Introduction
Cutaneus Squamous Cell Carcinoma (cSCC) is a common
malignancy in elderly, representing nearly 25% of non-melanoma skin
cancers. Its incidence is continuously rising due to aging
population and increased ultraviolet exposure (1).
Platinum-based combination therapies demonstrated
high efficacy in the locally advanced or metastatic settings.
However, they are not always able to guarantee durable responses
(2), highlighting the need to assess
new therapeutic options, especially for the recurrent disease no
more amenable to surgery.
So far, no targeted therapy has been introduced as
‘standard of care’ for cSCC. However, multiple studies have
reported deregulation of the EGFR-signaling cascades (RAS-MAPK and
PI3K-AKT-mTOR axes) and/or other pathways (i.e., Notch, p53,
CDKN2A) (3,4). A recent comprehensive genomic profiling
of 315 cancer genes in 122 cSCC cases showed that 88% harbored at
least one clinically relevant mutation, with an average of 2.5
actionable genomic alterations per patient (5). Thus, conventional and unconventional
deregulated pathways eventually amenable for therapeutic
intervention are being found also in cSCC. In example, anti-EGFR
monoclonal antibodies have been used in platinum-resistant advanced
cSCC patients with clinical benefit and improved toxicity profiles
(6).
Curcumin is a dietary polyphenol derived from the
root of the plant Curcuma Longa, which has been shown to possess
anti-inflammatory and anti-cancer activities both in vitro
and in vivo (7,8). In vitro, it causes cell growth
inhibition and/or apoptosis in multiple cancer cell models
(9) and has been reported to inhibit
several cancer-related pathways such as PI3K-AKT-mTOR and EGFR axes
(10). In particular, Curcumin
demonstrated to reduce the invasion and adhesive abilities of cSCC
A431 cells by the inhibition of Signal Transducer and Activator of
Transcription 3 (STAT3) activation (11). Furthermore, Phillips et al
reported that Curcumin was able to significantly reduce cSCC tumor
progression in vivo by inhibiting S6 phosphorylation and, as
a consequence, the mTOR pathway (12).
Here we report the first description of an elderly
cSCC patient, resistant to conventional treatments, but
successfully responding to an anti-EGFR pathway inibition with a
‘chemo-free’ combination of Cetuximab with a daily oral dose of
Curcumin phospholipid supplement.
Data collection methods
DNA extraction
DNA was extracted from Formalin-Fixed and
Paraffin-Embedded (FFPE) tumor tissue, obtained by skin lesion
biopsy. Xylene was added once and ethanol was added twice to remove
all paraffin from the tissue sample. The DNA was extracted using
QIAamp DNA FFPE Tissue kit (Qiagen GmbH, Hilden, Germany) according
to the manufacturer's instructions (13). Eluted DNA was quantified with Qubit
2.0 Fluorometer (Thermo fisher).
IT-PGM sequencing and variant
calling
Approximately 10 ng of DNA was required to construct
the barcoded and adaptor-ligated library using the Ion AmpliSeq
Library Kit 2.0 (Thermo fisher). The sample was analysed using the
Ion AmpliSeq Colon and Lung Cancer Research Panel V2 containing a
single primer pool to amplify hotspots and targeted regions of 22
cancer genes frequently mutated in CRCs and NSCLCs (https://www.ampliseq.com). Templated spheres were
prepared using 100 pM of the library using the Ion One Touch 2.0
machine. Template-positive spheres were loaded into Ion chip 314
and sequenced by IT-PGM machine (Thermo fisher). Sequencing data
were finally analysed with Coverage Analysis and Variant Caller
plugins available within the Ion Torrent Suite software. Variants
with a quality <30 were filtered out. Sequence reads were
finally visualized with IGV tool using HG19 as reference genome to
direct inspection of mutations.
Case report
An 83 years old Caucasian male patient was admitted
in our oncology department on March 2016 for recurrent cSCC of the
supraclavicular region. The patient had been previously treated
with a platinum-based chemotherapy regimen (Carboplatin
AUC4-5-Fluorouracil, 750 mg day 1–5; q21-3 cycles) with neoadjuvant
intent. After an early locoregional disease progression, he had
debulking surgery followed by locoregional radiotherapy. More
specifically, the patient underwent radical ipsilateral neck
dissection with resection of the sternocleidomastoid muscle, the
spinal accessory nerve, the whole collarbone and the internal
jugular vein, required to remove the vast (m.d. 15×8 cm) lesion
infiltrating the underlying tissue. The pathology report confirmed
the diagnosis of poorly differentiated cSCC obtained at the time of
first biopsy and revealed bone infiltration.
