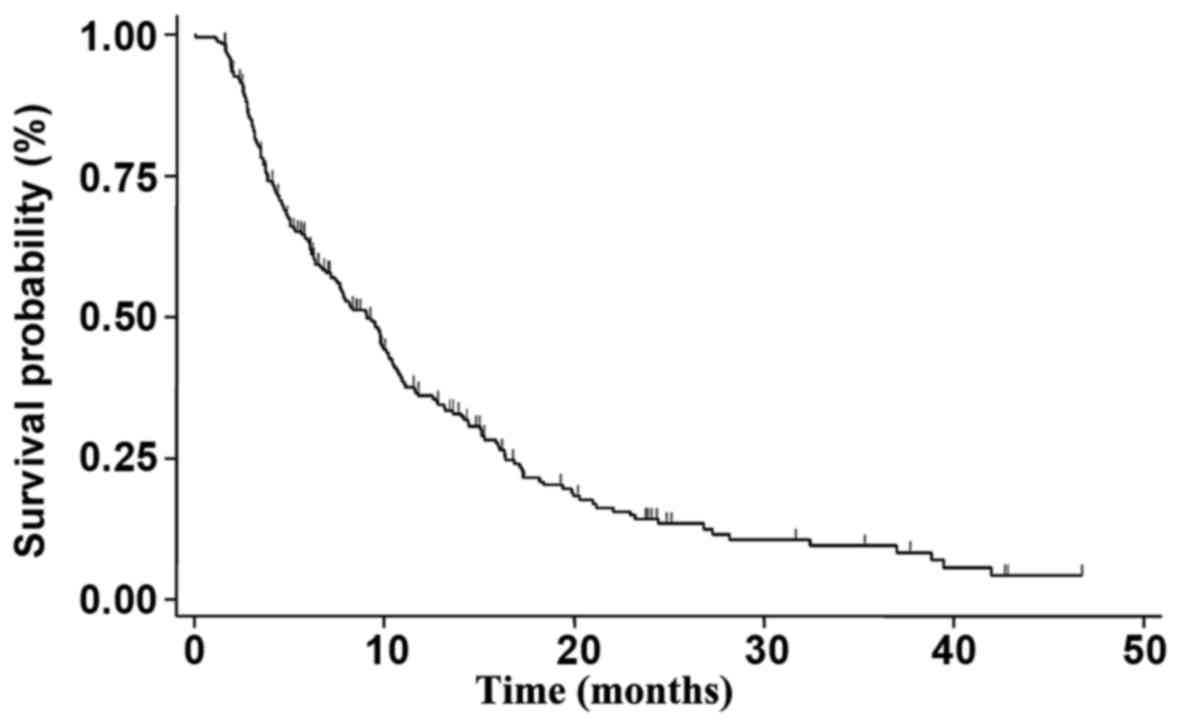
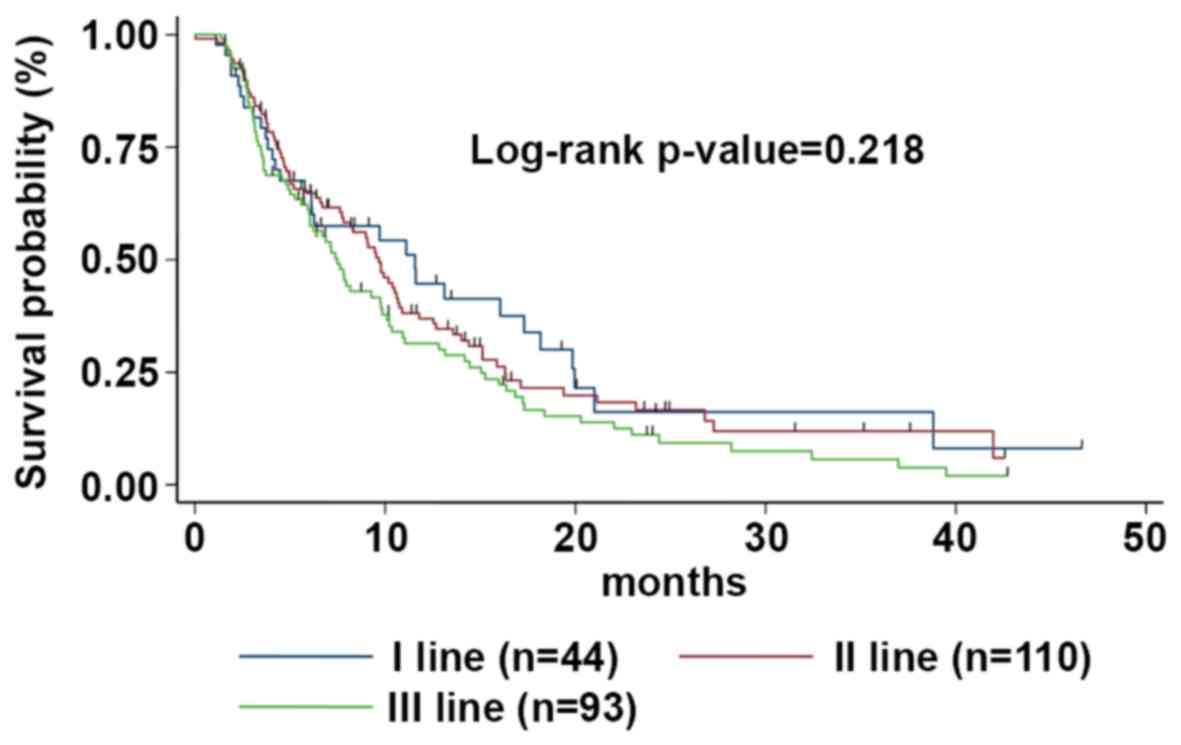
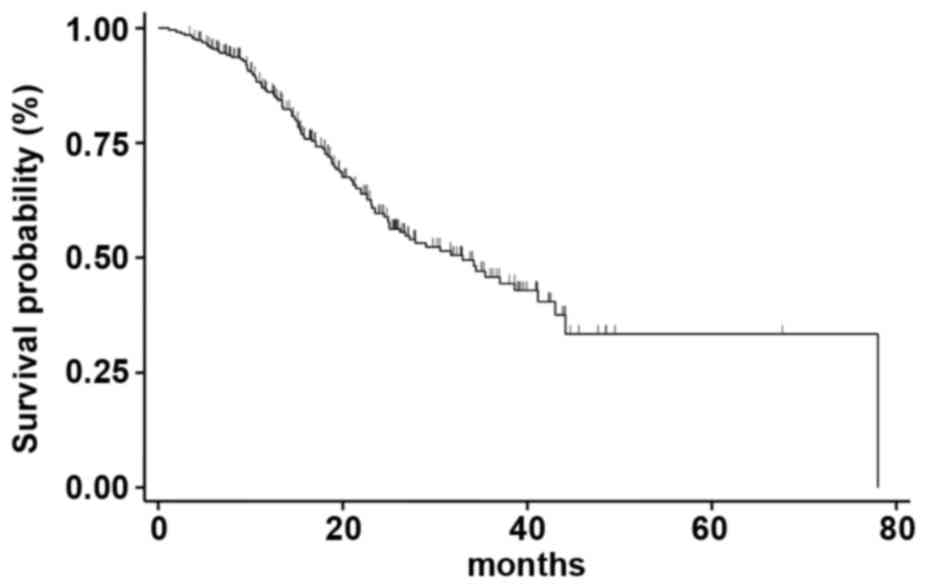
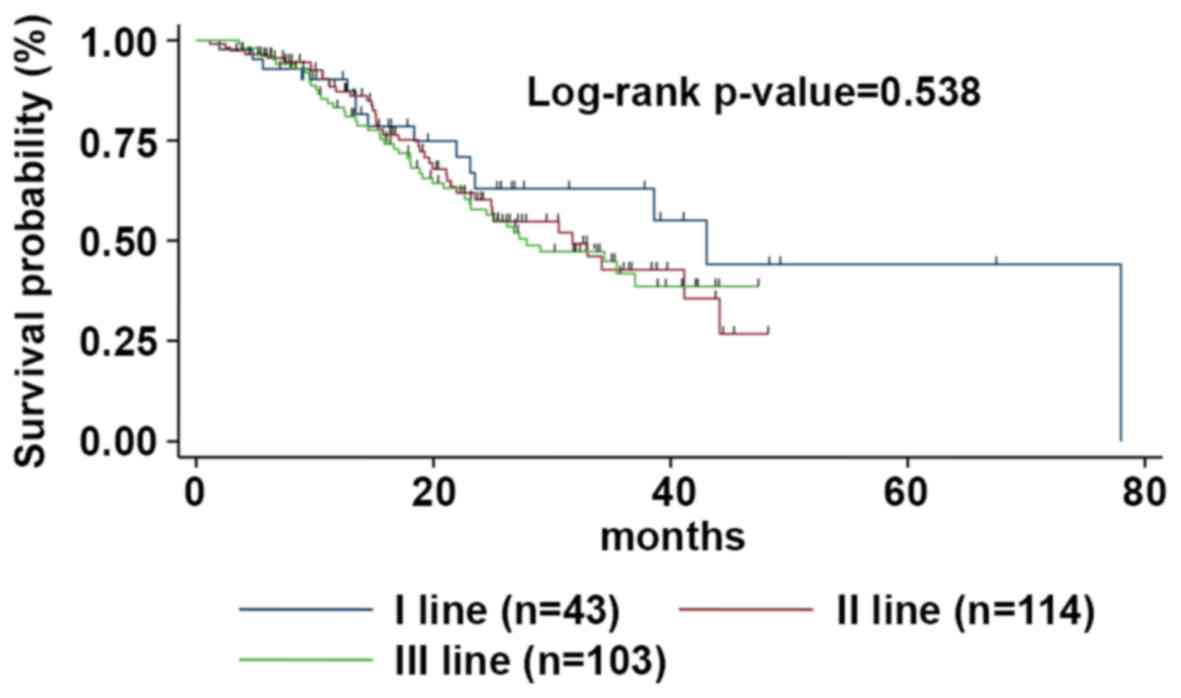
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Anderson WF, Chatterjee N, Ershler WB and
Brawley OW: Estrogen receptor breast cancer phenotypes in the
Surveillance, Epidemiology, and End Results database. Breast Cancer
Res Treat. 76:27–36. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cardoso F, Costa A, Senkus E, Aapro M,
André F, Barrios CH, Bergh J, Bhattacharyya G, Biganzoli L, Cardoso
MJ, et al: 3rd ESO-ESMO International Consensus Guidelines for
Advanced Breast Cancer (ABC 3). Ann Oncol. 28:16–33. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
National Comprehensive Cancer Network, :
NCCN Clinical Practice Guidelines in Oncology Breast Cancer Version
1.2018. https://www.nccn.org/professional/physician_gls/pdf/breast.pdfApril
6–2018
|
|
5
|
Osborne CK and Schiff R: Mechanisms of
endocrine resistance in breast cancer. Annu Rev Med. 62:233–247.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bachelot T, Bourgier C, Cropet C,
Ray-Coquard I, Ferrero JM, Freyer G, Abadie-Lacourtoisie S, Eymard
JC, Debled M, Spaëth D, et al: Randomized phase II trial of
