Introduction
Breast cancer is the most frequently diagnosed
cancer and the leading cause of cancer death in women worldwide,
accounting for 23% (1.4 million) of the total new cancer cases and
14% (458,400) of the total cancer deaths in 2008 (1,2).
Breast cancer is a heterogeneous disease and has
distinct morphological features and tumor subtypes (3–6). Breast
cancer is classified into 4 subtypes: Luminal A (hormone receptor
positive, HER2 negative), luminal B (hormone receptor positive,
HER2 positive), HER2-enriched (hormone receptor negative, HER2
positive), and triple negative (hormone receptor negative, HER2
negative) by microarray and hierarchical clustering analysis
(7–11). It is now classified into 5 subtypes
using Ki-67 expression. This classification has been used to
formulate guidelines for breast cancer therapy. It was used to
determine systemic adjuvant therapies by subtype and risk
categories in 2007 per the St. Gallen consensus meeting (12). The 11th St. Gallen (Switzerland)
expert consensus meeting on the primary treatment of early breast
cancer in March 2009 maintained an emphasis on targeting adjuvant
systemic therapies to subgroups as defined by these predictive
markers (13,14).
We followed these guidelines and based our breast
cancer therapy on expression of hormone receptors and HER2. Little
is known about the prognosis of Japanese breast cancer patients
treated according to subtype. There are no articles that described
long-term prognosis and compared more recent outcomes with those of
the 1990s. In this study, we examined prognosis of Japanese breast
cancer patients from the 2000s, during which time we followed
guidelines for therapy.
Patients and methods
A total of 662 patients with stage 0–III breast
cancer underwent surgical resection in the Kitasato University
Hospital between January 2006 and March 2009. We classified them
into 4 subtypes: Hormone receptor positive/HER2 negative, hormone
receptor positive/HER2 positive, hormone receptor negative/HER2
positive, and hormone receptor negative/HER2 negative known as
triple negative. Systemic therapy was consistent with
recommendations for each of the biological subtypes.
Characteristics of the 662 patients are shown in Table I. Median follow-up period was 77
(2–109) months. TNM classification was used based on the 7th
edition of the Union for International Cancer Control (UICC).
Clinical lymph node metastasis was considered more than 10 mm minor
axis based on computed tomography. The patients with neoadjuvant
chemotherapy (NAC) were also classified to pTNM. The positive
cut-off value of serum CEA was higher than 5 ng/ml.
 | Table I.Clinicopathologic characteristics of
the 662 patients. |
Table I.
Clinicopathologic characteristics of
the 662 patients.
| Factors | No. | % |
|---|
| Patient | 662 | 100.0 |
| Age (median) | 56 (24–93) |
|
| Sex |
|
|
|
Female | 657 | 99.3 |
|
Male | 5 | 0.7 |
| Neo adjuvant
therapy |
|
|
|
Yes | 75 | 11.3 |
| No | 587 | 88.7 |
| cTa |
|
|
| T0 | 104 | 15.7 |
| T1 | 280 | 42.3 |
| T2 | 215 | 32.5 |
| T3 | 32 | 4.8 |
| T4 | 31 | 4.7 |
| cN |
|
|
| N0 | 530 | 80.1 |
| N1 | 91 | 13.7 |
| N2 | 32 | 4.8 |
| N3 | 9 | 1.4 |
| cStage |
|
|
| 0 | 104 | 15.5 |
| I | 255 | 38.5 |
| II | 228 | 34.7 |
|
III | 75 | 11.3 |
| Serum CEA |
|
|
|
5> | 607 | 91.7 |
| 5≤ | 55 | 8.3 |
| Surgical
method |
|
|
|
Lumpectomy | 450 | 68.1 |
|
Mastectomy | 212 | 32.0 |
|
Lymphadenectomy |
|
|
|
Sentinel lymphnode biopsy | 360 | 54.4 |
|
Axillary lymphadenectomy | 267 | 40.3 |
| Not
performed | 35 | 5.3 |
| Pathological
type |
|
|
| Ductal
carcinoma in situ | 106 | 16.0 |
| Lobular
carcinoma in situ | 2 | 0.3 |
|
Invasive ductal carcinoma | 500 | 75.5 |
|
Invasive lobular
carcinoma | 54 | 8.2 |
| Nuclear grade |
|
|
| Grade
1 | 205 | 31.0 |
| Grade
2 | 98 | 14.8 |
| Grade
3 | 176 | 26.6 |
|
Unknown | 183 | 27.6 |
| pT |
|
|
| T0 | 10 | 1.5 |
|
Tis | 108 | 16.3 |
| T1 | 295 | 44.6 |
| T2 | 189 | 28.6 |
| T3 | 28 | 4.2 |
| T4 | 32 | 4.8 |
| pN |
|
|
|
pN0 | 451 | 68.1 |
|
pN1 | 138 | 20.8 |
|
pN2 | 39 | 5.9 |
|
pN3 | 22 | 3.3 |
|
Unknown | 12 | 1.8 |
| pStage |
|
|
| 0 | 116 | 17.5 |
| I | 225 | 34.0 |
| II | 227 | 34.3 |
|
III | 94 | 14.2 |
| HR (IHC) |
|
|
|
Positive | 522 | 78.9 |
|
Negative | 140 | 21.1 |
| HER 2 (IHC and/or
FISH) |
|
|
|
Positive | 67 | 10.2 |
|
Negative | 593 | 89.6 |
|
Unknown | 2 | 0.2 |
| Subtype |
|
|
|
HR+/HER2− | 503 | 76.0 |
|
HR+/HER2+ | 19 | 2.9 |
|
HR−/HER2+ | 48 | 7.3 |
|
HR−/HER2− | 92 | 13.8 |
| Postoperative
adjuvant therapy |
|
|
|
Yes | 597 | 90.2 |
| No | 65 | 9.8 |
| Recurrence |
|
|
|
Yes | 69 | 10.4 |
| No | 593 | 89.6 |
| Succumbed |
|
|
|
Yes | 26 | 4.0 |
| No | 636 | 96.0 |
We extracted 87 cases with hormone receptor
positive/HER2 negative and cN positive/pN positive between April,
2009 and December, 2014 as the validation group. Median follow-up
was 51 (5–96) months. The present study was approved by the Ethics
Committee of Kitasato University.
