality risk, compared with control populations,
despite the SMR being <1. Unfortunately, the large international
cohort studies assessed the excess mortality risk of cancer using
excess relative risk (ERR) rather than SMR (15,31–33). We
found that the high heterogeneity (I2=94.6%, P=0.00) in
solid cancer mortality risk was attributed to the 6 complete
studies, but there was enough evidence to conclude that LDIR could
significantly increase brain and CNS cancer mortality risk
(combined SMR=1.16; 95% CI: 1.02–1.31), regardless of whether the
fixed-effects or random-effects model was used. Subgroup analysis
was not performed, as its over stratification would substantially
reduce the subject-pool size, but the result of sensitivity
analysis of brain and CNS, colorectal and kidney cancers, it was
statistically significant with little heterogeneity (17,28,29). The
90% CI of SMR was applied in 2 studies (34,35);
this value was difficult to convert to 95% CI for the present
meta-analysis, and the calculation of skewed distribution may have
reduced the precision. Another source of bias was the combined
colorectal SMR in three individual parts of the colon, small
intestines and rectum in several studies (17,26,36–47),
which could increase heterogeneity. Unfortunately, while the type
of work-related exposure may be similar, a large-scale study also
has significant differences in sensitivity and may have skewed the
results of the meta-analysis.
As the observed populations were not limited only to
uranium workers, but included subjects whose primary duties were
not mining, such as office administrators, it was quite difficult
to determine the effect outcomes of solid cancer mortality
resulting from LDIR in the nuclear industry based only on the
current studies using non-standard protocols (48). An epidemiological study (11) published in 2014 reported that
exposure to the physicochemical properties of uranium could
increase the lung cancer mortality risk of nuclear industrial
workers, compared with the general population. Similarly, our
results demonstrated the relative SMR of lung cancer in
uranium-processing workers. There was no statistical significance
of SMR for increasing total and solid cancer mortality risk in
uranium facility workers when combined with nuclear power plant and
medicinal research (49), and the
health worker effect was observed. This effect consists of three
components, namely the health worker survival (17–19),
health worker exposure (52–55) and health worker selection (55,56)
effects in occupational exposure epidemiology, and leads to the
selection of a working population that is healthier compared with
the general population. As a result, the observed SMR of the
cancers of interest in this study was lower compared with the
control population. Therefore, it is necessary to control the
deviation observed in healthcare workers and adjust the sensitivity
indicators when comparing the health effects of LDIR exposure of
these subjects to uranium-processing workers.
While the absorbed dose of uranium is widely
considered as benchmark data to analyze the dose-response
association between LDIR and cancer mortality risk, we did not
address LDIR dosimetry with ERR in analyzing the influence on
uranium workers in this meta-analysis. During the initial study
design, it was intended to collect and compare the ERR among the
target tumors. In the final study design, however, acquisition and
expression of these data and the effect on tumor outcomes was
exceedingly difficult and unsatisfactory, as it was also reported
by Zhivin et al (11). Dose
level, radiation category, dose monitor standard and particle size
associated with LDIR all affect cancer mortality risk, but these
factors are often overlooked in basic epidemiological studies.
Additionally, classical epidemiological methods rely on risk
stratification rather than adjustment for complex factors, and may
lead to errors in the analysis of cancer mortality risk.
In the present study, we evaluated the health
outcomes due to tumor-related mortality due to LDIR exposure in the
uranium industry, despite the fact that the results were complex.
As most of the occupational environmental epidemiological findings
combined SMR, we did not obtain a positive result. This should be
attributed to the collection and analysis of raw data from multiple
studies using multiple collection and reporting methodologies. In
summary, the results of analytical epidemiological studies lacking
statistical efficacy are unsubstantiated. Similarly, the
significance of the results gained from a hybrid study that
increases statistical performance, but lacks a unified theoretical
basis, is also limited.
In summary, the present epidemiological study cannot
report definitive findings on the association between LDIR and
cancer mortality risk. Based on the available data, a preliminary
conclusion could be proffered, using meta-analysis with SMR, that
exposure to uranium IR may increase cancer mortality risk,
particularly from solid cancers, lung cancer, brain and CNS cancer,
colorectal cancer, kidney cancer, bladder cancer and prostate
cancer. A convincing and exact outcome could be reached if a more
complete study was performed and results that are more precise
could be calculated using commonly accepted statistical methods
with standardized protocols.
Acknowledgements
Not applicable.
Funding
The present study was supported by Chinese Science
and Technology Plan Project (no. 2014GB112006), National Natural
Science Foundation of China (no. 81372921), The Nuclaer Eneragy
Devolopemnt Project (no. 2016-1295) and Postgraduate Research and
Practice Innovation Program of Jiangsu Province (KYCX17-2017).
