Introduction
Co-occurrence of carcinoma in the uterus and ovary
is found in ~5% of cases of endometrial cancer and 10% of ovarian
cancer (1,2). Synchronous endometrial and ovarian
cancer (SEOC) accounts for 50–70% of all synchronous female genital
cancers, and ~1–2% of all women with gynecological cancers have
simultaneous primary tumors involving the genital tract (3,4). SEOC is
usually diagnosed at its early stages, which results in a good
prognosis (5,6). Another characteristic of SEOC is that
both the endometrial and ovarian cancers have mainly endometrioid
histology (2). In such cases,
differential diagnoses include primary SEOC with stage I
endometrioid endometrial cancer and endometrioid ovarian cancer,
stage III metastatic endometrioid endometrial cancer to the ovary,
and stage II metastatic endometrioid ovarian cancer to the
endometrium. The Ulbright and Roth criteria are commonly used to
distinguish SEOC from metastatic endometrial or ovarian cancer
(7).
The correlation of Lynch syndrome with SEOC is not
well understood. Several studies have reported that Lynch syndrome
is not common in patients with SEOC, and it is estimated that
~3–14% of SEOC cases are caused by Lynch syndrome (8–10). By
contrast, 17–30% of cases of synchronous or metachronous
endometrial and colorectal cancers are caused by Lynch syndrome
(11,12). However, the prevalence of Lynch
syndrome in SEOC is more frequent compared with that in endometrial
or ovarian cancer. Furthermore, a double primary Lynch-associated
cancer with a family history shows a high prevalence of Lynch
syndrome, and it is important to test for microsatellite