everolimus in combination with tamoxifen in patients with hormone
receptor-positive, human epidermal growth factor receptor
2-negative metastatic breast cancer with prior exposure to
aromatase inhibitors: A GINECO study. J Clin Oncol. 30:2718–2724.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baselga J, Semiglazov V, van Dam P,
Manikhas A, Bellet M, Mayordomo J, Campone M, Kubista E, Greil R,
Bianchi G, et al: Phase II randomized study of neoadjuvant
everolimus plus letrozole compared with placebo plus letrozole in
patients with estrogen receptor-positive breast cancer. J Clin
Oncol. 27:2630–2637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boulay A, Rudloff J, Ye J, Zumstein-Mecker
S, O'Reilly T, Evans DB, Chen S and Lane HA: Dual inhibition of
mTOR and estrogen receptor signaling in vitro induces cell death in
models of breast cancer. Clin Cancer Res. 11:5319–5328. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baselga J, Campone M, Piccart M, Burris HA
III, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun
F, et al: Everolimus in postmenopausal hormone-receptor-positive
advanced breast cancer. N Engl J Med. 366:520–529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yardley DA, Noguchi S, Pritchard KI,
Burris HA III, Baselga J, Gnant M, Hortobagyi GN, Campone M,
Pistilli B, Piccart M, et al: Everolimus plus exemestane in
postmenopausal patients with HR(+) breast cancer: BOLERO-2 final
progression-free survival analysis. Adv Ther. 30:870–884. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Burris HA III, Lebrun F, Rugo HS, Beck JT,
Piccart M, Neven P, Baselga J, Petrakova K, Hortobagyi GN,
Komorowski A, et al: Health-related quality of life of patients
with advanced breast cancer treated with everolimus plus exemestane
versus placebo plus exemestane in the phase 3, randomized,
controlled, BOLERO-2 trial. Cancer. 119:1908–1915. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Turner NC, Ro J, André F, Loi S, Verma S,
Iwata H, Harbeck N, Loibl S, Huang Bartlett C, Zhang K, et al:
Palbociclib in hormone-receptor-positive advanced breast cancer. N
Engl J Med. 373:209–219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cristofanilli M, Turner NC, Bondarenko I,
Ro J, Im SA, Masuda N, Colleoni M, De Michele A, Loi S, Verma S, et
al: Fulvestrant plus palbociclib versus fulvestrant plus placebo
for treatment of hormone-receptor-positive, HER2-negative
metastatic breast cancer that progressed on previous endocrine
therapy (PALOMA-3): Final analysis of the multicentre,
double-blind, phase 3 randomised controlled trial. Lancet Oncol.