Statistical analysis
Recurrence free survival (RFS) and disease specific
survival (DSS) were analysed based on clinicopathologic
characteristics. RFS and DSS were calculated using the Kaplan-Meier
method, and survival differences were assessed using a log-rank
test. Variables suggested to be prognostic factors on univariate
analysis were subjected to multivariate analysis using a Cox
proportional-hazards model. The P-value <0.05 was considered to
indicate statistical significance. All statistical analyses were
conducted with SAS software package (JMP Pro11, SAS Institute,
Cary, NC, USA).
Results
Univariate analysis for RFS and
DSS
5-year RFS of surgically treated breast cancer was
94.9%. Univariate prognostic factors for recurrence were serum
value of CEA≥5.0 ng/ml (CEA positive) before cancer therapy
(P<0.05), cT2-4 (P<0.001), cN positive (P<0.0001), NAC
(P<0.0001), mastectomy (P<0.001), hormone receptor negative
(P<0.001), pT2-4 (P<0.01), and pN positive (P<0.0001)
(Table II). 5-year DSS was 98.4%.
Univariate prognostic factors for DSS were serum value of CEA
positive (P<0.01), cT2-4 (P<0.001), cN positive
(P<0.0001), hormone receptor negative (P<0.01), pT2-4
(P=0.01), and pN positive (P<0.0001) (Table III).
 | Table II.Univariate and multivariate analysis
for recurrence free survival (RFS). |
Table II.
Univariate and multivariate analysis
for recurrence free survival (RFS).
|
| RFS |
|---|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factors | Patient number | RFS (%) | P-value | HR | 95% CI | P-value |
|---|
| Age, years |
|
| 0.87 |
|
|
|
|
>56 | 322 | 86 |
|
|
|
|
|
<56 | 340 | 80 |
|
|
|
|
| Sex |
|
| 0.34 |
|
|
|
|
Female | 657 | 84 |
|
|
|
|
|
Male | 5 | 100 |
|
|
|
|
| CEA |
|
| <0.05 |
|
| NS |
|
5> | 607 | 85 |
| 1.79 | 0.86–3.37 |
|
| 5≤ | 55 | 81 |
|
|
|
|
| cT |
|
| <0.001 |
|
| NS |
|
T0-1 | 384 | 90 |
| 1.14 | 0.41–2.90 |
|
|
T2-4 | 278 | 77 |
|
|
|
|
| cN |
|
| <0.0001 |
|
| <0.01 |
|
Negative | 530 | 88 |
| 2.28 | 1.26–4.28 |
|
|
Positive | 132 | 70 |
|
|
|
|
| cStage (7th
UICC) |
|
| <0.0001 |
|
|
|
| 0 | 102 | 79 |
|
|
|
|
| I | 255 | 91 |
|
|
|
|
| II | 230 | 81 |
|
|
|
|
|
III | 75 | 71 |
|
|
|
|
| Neo adjuvant
therapy |
|
| <0.0001 |
|
|
|
| No | 587 | 86 |
|
|
|
|
|
Yes | 75 | 72 |
|
|
|
|
| Surgical
method |
|
| <0.001 |
|
|
|
|
Lumpectomy | 450 | 91 |
|
|
|
|
|
Mastectomy | 212 | 73 |
|
|
|
|
| Pathological
type |
|
| 0.1 |
|
|
|
|
DCIS | 106 | 74 |
|
|
|
|
|
LCIS | 2 | 100 |
|
|
|
|
|
IDC | 500 | 86 |
|
|
|
|
|
ILC | 54 | 45 |
|
|
|
|
| Hormone
receptor |
|
| <0.001 |
|
| <0.05 |
|
Negative | 140 | 71 |
| 1.83 | 1.08–3.01 |
|
|
Positive | 522 | 89 |
|
|
|
|
| HER2 receptor |
|
| 0.08 |
|
|
|
|
Positive | 67 | 83 |
|
|
|
|
|
Negative | 593 | 85 |
|
|
|
|
| Subtype |
|
|
|
|
|
|
|
HR+/HER2− | 503 | 89 | 0.01 |
|
|
|
|
HR+/HER2+ | 19 | 83 |
|
|
|
|
|
HR−/HER2+ | 48 | 83 |
|
|
|
|
|
HR−/HER2− | 92 | 68 |
|
|
|
|
| pT |
|
| <0.01 |
|
| NS |
|
T0-1 | 413 | 89 |
| 1.02 | 0.42–2.79 |
|
|
T2-4 | 249 | 77 |
|
|
|
|
| pN |
|
| <0.0001 |
|
| 0.001 |
|
Negative | 451 | 92 |
| 2.85 | 1.52–5.30 |
|
|
Positive | 199 | 68 |
|
|
|
|
| pStage (7th
UICC) |
|
| <0.0001 |
|
|
|
| 0 | 116 | 84 |
|
|
|
|
| I | 225 | 90 |
|
|
|
|
| II | 227 | 92 |
|
|
|
|
|
III | 94 | 41 |
|
|
|
|
| Adjuvant
therapy |
|
| 0.2 |
|
|
|
| No | 65 | 94 |
|
|
|
|
|
Yes | 597 | 83 |
|
|
|
|
 | Table III.Univariate and multivariate analysis
for disease specific survival (DSS). |
Table III.