instability (MSI) or expression of mismatch repair (MMR) proteins
by immunohistochemistry (IHC) in such cases (13). We herein report a case of SEOC
(endometrial endometrioid adenocarcinoma and ovarian mixed
endometrioid and clear cell carcinoma) with a MSH2
mutation.
Case report
A 41-year-old woman with abnormal genital bleeding
and hypermenorrhea was referred to the Department of Obstetrics and
Gynecology of Keio University School of Medicine (Tokyo, Japan)
from a gynecological outpatient clinic in May, 2016. The patient
had anemia (hemoglobin 6.5 g/dl), and transvaginal ultrasonography
revealed thickened (20 mm) endometrium and swelling (34 mm) of the
left ovary with a solid component. The findings on cervical
cytology were atypical glandular cells, favor neoplastic (AGC-FN),
indicating contamination by endometrial cells, and endometrial
cytology was positive, suggesting endometrioid adenocarcinoma.
Endometrial curettage revealed endometrial endometrioid
adenocarcinoma grade 2. The tumor markers carbohydrate antigen (CA)
19-9 and CA125 were elevated (118 and 52 U/ml, respectively; normal
range: <37 and <35 U/ml, respectively), whereas the
carcinoembryonic antigen level was normal (0.9 ng/ml). Positron
emission tomography/computed tomography revealed no distant
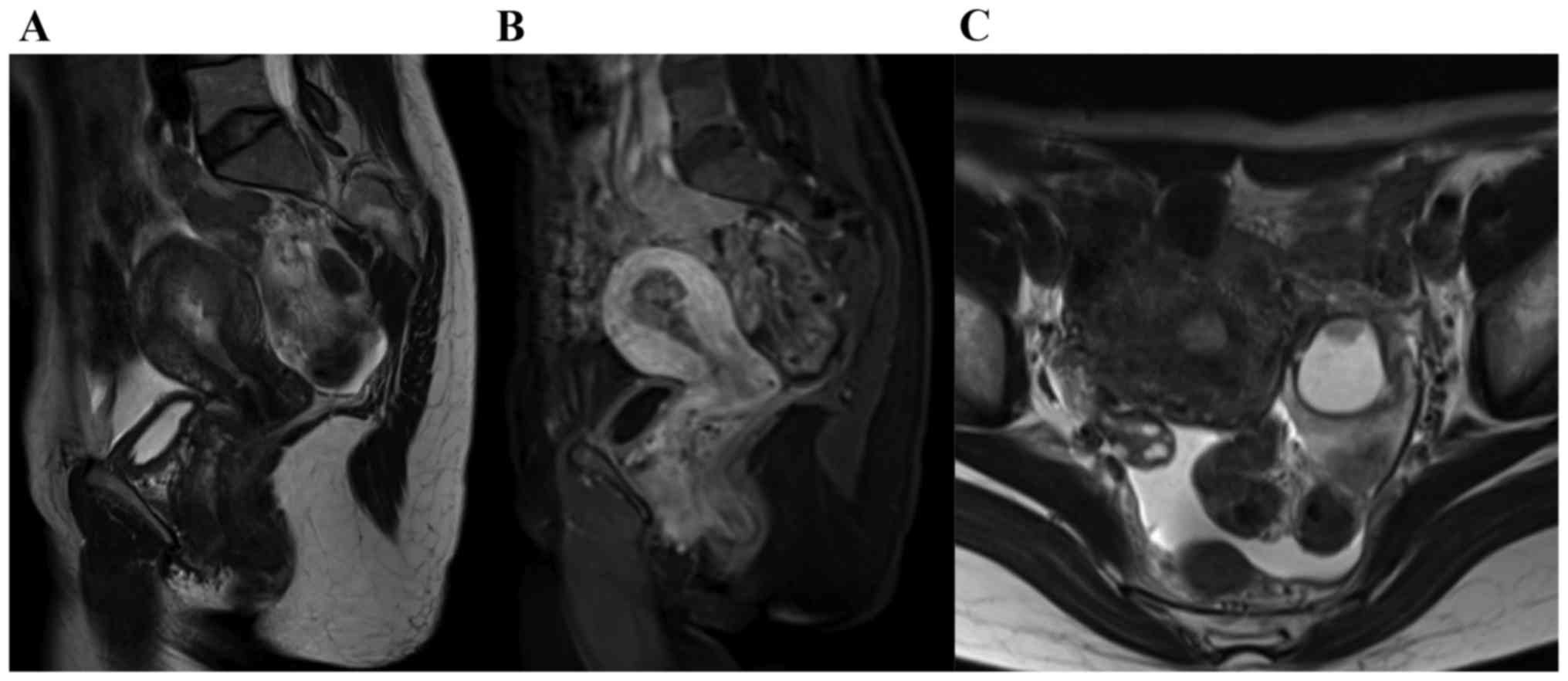
metastasis, but contrast-enhanced pelvic magnetic resonance imaging
examination revealed invasion of over half of the thickness of the
myometrium by endometrial cancer and enlargement of the left ovary
to 30 mm with an enhanced solid component (Fig. 1). These findings indicated that the
pelvic tumor was SEOC, or endometrial cancer with ovarian
metastasis.