Author contributions
SGQ and JG performed the meta-analysis and BY
performed the data analysis. SGQ, JG and BT participated in writing
this paper, YPS and YT designed the study and participated in
writing the paper. All authors have read and approved this
manuscript.
Availability of data and materials
The analysed data sets generated during the study
are available from the corresponding authors on reasonable
request.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that that they have no competing
interests.
References
|
1
|
Ozasa K, Shimizu Y, Suyama A, Kasagi F,
Soda M, Grant EJ, Sakata R, Sugiyama H and Kodama K: Studies of the
mortality of atomic bomb survivors, Report 14, 1950–2003: An
overview of cancer and noncancer diseases. Radiat Res. 177:229–243.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kamiya K, Ozasa K, Akiba S, Niwa O, Kodama
K, Takamura N, Zaharieva EK, Kimura Y and Wakeford R: Long-term
effects of radiation exposure on health. Lancet. 386:469–478. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gorsky AI, Maksioutov MA, Tumanov KA,
Shchukina NV, Chekin SY and Ivanov VK: Non-parametric analysis of
radiation risks of mortality among chernobyl clean-up workers.
Radiats Biol Radioecol. 56:138–148. 2016.(In Russian). PubMed/NCBI
|
|
4
|
Abbott A: Researchers pin down risks of
low-dose radiation. Nature. 523:17–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leuraud K, Richardson DB, Cardis E,
Daniels RD, Gillies M, O'Hagan JA, Hamra GB, Haylock R, Laurier D,
Moissonnier M, et al: Ionising radiation and risk of death from
leukaemia and lymphoma in radiation-monitored workers (INWORKS): An
international cohort study. Lancet Haematol. 2:e276–e281. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cardis E, Vrijheid M, Blettner M, Gilbert
E, Hakama M, Hill C, Howe G, Kaldor J, Muirhead CR,
Schubauer-Berigan M, et al: The 15-country collaborative study of
cancer risk among radiation workers in the nuclear industry:
Estimates of radiation-related cancer risks. Radiat Res.
167:396–416. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hall J, Jeggo PA, West C, Gomolka M,
Quintens R, Badie C, Laurent O, Aerts A, Anastasov N, Azimzadeh O,
et al: Ionizing radiation biomarkers in epidemiological studies-An
update. Mutat Res. 771:59–84. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harbron RW: Cancer risks from low dose
exposure to ionising radiation-is the linear no-threshold model
still relevant? Radiography. 18:28–33. 2012. View Article : Google Scholar
|
|
9
|
Fornalski KW and Dobrzyński L: Pooled
Bayesian analysis of twenty-eight studies on radon induced lung
cancers. Health Phys. 101:265–273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Laurier D, Canu IG, Baatout S, Bertho
J.-M, Blanchardon E, Bouffler S, Cardis E, Gomolka M, Hall J,
Kesminiene A, et al: DoReMi workshop on multidisciplinary
approaches to evaluating cancer risks associated with low-dose
internal contamination. Radioprotection. 47:119–148. 2012.
View Article : Google Scholar
|
|
11
|
Zhivin S, Laurier D and Canu Guseva I:
Health effects of occupational exposure to uranium: Do
physicochemical properties matter? Int J Radiat Biol. 90:1104–1113.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
López-Abente G, Aragonés N and Pollán M:
Solid-tumor mortality in the vicinity of uranium cycle facilities
and nuclear power plants in Spain. Environ Health Perspect.
109:721–729. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Krestinina LY, Preston DL, Ostroumova EV,
Degteva MO, Ron E, Vyushkova OV, Startsev NV, Kossenko MM and
Akleyev AV: Protracted radiation exposure and cancer mortality in
the Techa River Cohort. Radiat Res. 164:602–611. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eidemüller M, Ostroumova E, Krestinina L,
Epiphanova S, Akleyev A and Jacob P: Comparison of mortality and
incidence solid cancer risk after radiation exposure in the Techa
River Cohort. Radiat Environ Biophys. 49:477–490. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Richardson DB, Cardis E, Daniels RD,
Gillies M, O'Hagan JA, Hamra GB, Haylock R, Laurier D, Leuraud K,
Moissonnier M, et al: Risk of cancer from occupational exposure to
ionising radiation: Retrospective cohort study of workers in
France, the United Kingdom and the United States (INWORKS). BMJ.