Univariate and multivariate analysis
for disease specific survival (DSS).
|
| DSS |
|---|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factors | Patient number | DSS (%) | P-value | HR | 95% CI | P-value |
|---|
| Age, years |
|
| 0.66 |
|
|
|
|
>56 | 322 | 95 |
|
|
|
|
|
<56 | 340 | 96 |
|
|
|
|
| Sex |
|
| 0.57 |
|
|
|
|
Female | 657 | 96 |
|
|
|
|
|
Male | 5 | 100 |
|
|
|
|
| CEA |
|
| <0.01 |
|
| <0.05 |
|
5> | 607 | 97 |
| 3.04 | 1.17–7.03 |
|
| 5≤ | 55 | 82 |
|
|
|
|
| cT |
|
| <0.001 |
|
| NS |
|
T0-1 | 384 | 98 |
| 2.31 | 0.53–9.45 |
|
|
T2-4 | 278 | 92 |
|
|
|
|
| cN |
|
| <0.0001 |
|
| <0.01 |
|
Negative | 530 | 99 |
| 4.55 | 1.64–15.18 |
|
|
Positive | 132 | 83 |
|
|
|
|
| cStage (7th
UICC) |
|
| <0.0001 |
|
|
|
| 0 | 102 | 100 |
|
|
|
|
| I | 255 | 99 |
|
|
|
|
| II | 230 | 93 |
|
|
|
|
|
III | 75 | 87 |
|
|
|
|
| Neo adjuvant
therapy |
|
| <0.001 |
|
|
|
| No | 587 | 97 |
|
|
|
|
|
Yes | 75 | 86 |
|
|
|
|
| Surgical
method |
|
| 0.01 |
|
|
|
|
Lumpectomy | 450 | 97 |
|
|
|
|
|
Mastectomy | 212 | 92 |
|
|
|
|
| Pathological
type |
|
| 0.33 |
|
|
|
|
DCIS | 106 | 100 |
|
|
|
|
|
LCIS | 2 | 100 |
|
|
|
|
|
IDC | 500 | 95 |
|
|
|
|
|
ILC | 54 | 96 |
|
|
|
|
| Hormone
receptor |
|
| <0.01 |
|
| <0.05 |
|
Negative | 140 | 91 |
| 2.32 | 1.02–5.17 |
|
|
Positive | 522 | 97 |
|
|
|
|
| HER2 receptor |
|
| 0.37 |
|
|
|
|
Positive | 67 | 94 |
|
|
|
|
|
Negative | 593 | 96 |
|
|
|
|
| Subtype |
|
| <0.05 |
|
|
|
|
HR+/HER2− | 503 | 97 |
|
|
|
|
|
HR+/HER2+ | 19 | 94 |
|
|
|
|
|
HR-/HER2+ | 48 | 93 |
|
|
|
|
|
HR-/HER2− | 92 | 89 |
|
|
|
|
| pT |
|
| 0.01 |
|
| NS |
|
T0-1 | 413 | 97 |
| 1.51 | 0.38–5.01 |
|
|
T2-4 | 249 | 93 |
|
|
|
|
| pN |
|
| <0.0001 |
|
| <0.05 |
|
Negative | 451 | 98 |
| 3.40 | 1.08–11.17 |
|
|
Positive | 198 | 89 |
|
|
|
|
| pStage (7th
UICC) |
|
| <0.0001 |
|
|
|
| 0 | 116 | 100 |
|
|
|
|
| I | 225 | 97 |
|
|
|
|
| II | 227 | 98 |
|
|
|
|
|
III | 94 | 82 |
|
|
|
|
| Adjuvant
therapy |
|
| 0.01 |
|
|
|
| No | 65 | 93 |
|
|
|
|
|
Yes | 597 | 96 |
|
|
|
|
Multivariate analysis for RFS and
DSS
Among the 662 patients, multivariate Cox
proportional hazards model identified pN positive (hazards ratio
(HR)=2.85, P=0.001), cN positive (HR=2.28, P<0.01), and hormone
receptor negative (HR=1.83, P<0.05) as significant independent
factors for recurrence (Table II).