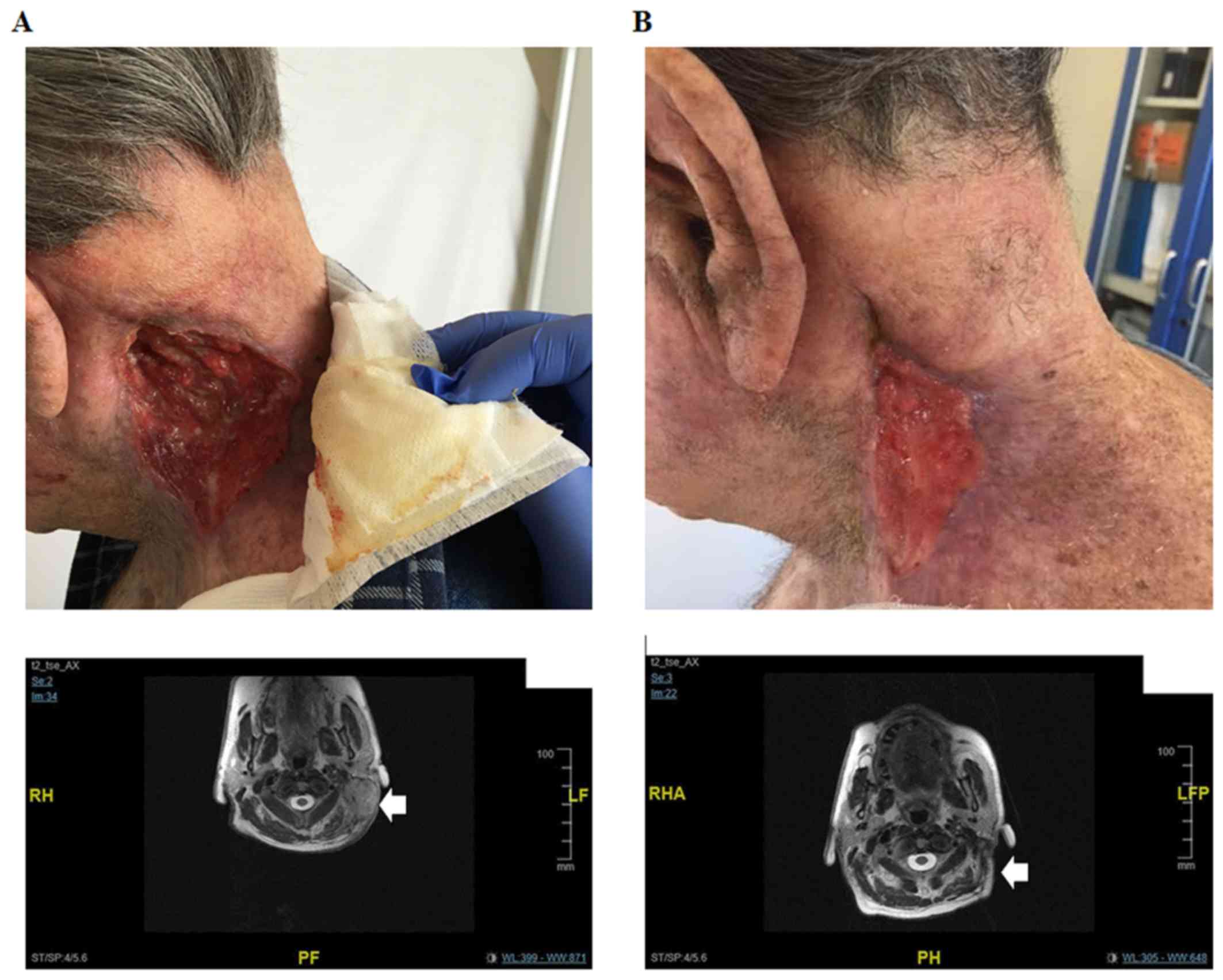
After 9 months, a head and neck MRI revealed a
locoregional disease progression characterized by a 6.4×3.6 cm
nuchal lesion and a 10.5×4.8 cm lesion in the supraclavicular
region having a cranium-caudal extension of 15 cm. The tumor had
also infiltrated the left paravertebral muscle, the left parotid
gland and the lax cellular tissue of the supraclavicular region. An
increase in cervical, submandibular and nuchal lymph nodes
dimension was also detectable. Moreover, the supraclavicular 10 cm
lesion appeared ulcerated at the medical examination (Fig. 1A).