351:h53592015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hunter N, Kuznetsova IS, Labutina EV and
Harrison JD: Solid cancer incidence other than lung, liver and bone
in Mayak workers: 1948–2004. Br J Cancer. 109:1989–1996. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Muirhead CR, O'Hagan JA, Haylock RG,
Phillipson MA, Willcock T, Berridge GL and Zhang W: Mortality and
cancer incidence following occupational radiation exposure: Third
analysis of the National Registry for Radiation Workers. Br J
Cancer. 100:206–212. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peterson LE and Kovyrshina T: Adjustment
of lifetime risks of space radiation-induced cancer by the healthy
worker effect and cancer misclassification. Heliyon. 1:e000482015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Keil AP, Richardson DB and Troester MA:
Healthy worker survivor bias in the Colorado Plateau uranium miners
cohort. Am J Epidemiol. 181:762–770. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Metz-Flamant C, Laurent O, Samson E,
Caër-Lorho S, Acker A, Hubert D, Richardson DB and Laurier D:
Mortality associated with chronic external radiation exposure in
the French combined cohort of nuclear workers. Occup Environ Med.
70:630–638. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bahr DE, Aldrich TE, Seidu D, Brion GM and
Tollerud DJ: Paducah Gaseous Diffusion Plant Project Team, Muldoon
S, Reinhart N, Youseefagha A and McKinney P Occupational exposure
to trichloroethylene and cancer risk for workers at the Paducah
Gaseous Diffusion Plant. Int J Occup Med Environ Health. 24:67–77.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Samson E, Telle-Lamberton M, Caër-Lorho S,
Bard D, Giraud JM, Metz-Flamant C, Neron MO, Quesne B, Acker A,
Tirmarche M and Hill C: Cancer mortality among two different
populations of French nuclear workers. Int Arch Occup Environ
Health. 84:627–634. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Little MP, Azizova TV, Bazyka D, Bouffler
SD, Cardis E, Chekin S, Chumak VV, Cucinotta FA, de Vathaire F,
Hall P, et al: Systematic review and meta-analysis of circulatory
disease from exposure to low-level ionizing radiation and estimates
of potential population mortality risks. Environ Health Perspect.
120:1503–1511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fournier L, Laurent O, Samson E,
Caër-Lorho S, Laroche P, Le Guen B, Laurier D and Leuraud K:
External radiation dose and cancer mortality among French nuclear
workers: Considering potential confounding by internal radiation
exposure. Int Arch Occup Environ Health. 89:1183–1191. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Figgs LW: Lung cancer mortality among
uranium gaseous diffusion plant workers: A cohort study 1952–2004.
Int J Occup Environ Med. 4:128–140. 2013.PubMed/NCBI
|
|
26
|
Richardson DB, Wing S, Keil A and Wolf S:
Mortality among workers at Oak Ridge National Laboratory. Am J Ind
Med. 56:725–732. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schubauer-Berigan MK, Daniels RD, Bertke
SJ, Tseng CY and Richardson DB: Cancer mortality through 2005 among
a Pooled Cohort of U.S. Nuclear Workers Exposed to External
Ionizing Radiation. Radiat Res. 183:620–631. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gun RT, Parsons J, Crouch P, Ryan P and
Hiller JE: Mortality and cancer incidence of Australian
participants in the British nuclear tests in Australia. Occup
Environ Med. 65:843–848. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rage E, Caër-Lorho S, Drubay D, Ancelet S,
Laroche P and Laurier D: Mortality analyses in the updated French
cohort of uranium miners (1946-2007). Int Arch Occup Environ
Health. 88:717–730. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Drubay D, Ancelet S, Acker A, Kreuzer M,
Laurier D and Rage E: Kidney cancer mortality and ionizing
radiation among French and German uranium miners. Radiat Environ
Biophys. 53:505–513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sokolnikov M, Preston D, Gilbert E,
Schonfeld S and Koshurnikova N: Radiation Effects on Mortality from
Solid Cancers Other than Lung, Liver, and Bone Cancer in the Mayak
Worker Cohort: 1948–2008. PLoS ONE. 10:e01177842015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zablotska LB, Lane RSD and Thompson PA: A
reanalysis of cancer mortality in Canadian nuclear workers
(1956–1994) based on revised exposure and cohort data. Br J Cancer.
110:214–223. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gilbert ES, Sokolnikov ME, Preston DL,
Schonfeld SJ, Schadilov AE, Vasilenko EK and Koshurnikova NA: Lung
Cancer Risks from Plutonium: An Updated Analysis of Data from the
Mayak Worker Cohort. Radiat Res. 179:332–342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Telle-Lamberton M, Samson E, Caër S,
Bergot D, Bard D, Bermann F, Gélas JM, Giraud JM, Hubert P,
Metz-Flamant C, et al: External radiation exposure and mortality in
a cohort of French nuclear workers. Occup Environ Med. 64:694–700.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shilnikova NS, Preston DL, Ron E, Gilbert
ES, Vassilenko EK, Romanov SA, Kuznetsova IS, Sokolnikov ME,
Okatenko PV, Kreslov VV and Koshurnikova NA: Cancer mortality risk
among workers at the Mayak nuclear complex. Radiat Res.