In the multivariate model for DSS, cN positive (HR=4.55,
P<0.01), pN positive (HR=3.40, P<0.05), serum value of CEA
positive (HR=3.04, P<0.05) and hormone receptor negative
(HR2.32, P<0.05) were identified as independent prognostic
factors (Table III).
Kaplan-Meier curve of RFS and DSS by
independent prognostic factors based on multivariate analysis
cN status was significantly different in recurrent
cases (RFS of cN positive and negative were 70 and 88%
respectively, P<0.0001). pN status was significantly different
in recurrent cases (RFS of pN positive and negative were 68 and 92%
respectively, P<0.0001). RFS of hormone receptor negative breast
cancer was worse than that of the hormone receptor positive group
(71 and 89% respectively, P<0.001).
cN positive cases had significantly poorer prognoses
than the cN negative group (83 and 99% respectively, P<0.0001).
DSS of hormone receptor negative breast cancers was 91%, whereas
DSS of hormone receptor positive breast cancer was 97% (P<0.01).
The pN positive group showed poorer prognoses than the pN negative
group (89 and 98% respectively, P<0.0001). DSS of the serum CEA
positive group was worse compared with that of the negative group
(83 and 97% respectively, P=0.0001).
Intersection of clinical and
pathological lymph node metastasis
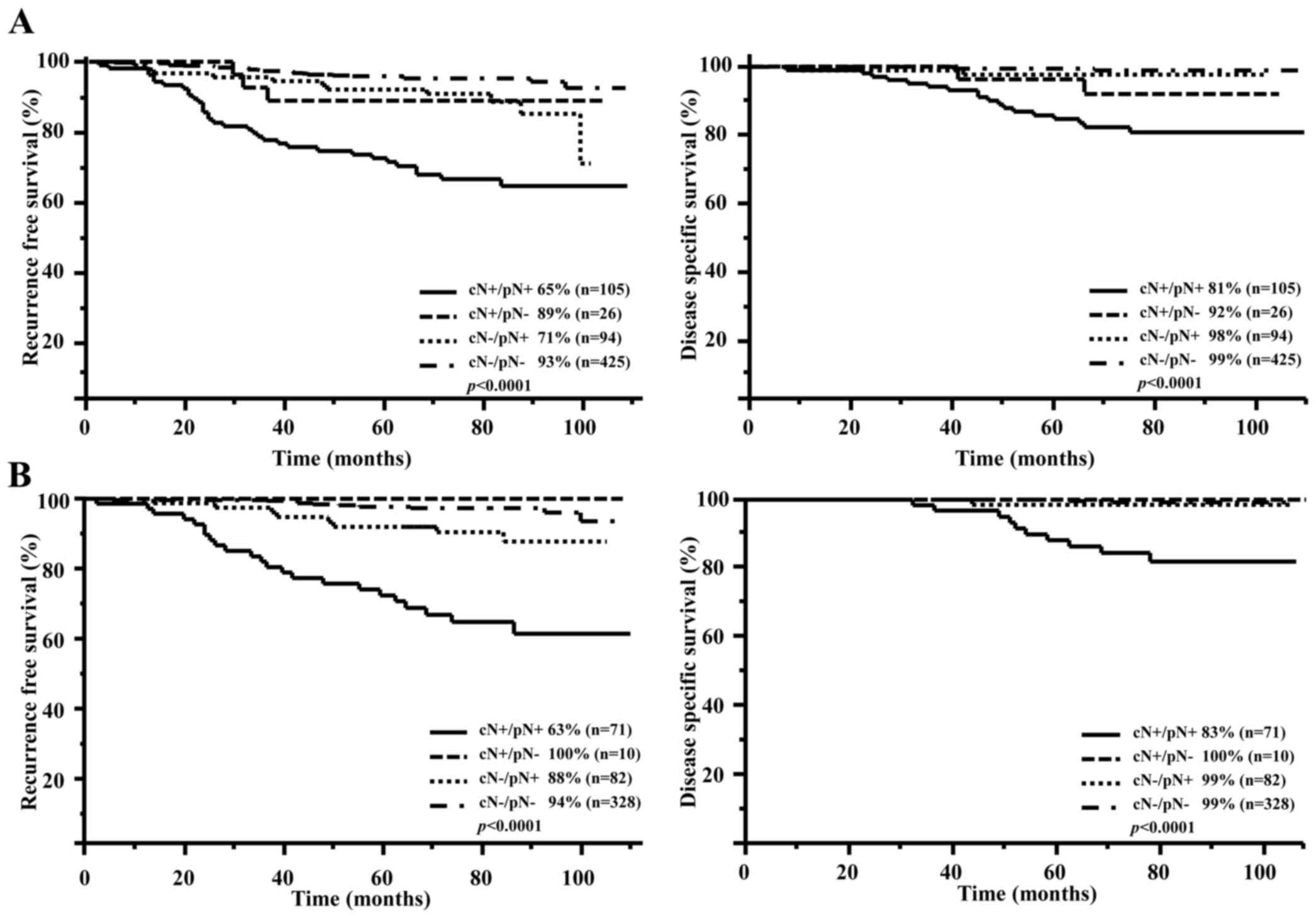
Fig. 1A shows
differences in RFS and DSS based on the intersection of cN and pN
factors. The RFS and DSS of the cN positive/pN positive group were
65 and 81%, respectively. This group was significantly worse off
than the other groups.