Given the extent of disease and its lack of response
to previous treatments, a new systemic therapy appeared necessary
for the patient. In order to consider alternative treatment
opportunities dictated by tumor biology, we evaluated the
mutational profile of the tumor at the time of the latest
progression. DNA was extracted from FFPE tumor tissue, obtained by
skin lesion biopsy. This was sequenced on an Ion Torrent PGM
platform by the Ion AmpliSeq Colon and Lung research Panel V2. The
tumor sample showed no mutation in most relevant EGFR signal
transducers. In contrast, it harbored pathogenic mutations in the
FGFR3 and TP53 genes and a likely pathogenic mutation in the MAP2K1
gene (Table I).
 | Table I.Genetic variants found in the cSCC
patient by IT-PGM sequencing. |
Table I.
Genetic variants found in the cSCC
patient by IT-PGM sequencing.
| Genea | Mutationb | AFc (%) |
Relevanced | Prediction
testj | (Refs.) |
|---|
| FGFR3 | c.1138G>A
p.Gly380Arg (COSM24842) | 28.7 |
Pathogenice,f |
| (23,24) |
|
| c.1953G>A
p.Thr651=(rs7688609) | 100.0 | Benigne,g |
|
|
| EGFR | c.1498+22 A>T
(rs1558544) | 100.0 | NCg,i | NNSPLICE:
Unchanged |
|
|
| c.2361G>A
p.Gln787=(rs1050171) | 100.0 | Benigne,g |
|
|
| MET | c.534C>T
p.Ser178Ser (rs35775721) | 67.2 | Benigng |
|
|
| MAP2K1 | c.174G>C
p.Gln58His | 15.8 | Likely
pathogenici | PROVEAN:
Deleterius; |
|
|
|
|
|
| SIFT:
Damaging; |
|
|
|
|
|
| PolyPhen: Possibly
damaging; |
|
|
|
|
|
| CRAVAT: High
pathogenicity cancer driver impact |
|
| TP53 | c.743G>A p.
Arg248Gln (COSM10662) | 21.1 |
Pathogenice,f,h |
| (25–27) |
|
| c.836_861del27 | 31.1 | Likely
pathogenici | PROVEAN:
Deleterius |
|
|
|
p.Gly279_Asn288delinsAsp |
|
| CRAVAT: High
pathogenicity cancer driver impact |
|
|
| c.215 C>G
p.Pro72Arg, (rs1042522, COSM250061) | 60.2 | Uncertain
significancee,f,i |
| (30) |
|
| c.1-46C>T | 21.2 | NA | NNSPLICE:
Unchanged |
|
The lack of other established therapeutic
alternatives, the positive results obtained by a recent phase II
Study (14) employing Cetuximab and
the absence of mutations on major components of the EGFR signal
transduction pathway met the ethical constraints of a tailored
therapy, and prompted us to propose an off-label Cetuximab-based
combination treatment.
Based on its known anti-inflammatory and anti-tumor
properties and in consideration of its ability to inhibit
cancer-related pathways, in this particular case we added a daily
supplementation of oral Curcumin phospholipid (Meriva, 500 mg
orally once daily) to the conventional Cetuximab schedule (400
mg/mq, followed by subsequent weekly doses of 250 mg/mq). The
protocol was approved by the institutional review board (Ospedale
Sant'Andrea, Sapienza University of Rome) on March 2016, and the
patient provided written informed consent. Already after 4 weeks of
this treatment, a significant clinical benefit was reported by the
patient. In particular, the lesion's bleeding stopped and a 5 cm
reduction in size was reported at the medical examination. After 12
weeks, a head and neck MRI confirmed the clinical response and
showed an ~50% reduction of the lesions. The infiltration of the
left parotid gland was no longer detectable and a dramatic
regression in cervical, submandibular and nuchal lymph nodes was
evidenced (Fig. 1B). Thus, the
patient responded to treatment and experienced no recurrence for 11
months. After 12 weeks, no major therapy-related toxicity had been
observed. On the contrary, treatment regimen had been well
tolerated and patient complained only for minor skin-related
toxicity (maximum grade 2 according to CTCAE version 4.0).