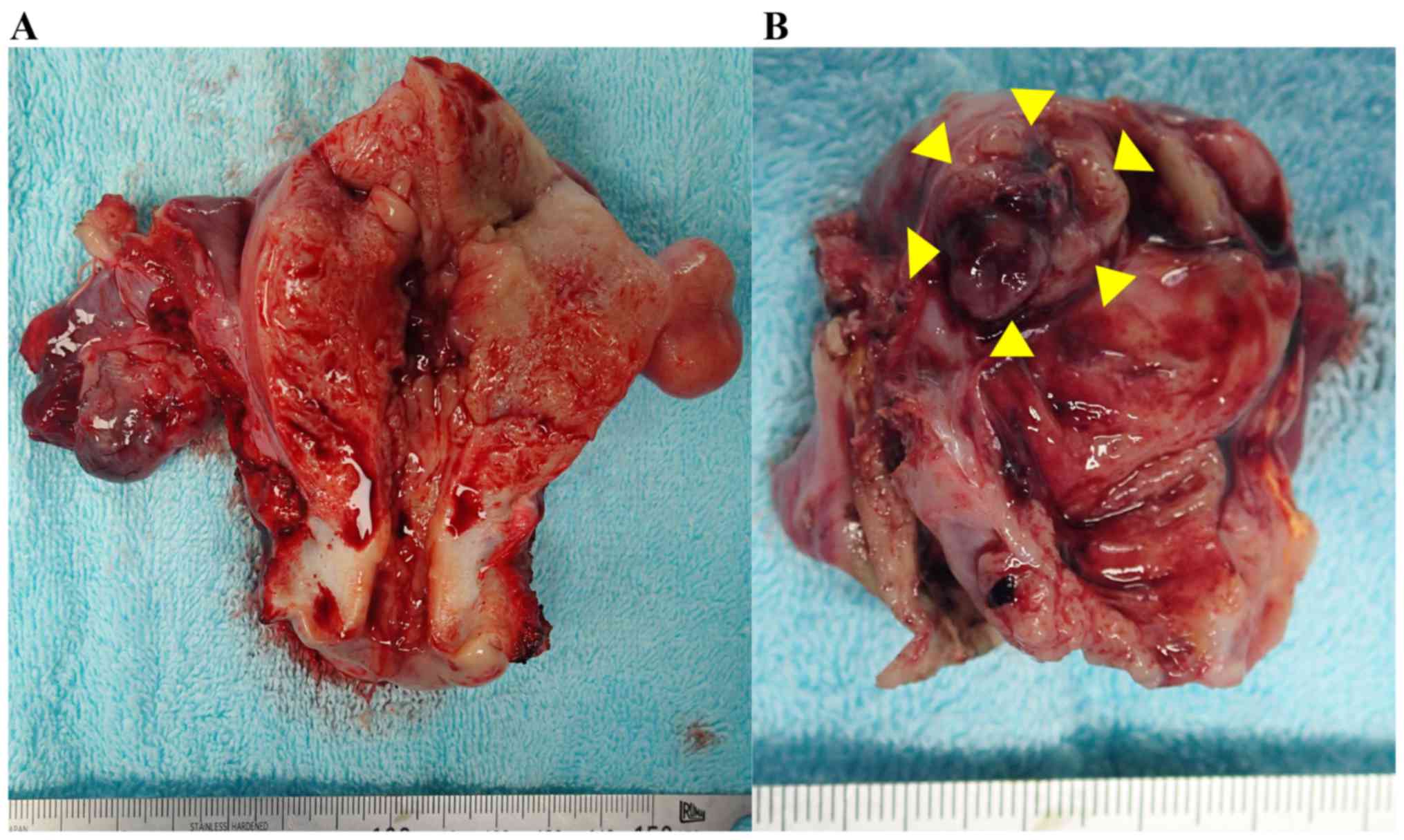
During surgery, the uterus and left ovary were
grossly enlarged and there was no abdominal metastasis. A frozen
section diagnosis of the left ovarian tumor revealed adenocarcinoma
with a clear cell component, indicating primary ovarian cancer.
Extended total hysterectomy, bilateral salpingo-oophorectomy,
pelvic and para-aortic lymphadenectomy, omentectomy, and peritoneal
biopsies were performed (Fig. 2).
Surgery was completed without any complications or the need for
blood transfusion, and the patient was discharged from the hospital
1 week after the operation.
The pathological findings included endometrial
endometrioid adenocarcinoma G2 and ovarian mixed endometrioid and
clear cell carcinoma. There was no metastasis to the omentum or
peritoneum, but there were metastases to the right obturator and
left para-aortic lymph nodes. Endometrial adenocarcinoma had
invaded almost half of the myometrium and also exhibited
lymphovascular invasion. Finally, the diagnosis was synchronous
International Federation of Gynecology and Obstetrics (FIGO) stage
IIIC2 endometrial endometrioid adenocarcinoma, and FIGO stage IA
ovarian endometrioid and clear cell carcinoma (Table I). Due to the high risk of
recurrence, adjuvant chemotherapy with paclitaxel (175
mg/m2) and carboplatin (area under the curve=6) was
administered once every three weeks for six cycles. The patient is
currently being followed up and remained recurrence-free at the
most recent follow-up appointment in April, 2018.
 | Table I.Pathological findings and the
diagnosis of this case. |
Table I.
Pathological findings and the
diagnosis of this case.