Fig. 1B shows the
prognosis of hormone receptor positive/HER2 negative according to
intersection of cN and pN. The cN positive/pN positive group showed
poorer prognoses than the other groups in hormone receptor
positive/HER2 negative types (RFS P<0.0001/DSS P<0.0001).
Lymph node metastasis was not associated with recurrence and was
not a prognostic factor in the other subtype groups.
Kaplan-Meier curve of RFS and DSS by
serum value of CEA before cancer therapy
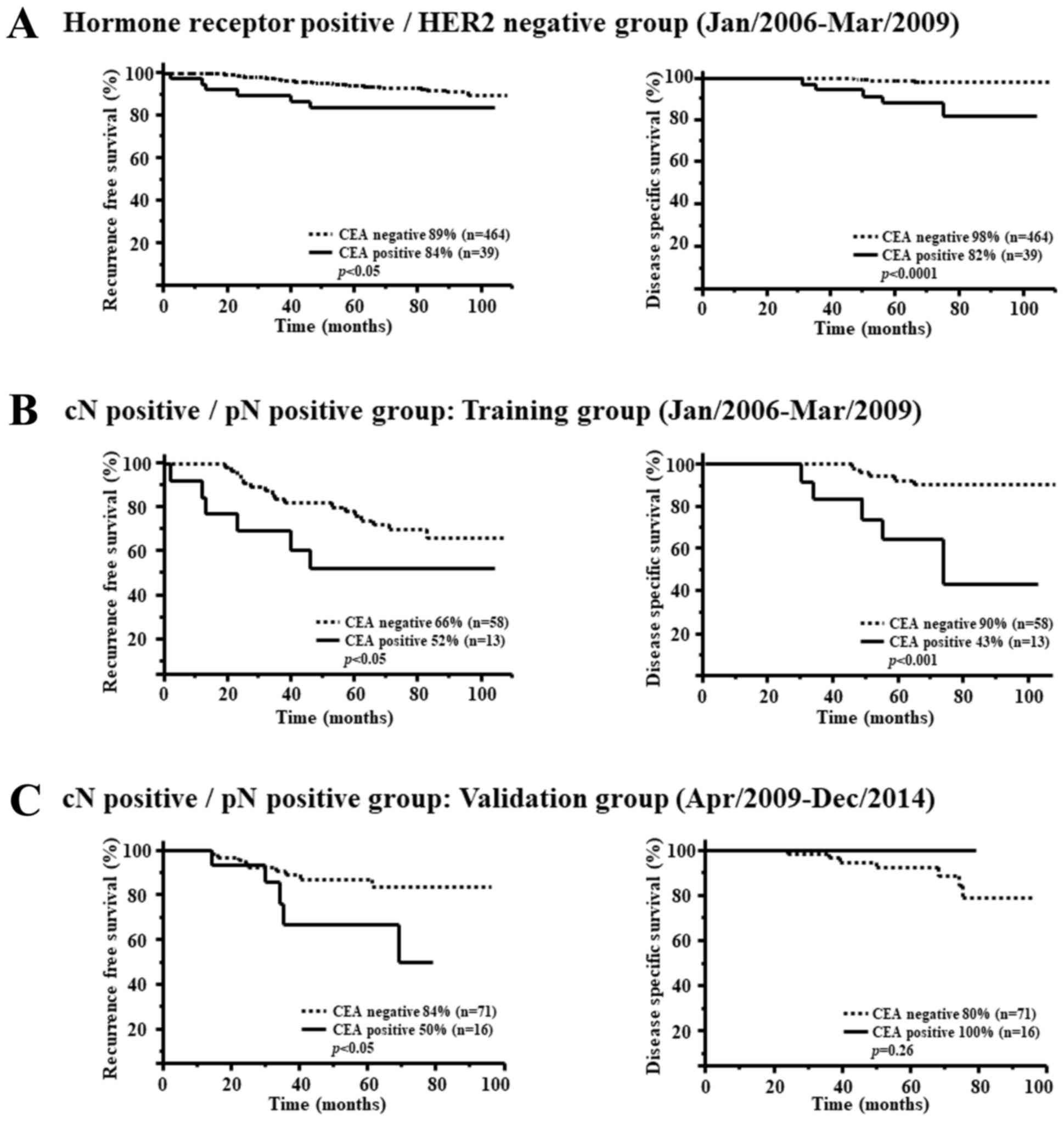
In hormone receptor positive/HER2 negative breast
cancers, RFS and DSS for the serum CEA positive group were
significantly worse compared with the CEA negative group (RFS
P<0.05, DSS P<0.0001) (Fig.
2A).
In the cN positive/pN positive group, the RFS of CEA
positive cases was 52% and for CEA negative cases was 66%
(P<0.05). The DSS of CEA positive cases was 43% and CEA negative
cases was 90% (P<0.001) (Fig.
2B).
The RFS of CEA positive cases was 50% and that of
CEA negative cases was 84% (P<0.05) in the validation group.