Discussion
In recent years, given the limited efficacy of
standard-of-care chemotherapy and radiotherapy for patients with
locally advanced or systemic disease, several investigators have
begun to study the genomic background of cSCC looking for novel
actionable targets. In particular, the widespread employment of NGS
techniques allowed to identify a large number of potentially
actionable driver genes (15,16). As
previously recognized for other tumors of epithelial origin, the
RAS-RAF-MEK-ERK and the PI3K/AKT branches acting downstream of RTKs
such as EGFR are very frequently mutated/deregulated also in cSCC
(17). Nonetheless, no targeted
therapy options have entered the routine and wide use for patients
with cSCC, yet (18,19). EGFR inhibitors should, in principle,
be considered a valid therapeutic option also for cSCC, since EGFR
seems to be overexpressed in a high proportion of primary tumors of
this type, which acquire a metastatic phenotype (20). The absence of mutations in most
relevant RTK-signal transducers revealed by our molecular profiling
allowed us to offer the patient a therapeutic intervention based on
Cetuximab (chimeric mouse-human anti-EGFR IgG1 monoclonal
antibody). Initially approved for the treatment of advanced
colorectal cancer, and subsequently employed in advanced or
platinum-refractory Head and Neck Squamous Cell Carcinoma (HNSCC),
Cetuximab monotherapy has also proved its efficacy and low toxicity
profile in advanced and metastatic cSCC, as described in several
case reports or case series (21,22).
In particular, in a recent prospective phase II
trial carried out by Maubec et al, comprehensive of 36
patients, a 67% rate of 6-weeks disease control supported the use
of single agent Cetuximab as first-line treatment for unresectable
cSCC (14).
Nonetheless, the activation of alternative
intracellular networks involved in the maintenance of the malignant
phenotype could also limit the benefit of anti-EGFR treatment.
In our specific case, genetic profiling of the tumor
tissue showed pathogenic mutations in FGFR3, TP53 and MAP2K1 genes.
The p.Gly380Arg FGFR3 mutation has been reported in patients
affected with achondroplasia, the most common form of human
dwarfism (23), and in cancer
patients (24). It causes an
increased and ligand-independent phosphorylation of FGFR3 leading
to constitutive activation of the downstream pathway, largely
accounted for by MAPK and PI3K/AKT activity. Two different TP53
mutations coexisted in our cSCC case: The p.Arg248Gln is a known
gain of function p53 mutation (25–27),
while the p.Gly279_Asn288delinsAsp is a previously undescribed
in-frame deletion which is predicted to be highly pathogenic. The
low allelic frequency of both TP53 mutations suggests that both
alleles of this gene might be destroyed in the tumor tissue. The
MAP2K1 p.Gln58His mutation is also previously undescribed, but
multiple prediction tools indicate it is most likely pathogenic
(Table I). In principle, both the
FGFR3 and the MAP2K1 mutations could have impaired the effect of
anti-EGFR treatment, in this patient.
Interestingly, Curcumin is endowed with potent
antinflammatory and cancer chemopreventive and therapeutic
properties. Although the precise molecular mechanism of Curcumin
action is far from being completely understood, it regulates the
expression of several genes involved in cytokines production,
cellular proliferation and cell survival (28), part of which are known TP53
targets.
Recent studies have pointed out that pharmacological
doses of Curcumin can inhibit EGFR pathway in different squamous
malignancies (8). Moreover, it has
also been shown to enhance the inhibitory effect generated by drugs
directly targeting EGFR, while also improving their toxicity
profile (9). Preclinical evidences
further indicated its ability to overcome anti-EGFR therapy
resistance (29). All of these
observations provided the rational for its use in combination with
Cetuximab in the reported case. While we are aware that the
description of a single cSCC case does not allow us to draw major
conclusion on the possibility that Curcumin phospholipid oral
supplement might potentiate the efficacy of the anti-EGFR
treatment, it is important to notice that this strategy was well
tolerated and resulted in a highly effective control of the disease
in a heavily pretreated cSCC patient.