17:425–439. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hortobagyi GN, Stemmer SM, Burris HA, Yap
YS, Sonke GS, Paluch-Shimon S, Campone M, Blackwell KL, André F,
Winer EP, et al: Ribociclib as first-line therapy for HR-positive,
advanced breast cancer. N Engl J Med. 375:1738–1748. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sledge GW Jr, Toi M, Neven P, Sohn J,
Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, et al:
MONARCH 2: Abemaciclib in combination with fulvestrant in women
with HR+/HER2-advanced breast cancer who had progressed while
receiving endocrine therapy. J Clin Oncol. 35:2875–2884. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Common Terminology Criteria for Adverse
Events (CTCAE), Version 4.03. June 14–2010, US Department of Health
and Human Services. National Institutes of Health National Cancer
Institute; http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_Quick
Reference_5×7.pdfOctober 8–2017
|
|
17
|
Kaplan EL and Meier P: Nonparametric
estimation from incomplete observations. J Am Stat Assoc.
53:457–481. 1958. View Article : Google Scholar
|
|
18
|
Piccart M, Hortobagyi GN, Campone M,
Pritchard KI, Lebrun F, Ito Y, Noguchi S, Perez A, Rugo HS, Deleu
I, et al: Everolimus plus exemestane for hormone-receptor-positive,
human epidermal growth factor receptor-2-negative advanced breast
cancer: Overall survival results from BOLERO-2. Ann Oncol.
25:2357–2362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rugo HS, Pritchard KI, Gnant M, Noguchi S,
Piccart M, Hortobagyi G, Baselga J, Perez A, Geberth M, Csoszi T,
et al: Incidence and time course of everolimus-related adverse
events in postmenopausal women with hormone receptor-positive
advanced breast cancer: Insights from BOLERO-2. Ann Oncol.
25:808–815. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jerusalem G, Mariani G, Ciruelos EM,
Martin M, Tjan-Heijnen VC, Neven P, Gavila JG, Michelotti A,
Montemurro F, Generali D, et al: Safety of everolimus plus
exemestane in patients with hormone-receptor-positive,
HER2-negative locally advanced or metastatic breast cancer
progressing on prior non-steroidal aromatase inhibitors: Primary
results of a phase IIIb, open-label, single-arm, expanded-access
multicenter trial (BALLET). Ann Oncol. 27:1719–1725. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moscetti L, Vici P, Gamucci T, Natoli C,
Cortesi E, Marchetti P, Santini D, Giuliani R, Sperduti I, Mauri M,
et al: Safety analysis, association with response and previous
treatments of everolimus and exemestane in 181 metastatic breast
cancer patients: A multicenter Italian experience. Breast.
29:96–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rugo HS, Hortobagyi GN, Yao J, Pavel M,
Ravaud A, Franz D, Ringeisen F, Gallo J, Rouyrre N, Anak O and
Motzer R: Meta-analysis of stomatitis in clinical studies of
everolimus: Incidence and relationship with efficacy. Ann Oncol.
27:519–525. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rugo HS, Seneviratne L, Beck JT, Glaspy
JA, Peguero JA, Pluard TJ, Dhillon N, Hwang LC, Nangia C, Mayer IA,
et al: Prevention of everolimus-related stomatitis in women with
hormone receptor-positive, HER2-negative metastatic breast cancer
using dexamethasone mouthwash (SWISH): A single-arm, phase 2 trial.
Lancet Oncol. 18:654–662. 2017. View Article : Google Scholar : PubMed/NCBI
|