There was no significant difference in DSS (P=0.26) (Fig. 2C).
Discussion
For the present research, we clarified breast cancer
treatment according to hormone receptor expression and HER2
expression. We reported treatment outcomes compared with 1995–1996
patients (15). According to results
reported by Nishimiya et al (15), recurrence rates for breast cancer in
1995–1996 was 31.2%. Mortality rate was 24.5%. These numbers were
10.4 and 4% respectively in recent years. For hormone receptor
positive breast cancers, RFS and DSS were 68.42 and 83.05%
respectively in the 1990s. In the 2000s, these numbers were 89 and
97%. These numbers were 60.20 and 64.88% respectively for hormone
receptor negative breast cancer. In the 2000s, these numbers were
71 and 91%. For HER2 positive breast cancer, RFS and DSS were 61.17
and 63.16% in the 1990s, and 83 and 94% in the 2000s respectively.
For HER2 negative breast cancers, these numbers were 67.40 and
81.26% and 85 and 96% respectively. Prognoses have improved in all
subtypes. In the 2000s, it became clear that prognosis was
improving dramatically due to more personalized medical treatment
for breast cancer. All in all, it is believed that the overall
prognosis of breast cancer has improved with diversification of
hormonal therapies and chemotherapy along with the advent of
molecular targeted therapeutic agents (16–23).
Also in Japan, the prognosis for recurrent breast cancer has
improved in the 2000s (24–26).
There is a limitation in this research. Ki-67
subtype was not analyzed yet. Ki-67 is a biomarker used for subtype
classification in hormone receptor positive breast cancers. It has
been drawing attention as a marker for possibility of recurrence
(14,15). Ki-67 cut-off values vary depending on
lab factors including antibodies, time, staining methods, and
methods for scoring. We believe that analysis according to Ki-67
will be necessary in the future.
Within the hormone receptor positive/HER2 negative
group, the cN negative group had a good prognosis. In these cases,
adjuvant hormone therapy was administered for 5 years. Recently,
studies such as ATLAS (27), attom
(28), and MA17R (29) demonstrated the effectiveness of 10
years of hormone therapy. Nevertheless, there is no research
determining the particular circumstances under which hormone
therapy should be administered. In the present study, in
multivariate analysis, factors relating to relapse included cN, pN,
and the presence or absence of hormone receptor. Factors relating
to prognosis were cN, pN, the presence or absence of hormone
receptor, and pretreatment serum CEA values. The analysis of the
Intersection of cN and pN was related to poor prognosis. Within the
hormone receptor positive/HER2 negative group, the prognosis for
the cN positive/pN positive group was particularly poor.
Additionally, within the cN positive/pN positive group in the
hormone receptor positive/HER2 negative group, there was a poor
prognosis for those with high pretreatment CEA levels (Fig. 2B). Serum CEA is a tumor marker used
for early detection and monitoring for recurrence of breast cancer,
and for evaluating progress of therapy in patients with
progressive, recurrent breast cancer in the clinical setting
(30–35). Within the 87 cases of the validation
group, there was a significant rate of recurrence in those with
high serum CEA levels compared with those with low levels of CEA
(P<0.05) (Fig. 2C). Nevertheless,
future surveillance is necessary, since the follow-up period is
insufficient. That is to say, in the cN positive/pN positive group,
contemporary treatments were inadequate. We believe it is an urgent
priority to develop stronger treatment algorithms or to develop new
treatments that exceed the current standard. Taking these results
into consideration, our facility plans to administer hormone
therapy for 10 years to those patients with high serum CEA levels
and cN positive/pN positive breast cancer. We will subsequently
study the therapeutic effects and side effects such as osteoporosis
of prolonged hormone therapy in this population.
Although the number of cases was small, the
prognosis was the same in the cN positive/pN negative, and cN
negative/pN negative groups. In these 10 cases, pretreatment
imaging showed axillary lymph node enlargement of 10 mm or more.
Eight out of the 10 cases received neoadjuvant chemotherapy, and
these became pN negative (ypN0) after surgery. von Minckwitz et
al (36) report a good prognosis
for patients with ypN0 induced by neoadjuvant chemotherapy. In the
hormone receptor positive/HER2 negative/high Ki-67 expression group
(Luminal B), the recurrence rate for pathological complete response
(pCR) cases was low (36). In the
NSABP B18 trial, good prognosis was reported for pCR cases
(37,38). There are additional reports of good
prognosis in pCR cases with neoadjuvant chemotherapy compared with
other cases (39,40). That is to say, aggressive neoadjuvant
chemotherapy should be performed for hormone receptor positive/HER2
negative cases that are cN positive and with high levels of Ki-67.
It is considered possible to selectively induce good prognosis via
neoadjuvant chemotherapy.
The cN positive/pN positive group had a poor overall
prognosis. In hormone receptor positive/HER2 negative breast
cancer, nodal metastasis was found to be a strong factor relating
to the prognosis of recurrence. Furthermore, cases with elevated
serum CEA had an especially poor prognosis; therefore the
prolongation of hormone therapy is necessary.
Acknowledgements
Not applicable.