Based on our preliminary observation, we believe
that the benefit of a combined anti-EGFR/Curcumin treatment on
molecularly stratified cSCC patients should be further addressed
with a specific clinical trial.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from:
Associazione Italiana per la Ricerca sul Cancro (grant no.
IG17734), Ministry of University and Research, PRIN projects and
Istituto Pasteur-Fondazione Cenci Bolognetti to GG.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CC, CP, GG, MF and PM were involved in patient
recruitment. FB, MP, VC, AT and AC were involved in sample
preparation and sequencing. CC, FB, MS, PM, GG were involved in
data analysis. CC, FB, MF, CP, MP, VC, AT, MS, AC, GG, and PM were
involved in manuscript writing and editing.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Written informed consent was obtained from the
patient for the publication of this case.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Green AC and Olsen CM: Cutaneous squamous
cell carcinoma: An epidemiological review. Br J Dermatol.
177:373–381. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Behan JW, Sutton A and Wysong A:
Management of skin cancer in the high-risk patient. Curr Treat
Options Oncol. 17:602016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Swick AD, Prabakaran PJ, Miller MC, Javaid
AM, Fisher MM, Sampene E, Ong IM, Hu R, Iida M, Nickel KP, et al:
Cotargeting mTORC and EGFR signaling as a therapeutic strategy in
HNSCC. Mol Cancer Ther. 16:1257–1268. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iglesias-Bartolome R, Martin D and Gutkind
JS: Exploiting the head and neck cancer oncogenome: Widespread
PI3K-mTOR pathway alterations and novel molecular targets. Cancer
Discov. 3:722–725. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Al-Rohil RN, Tarasen AJ, Carlson JA, Wang
K, Johnson A, Yelensky R, Lipson D, Elvin JA, Vergilio JA, Ali SM,
et al: Evaluation of 122 advanced-stage cutaneous squamous cell
carcinomas by comprehensive genomic profiling opens the door for
new routes to targeted therapies. Cancer. 122:249–257. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suen JK, Bressler L, Shord SS, Warso M and
Villano JL: Cutaneous squamous cell carcinoma responding serially
to single-agent cetuximab. Anticancer Drugs. 18:827–829. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang X, Li Z, Wang N, Li L, Song L, He T,
Sun L, Wang Z, Wu Q, Luo N, et al: Curcumin-encapsulated polymeric
micelles suppress the development of colon cancer in vitro and in
vivo. Sci Rep. 5:103222015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Starok M, Preira P, Vayssade M, Haupt K,
Salomé L and Rossi C: EGFR inhibition by curcumin in cancer cells:
A dual mode of action. Biomacromolecules. 16:1634–1642. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wada K, Lee JY, Hung HY, Shi Q, Lin L,
Zhao Y, Goto M, Yang PC, Kuo SC, Chen HW and Lee KH: Novel curcumin
analogs to overcome EGFR-TKI lung adenocarcinoma drug resistance
and reduce EGFR-TKI-induced GI adverse effects. Bioorg Med Chem.
23:1507–1514. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kasi PD, Tamilselvam R, Skalicka-Woźniak
K, Nabavi SF, Daglia M, Bishayee A, Pazoki-Toroudi H and Nabavi SM:
Molecular targets of curcumin for cancer therapy: An updated
review. Tumour Biol. 37:13017–13028. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu J, Lu WY and Cui LL: Inhibitory effect
of curcumin on invasion of skin squamous cell carcinoma A431 cells.
Asian Pac J Cancer Prev. 16:2813–2818. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Phillips JM, Clark C, Herman-Ferdinandez
L, Moore-Medlin T, Rong X, Gill JR, Clifford JL, Abreo F and Nathan
CO: Curcumin inhibits skin squamous cell carcinoma tumor growth in
vivo. Otolaryngol Head Neck Surg. 145:58–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giannini G, Capalbo C, Ottini L, Buffone
A, de Marchis L, Margaria E, Vitolo D, Ricevuto E, Rinaldi C, Zani
M, et al: Clinical classification of BRCA1 DNA missense variants:
H1686Q is a novel pathogenic mutation occurring in the
ontogenetically invariant THV motif of the N-terminal BRCT domain.