159:787–798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Habib RR, Abdallah SM, Law M and Kaldor J:
Mortality rates among nuclear industry workers at Lucas Heights
Science and Technology Centre. Aust N Z J Public Health.
29:229–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Engels H, Swaen GM, Slangen J, van
Amersvoort L, Holmstock L, Van Mieghem E, Van Regenmortel I and
Wambersie A: Radiation exposure and cause specific mortality among
nuclear workers in Belgium (1969-1994). Radiat Prot Dosimetry.
117:373–381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kreuzer M, Dufey F, Laurier D, Nowak D,
Marsh JW, Schnelzer M, Sogl M and Walsh L: Mortality from internal
and external radiation exposure in a cohort of male German uranium
millers, 1946–2008. Int Arch Occup Environ Health. 88:431–441.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zablotska LB, Lane RS and Frost SE:
Mortality (1950-1999) and cancer incidence (1969-1999) of workers
in the Port Hope cohort study exposed to a unique combination of
radium, uranium and γ-ray doses. BMJ Open. 3:pii: e002159. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Boice JD Jr, Cohen SS, Mumma MT, Chadda B
and Blot WJ: A cohort study of uranium millers and miners of
Grants, New Mexico, 1979–2005. J Radiol Prot. 28:303–325. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pinkerton LE, Bloom TF, Hein MJ and Ward
EM: Mortality among a cohort of uranium mill workers: An update.
Occup Environ Med. 61:57–64. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Silver SR, Bertke SJ, Hein MJ, Daniels RD,
Fleming DA, Anderson JL, Pinney SM, Hornung RW and Tseng CY:
Mortality and ionising radiation exposures among workers employed
at the Fernald Feed Materials Production Center (1951-1985). Occup
Environ Med. 70:453–463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dupree-Ellis E, Watkins J, Ingle JN and
Phillips J: External radiation exposure and mortality in a cohort
of uranium processing workers. Am J Epidemiol. 152:91–95. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Samson E, Piot I, Zhivin S, Richardson DB,
Laroche P, Serond AP, Laurier D and Laurent O: Cancer and
non-cancer mortality among French uranium cycle workers: The TRACY
cohort. BMJ Open. 6:e0103162016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Canu Guseva I, Cardis E, Metz-Flamant C,
Caër-Lorho S, Auriol B, Wild P, Laurier D and Tirmarche M: French
cohort of the uranium processing workers: Mortality pattern after
30-year follow-up. Int Arch Occup Environ Health. 83:301–308. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhivin S, Canu Guseva I, Samson E, Laurent
O, Grellier J, Collomb P, Zablotska LB and Laurier D: Mortality
(1968-2008) in a French cohort of uranium enrichment workers
potentially exposed to rapidly soluble uranium compounds. Occup
Environ Med. 73:167–174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Metz-Flamant C, Samson E, Caër-Lorho S,
Acker A and Laurier D: Solid cancer mortality associated with
chronic external radiation exposure at the French atomic energy
commission and nuclear fuel company. Radiat Res. 176:115–127. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chan C, Hughes TS, Muldoon S, Aldrich T,
Rice C, Hornung R and Tollerud DJ: Mortality Patterns Among Paducah
Gaseous Diffusion Plant Workers. J Occup Environ Med. 52:725–732.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ahn YS, Park RM and Koh DH: Cancer
admission and mortality in workers exposed to ionizing radiation in
Korea. J Occup Environ Med. 50:791–803. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dufey F, Walsh L, Sogl M, Tschense A,
Schnelzer M and Kreuzer M: Radiation dose dependent risk of liver
cancer mortality in the German uranium miners cohort 1946–2003. J
Radio Pro. 33:175–185. 2013. View Article : Google Scholar
|
|
51
|
Ritz B, Morgenstern H, Crawford-Brown D
and Young B: The effects of internal radiation exposure on cancer
mortality in nuclear workers at Rocketdyne. Environ Health
Perspect. 108:743–751. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Greenland S, Fischer HJ and Kheifets L:
Methods to explore uncertainty and bias introduced by job exposure
matrices. Risk Anal. 36:74–82. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nielsen MB and Knardahl S: The healthy
worker effect: Do health problems predict participation rates in,
and the results of, a follow-up survey? Int Arch Occup Environ
Health. 89:231–238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Burstyn I, Lavoué J and Van Tongeren M:
Aggregation of exposure level and probability into a single metric
in job-exposure matrices creates bias. Ann Occup Hyg. 56:1038–1050.
2012.PubMed/NCBI
|
|
55
|
Punnett L: Adjusting for the healthy
worker selection effect in cross-sectional studies. Int J
Epidemiol. 25:1068–1076. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Pearce N, Checkoway H and Kriebel D: Bias
in occupational epidemiology studies. Occup Environ Med.
64:562–568. 2007. View Article : Google Scholar : PubMed/NCBI
|