Conflicts of interest
None.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YK and NM performed the statistical analysis and
wrote the manuscript. YT, AS, MK, HN, MiW and HK participated in
the interpretation of data and the critical review of the
manuscript. TS, NS, HT, KY and MaW gave final approval of the
version to be published, and made substantial contributions to the
conception and design of the study. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Kitasato University and written informed consents were
obtained from the patients for publication of this study.
Patient consent for publication
Written informed consents were obtained from the
patients for publication of this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lam SW, Jimenez CR and Boven E: Breast
cancer classification by proteomic technologies: Current state of
knowledge. Cancer Treat Rev. 40:129–138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cleary AS, Leonard TL, Gestl SA and
Gunther EJ: Tumour cell heterogeneity maintained by cooperating
subclones in Wnt-driven mammary cancers. Nature. 508:113–117. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Geyer FC, Marchio C and Reis-Filho JS: The
role of molecular analysis in breast cancer. Pathology. 41:77–88.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weigelt B, Horlings HM, Kreike B, Hayes
MM, Hauptmann M, Wessels LF, de Jong D, Van de Vijver MJ, Van't
Veer LJ and bPeterse JL: Refinement of breast cancer classification
by molecular characterization of histological special types. J
Pathol. 216:141–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yersal O and Barutca S: Biological
subtypes of breast cancer: Prognostic and therapeutic implications.
World J Clin Oncol. 5:412–424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Parker JS, Mullins M, Cheang MC, Leung S,
Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z, et al:
Supervised risk predictor of breast cancer based on intrinsic
subtypes. J Clin Oncol. 27:1160–1167. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sorlie T, Tibshirani R, Parker J, Hastie
T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et
al: Repeated observation of breast tumor subtypes in independent
gene expression data sets. Proc Natl Acad Sci USA. 100:8418–8423.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fan C, Oh DS, Wessels L, Weigelt B, Nuyten
DS, Nobel AB, van't Veer LJ and Perou CM: Concordance among
gene-expression-based predictors for breast cancer. N Engl J Med.
355:560–569. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sørlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goldhirsch A, Wood WC, Gelber RD, Coates
AS, Thürlimann B and Senn HJ: 10th St. Gallen conference: Progress
and promise: Highlights of the international expert consensus on
the primary therapy of early breast cancer 2007. Ann Oncol.
18:1133–1144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goldhirsch A, Ingle JN, Gelber RD, Coates
AS, Thürlimann B and Senn HJ: Panel members: Thresholds for
therapies: Highlights of the St Gallen International expert
consensus on the primary therapy of early breast cancer 2009. Ann
Oncol. 20:1319–1329. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goldhirsch A, Wood WC, Coates AS, Gelber
RD, Thürlimann B and Senn HJ: Panel members: Strategies for
subtypes-dealing with the diversity of breast cancer: Highlights of
the St. Gallen International expert consensus on the primary
therapy of early breast cancer 2011. Ann Oncol. 22:1736–1747. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nishimiya H, Kosaka Y, Yamashita K,
Minatani N, Kikuchi M, Ema A, Nakamura K, Waraya M, Sengoku N,
Tanino H, et al: Prognostic significance of Ki-67 in
chemotherapy-naive breast cancer patients with 10-year follow-up.
Anticancer Res. 34:259–268. 2014.PubMed/NCBI
|
|
16
|
Aebi S, Sun Z, Braun D, Price KN,
Castiglione-Gertsch M, Rabaglio M, Gelber RD, Crivellari D,
Lindtner J, Snyder R, et al: Differential efficacy of three cycles
of CMF followed by tamoxifen in patients with ER-positive and
ER-negative tumors: Long-term follow up on IBCSG Trial IX. Ann
Oncol. 22:1981–1987. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dafni U, Grimani I, Xyrafas A, Eleftheraki
AG and Fountzilas G: Fifteen-year trends in metastatic breast
cancer survival in Greece. Breast Cancer Res Treat. 119:621–631.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dawood S, Broglio K, Gonzalez-Angulo AM,
Buzdar AU, Hortobagyi GN and Giordano SH: Trends in survival over
the past two decades among white and black patients with newly
diagnosed stage IV breast cancer. J Clin Oncol. 26:4891–4898. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gennari A, Conte P, Rosso R, Orlandini C
and Bruzzi P: Survival of metastatic breast carcinoma patients over
a 20-year period: A retrospective analysis based on individual
patient data from six consecutive studies. Cancer. 104:1742–1750.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), . Effects of chemotherapy and
hormonal therapy for early breast cancer on recurrence and 15-year
survival: An overview of the randomised trials. Lancet.
365:1687–1717. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arimidex, Tamoxifen, Alone or in
Combination (ATAC) Trialists' Group, . Forbes JF, Cuzick J, Buzdar
A, Howell A, Tobias JS and Baum M: Effect of anastrozole and
tamoxifen as adjuvant treatment for early-stage breast cancer:
100-month analysis of the ATAC trial. Lancet Oncol. 9:45–53. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Smith I, Procter M, Gelber RD, Guillaume
S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G,
Baselga J, et al: 2-year follow-up of trastuzumab after adjuvant
chemotherapy in HER2-positive breast cancer: A randomised
controlled trial. Lancet. 369:29–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Piccart-Gebhart MJ, Procter M,
Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga
J, Bell R, Jackisch C, et al: Trastuzumab after adjuvant
chemotherapy in HER2-positive breast cancer. N Engl J Med.