J Clin Oncol. 26:4212–4214; author reply, 4214–4215. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maubec E, Petrow P, Scheer-Senyarich I,
Duvillard P, Lacroix L, Gelly J, Certain A, Duval X, Crickx B,
Buffard V, et al: Phase II study of cetuximab as first-line
single-drug therapy in patients with unresectable squamous cell
carcinoma of the skin. J Clin Oncol. 29:3419–3426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Belardinilli F, Capalbo C, Buffone A,
Petroni M, Colicchia V, Ferraro S, Zani M, Nicolussi A, D'Inzeo S,
Coppa A, et al: Validation of the Ion Torrent PGM sequencing for
the prospective routine molecular diagnostic of colorectal cancer.
Clin Biochem. 48:908–910. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Capalbo C, Marchetti P, Coppa A, Calogero
A, Anastasi E, Buffone A, Belardinilli F, Gulino M, Frati P,
Catalano C, et al: Vemurafenib and panitumumab combination tailored
therapy in BRAF-mutated metastatic colorectal cancer: A case
report. Cancer Biol Ther. 15:826–831. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Toll A, Salgado R, Yébenes M,
Martín-Ezquerra G, Gilaberte M, Baró T, Solé F, Alameda F, Espinet
B and Pujol RM: Epidermal growth factor receptor gene numerical
aberrations are frequent events in actinic keratoses and invasive
cutaneous squamous cell carcinomas. Exp Dermatol. 19:151–153. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schwaederle M, Elkin SK, Tomson BN, Carter
JL and Kurzrock R: Squamousness: Next-generation sequencing reveals
shared molecular features across squamous tumor types. Cell Cycle.
14:2355–2361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pickering CR, Zhou JH, Lee JJ, Drummond
JA, Peng SA, Saade RE, Tsai KY, Curry JL, Tetzlaff MT, Lai SY, et
al: Mutational landscape of aggressive cutaneous squamous cell
carcinoma. Clin Cancer Res. 20:6582–6592. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shimizu T, Izumi H, Oga A, Furumoto H,
Murakami T, Ofuji R, Muto M and Sasaki K: Epidermal growth factor
receptor overexpression and genetic aberrations in metastatic
squamous-cell carcinoma of the skin. Dermatology. 202:203–206.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wollina U: Cetuximab in non-melanoma skin
cancer. Expert Opin Biol Ther. 12:949–956. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Seber S, Gonultas A, Ozturk O and
Yetisyigit T: Recurrent squamous cell carcinoma of the skin treated
successfully with single agent cetuximab therapy. Onco Targets
Ther. 9:945–948. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takagi M, Kouwaki M, Kawase K, Shinohara
H, Hasegawa Y, Yamada T, Fujiwara I, Sawai H, Nishimura G and
Hasegawa T: A novel mutation Ser344Cys in FGFR3 causes
achondroplasia with severe platyspondyly. Am J Med Genet A.
167A:2851–2854. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
L'Hôte CG and Knowles MA: Cell responses
to FGFR3 signalling: Growth, differentiation and apoptosis. Exp
Cell Res. 304:417–431. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shtraizent N, Matsui H, Polotskaia A and
Bargonetti J: Hot spot mutation in TP53 (R248Q) causes oncogenic
gain-of-function phenotypes in a breast cancer cell line derived
from an African American patient. Int J Environ Res Public Health.
13:ijerph130100222015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Muller PA and Vousden KH: Mutant p53 in
cancer: New functions and therapeutic opportunities. Cancer Cell.
25:304–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu J, Wang J, Hu Y, Qian J, Xu B, Chen H,
Zou W and Fang JY: Unequal prognostic potentials of p53
gain-of-function mutations in human cancers associate with
drug-metabolizing activity. Cell Death Dis. 5:e11082014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jobin C, Bradham CA, Russo MP, Juma B,
Narula AS, Brenner DA and Sartor RB: Curcumin blocks
cytokine-mediated NF-kappa B activation and proinflammatory gene
expression by inhibiting inhibitory factor I-kappa B kinase
activity. J Immunol. 163:3474–3483. 1999.PubMed/NCBI
|
|
29
|
Li S, Liu Z, Zhu F, Fan X, Wu X, Zhao H
and Jiang L: Curcumin lowers erlotinib resistance in non-small cell
lung carcinoma cells with mutated EGF receptor. Oncol Res.
21:137–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Olivier M, Hollstein M and Hainaut P: TP53
mutations in human cancers: Origins, consequences, and clinical
use. Cold Spring Harb Perspect Biol. 2:a0010082010. View Article : Google Scholar : PubMed/NCBI
|