353:1659–1672. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shigematsu H, Kawaguchi H, Nakamura Y,
Tanaka K, Shiotani S, Koga C, Nishimura S, Taguchi K, Nishiyama K
and Ohno S: Significant survival improvement of patients with
recurrent breast cancer in the periods 2001–2008 vs. 1992–2000. BMC
Cancer. 11:1182011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kurebayashi J, Miyoshi Y, Ishikawa T, Saji
S, Sugie T, Suzuki T, Takahashi S, Nozaki M, Yamashita H, Tokuda Y
and Nakamura S: Clinicopathological characteristics of breast
cancer and trends in the management of breast cancer patients in
Japan: Based on the Breast Cancer Registry of the Japanese Breast
Cancer Society between 2004 and 2011. Breast Cancer. 22:235–244.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tamaki M, Kamio T, Kameoka S, Kojimahara N
and Nishikawa T: The relevance of the intrinsic subtype to the
clinicopathological features and biomarkers in Japanese breast
cancer patients. World J Surg Oncol. 11:2932013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Davies C, Pan H, Godwin J, Gray R,
Arriagada R, Raina V, Abraham M, Alencar Medeiros VH, Badran A,
Bonfill X, et al: Long-term effects of continuing adjuvant
tamoxifen to 10 years versus stopping at 5 years after diagnosis of
oestrogen receptor-positive breast cancer: ATLAS, a randomised
trial. Lancet. 381:805–816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gray R, Davies C and Perry P: Tamoxifen
for early breast cancer: Better late than never. Ann Oncol.
11:505–507. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Goss PE, Ingle JN, Pritchard KI, Robert
NJ, Muss H, Gralow J, Gelmon K, Whelan T, Strasser-Weippl K, Rubin
S, et al: Extending aromatase-inhibitor adjuvant therapy to 10
years. N Engl J Med. 375:209–219. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Uehara M, Kinoshita T, Hojo T,
Akashi-Tanaka S, Iwamoto E and Fukutomi T: Long-term prognostic
study of carcinoembryonic antigen (CEA) and carbohydrate antigen
15-3 (CA 15-3) in breast cancer. Int J Clin Oncol. 13:447–451.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shao Y, Sun X, He Y, Liu C and Liu H:
Elevated levels of serum tumor markers CEA and CA15-3 are
prognostic parameters for different molecular subtypes of breast
cancer. PLoS One. 10:e01338302015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee JS, Park S, Park JM, Cho JH, Kim SI
and Park BW: Elevated levels of preoperative CA 15-3 and CEA serum
levels have independently poor prognostic significance in breast
cancer. Ann Oncol. 24:1225–1231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pedersen AC, Sørensen PD, Jacobsen EH,
Madsen JS and Brandslund I: Sensitivity of CA 15-3, CEA and serum
HER2 in the early detection of recurrence of breast cancer. Clin
Chem Lab Med. 51:1511–1519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Park BW, Oh JW, Kim JH, Park SH, Kim KS,
Kim JH and Lee KS: Preoperative CA 15-3 and CEA serum levels as
predictor for breast cancer outcomes. Ann Oncol. 19:675–681. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang Y, Zhang H, Zhang M, Meng Q, Cai L
and Zhang Q: Elevation of serum CEA and CA15-3 levels during
antitumor therapy predicts poor therapeutic response in advanced
breast cancer patients. Oncol Lett. 14:7549–7556. 2017.PubMed/NCBI
|
|
36
|
von Minckwitz G, Untch M, Blohmer JU,
Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich
J, Huober J, et al: Definition and impact of pathologic complete
response on prognosis after neoadjuvant chemotherapy in various
intrinsic breast cancer subtypes. J Clin Oncol. 30:1796–1804. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wolmark N, Wang J, Mamounas E, Bryant J
and Fisher B: Preoperative chemotherapy in patients with operable
breast cancer: Nine-year results from National Surgical Adjuvant
Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 1–102.
2001.
|
|
38
|
Rastogi P, Anderson SJ, Bear HD, Geyer CE,
Kahlenberg MS, Robidoux A, Margolese RG, Hoehn JL, Vogel VG, Dakhil
SR, et al: Preoperative chemotherapy: Updates of National surgical
adjuvant breast and bowel project protocols B-18 and B-27. J Clin
Oncol. 26:778–785. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fisher B, Bryant J, Wolmark N, Mamounas E,
Brown A, Fisher ER, Wickerham DL, Begovic M, DeCillis A, Robidoux
A, et al: Effect of preoperative chemotherapy on the outcome of
women with operable breast cancer. J Clin Oncol. 16:2672–2685.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Carey LA, Metzger R, Dees EC, Collichio F,
Sartor CI, Ollila DW, Klauber-DeMore N, Halle J, Sawyer L, Moore DT
and Graham ML: American Joint Committee on Cancer
tumor-node-metastasis stage after neoadjuvant chemotherapy and
breast cancer outcome. J Natl Cancer Inst. 97:1137–1142. 2005.
View Article : Google Scholar : PubMed/NCBI
|