| Location | Tumor
characteristics |
|---|
| Uterus | Endometrioid
adenocarcinoma G2 with squamous differentiation, 45×42 mm in size,
myometrial invasion 12/26 mm ly (+), v (−), invasion to cervix (−),
surface exposure (−), margin (−) |
| Ovary (left) | Mixed epithelial
carcinoma (endometrioid G1+ clear cell carcinoma), 30×25×19 mm in
size, ly (−), v (−), surface exposure (−) |
| Ovary (right) | Borderline clear cell
adenofibroma (4×3 mm in size) |
| Omentum | No metastasis |
| Lymph nodes
(pelvic) | Metastasis to a right
obturator lymph node (1/37) |
| Lymph nodes
(para-aortic) | Metastasis of
carcinoma to left 326b1 (above inferior mesenteric artery) Lymph
node status, 1/27 |
| Peritoneal
biopsy | No metastasis |
| Ascites | Negative |
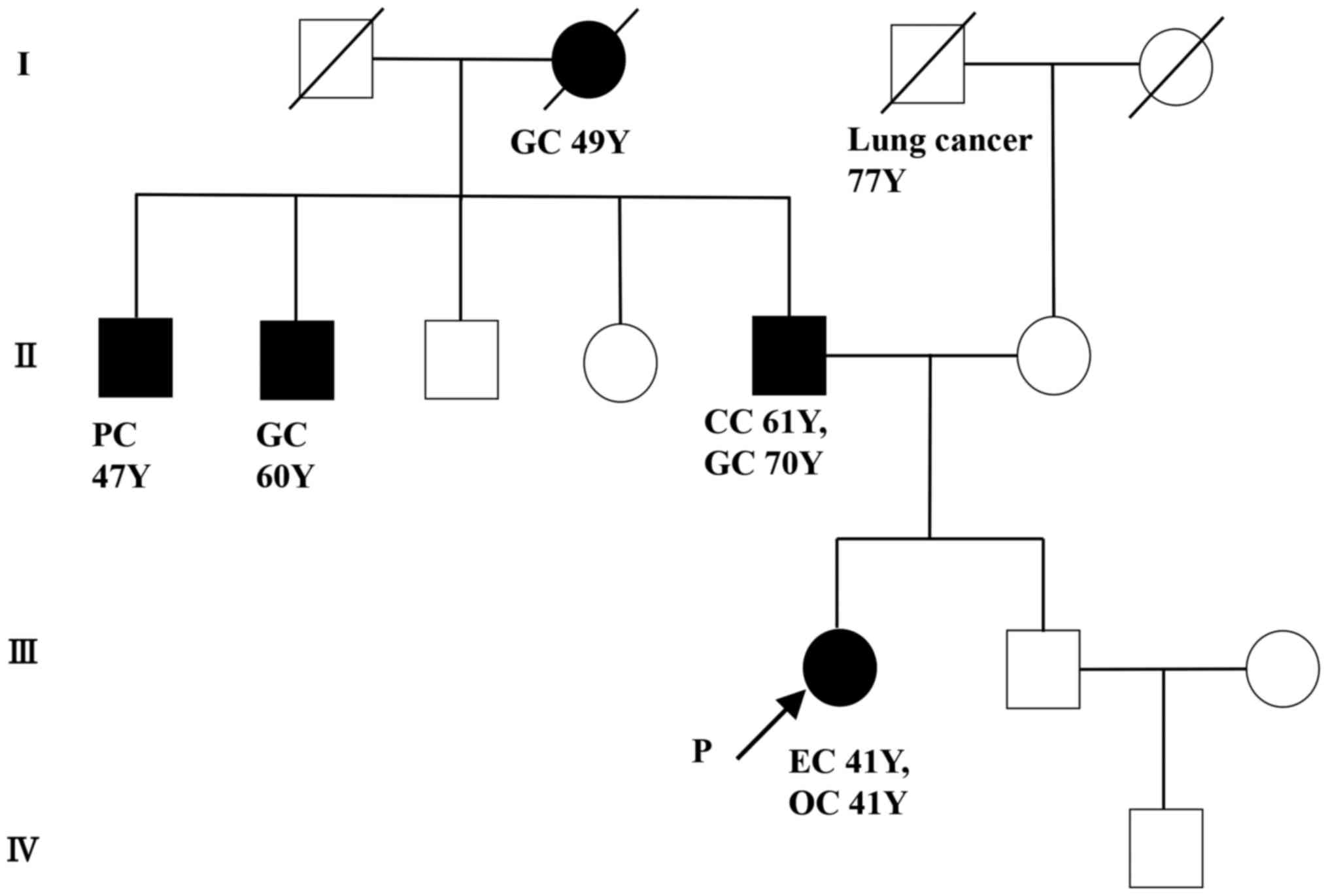
The patient had a family history of colorectal and
gastric cancers, as well as young onset of SEOC, and Lynch syndrome
was suspected (Fig. 3). Therefore,
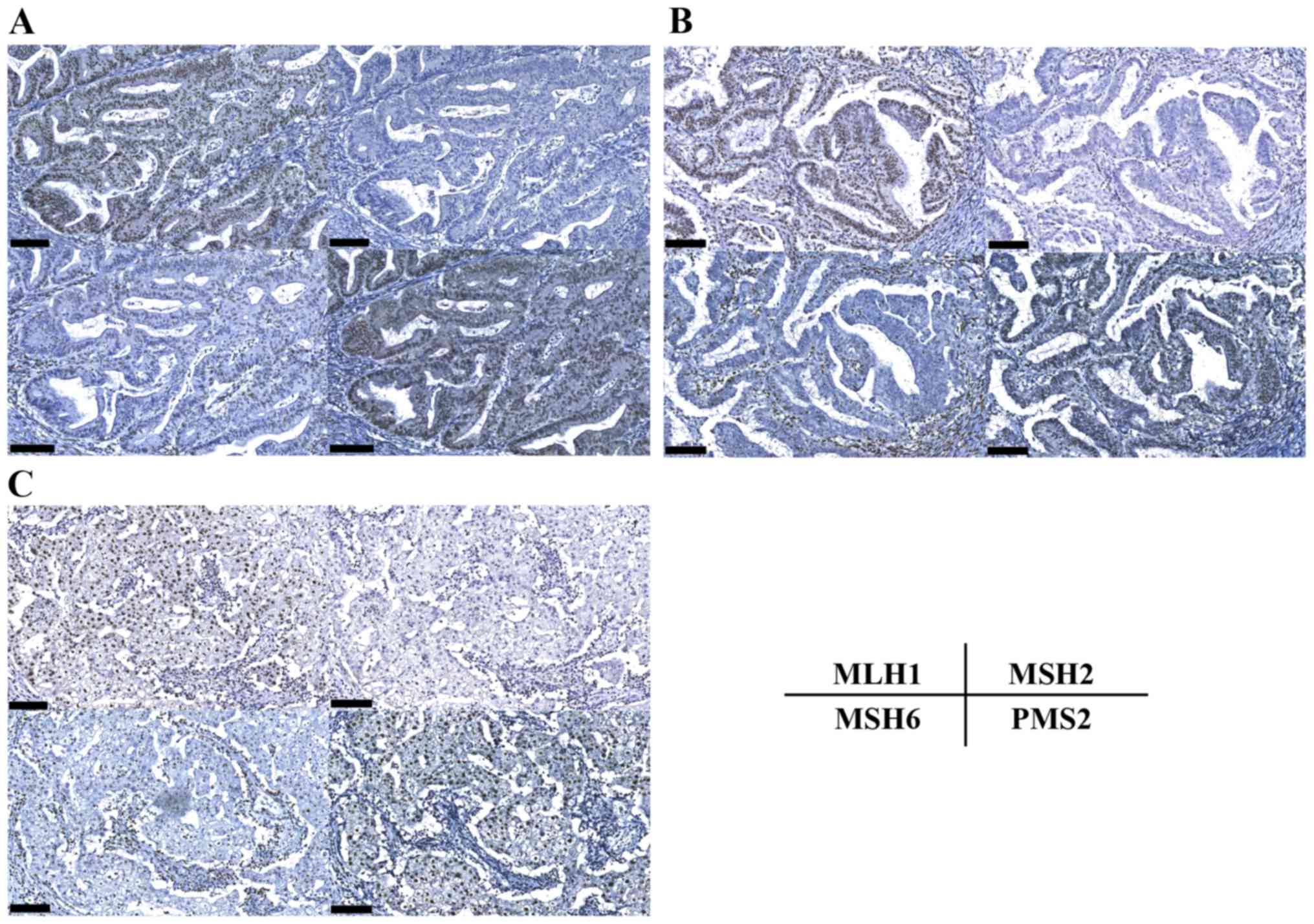
MSI was analyzed for 5 markers (NR21, NR24, BAT25, BAT26, MONO27)
and all were positive (MSI-high). IHC was performed for MLH1, MSH2,
MSH6 and PMS2. All the cancer components (endometrial endometrioid
adenocarcinoma, ovarian endometrioid carcinoma and ovarian clear
cell carcinoma) exhibited loss of MSH2 and MSH6 expression
(Fig. 4). These results indicated a
MSH2 germline mutation or MSH2 epimutation due to an
EPCAM mutation. Following detailed genetic counseling, we performed
a genetic test for MMR genes, namely MLH1, MSH2, MSH6 and
PMS2. reverse transcription-polymerase chain reaction (PCR)
was performed, and the results were confirmed by direct sequencing
using genomic DNA. Furthermore, we checked for a large
rearrangement by multiplex ligation-dependent probe amplification
(MLPA) for MLH1, MSH2, MSH6 and PMS2. Additionally,
the methylation status of CpG islands in the MLH1, MSH2 and
MSH6 promoters was analyzed by methylation-specific PCR.
There was no large rearrangement, and methylation for tested genes
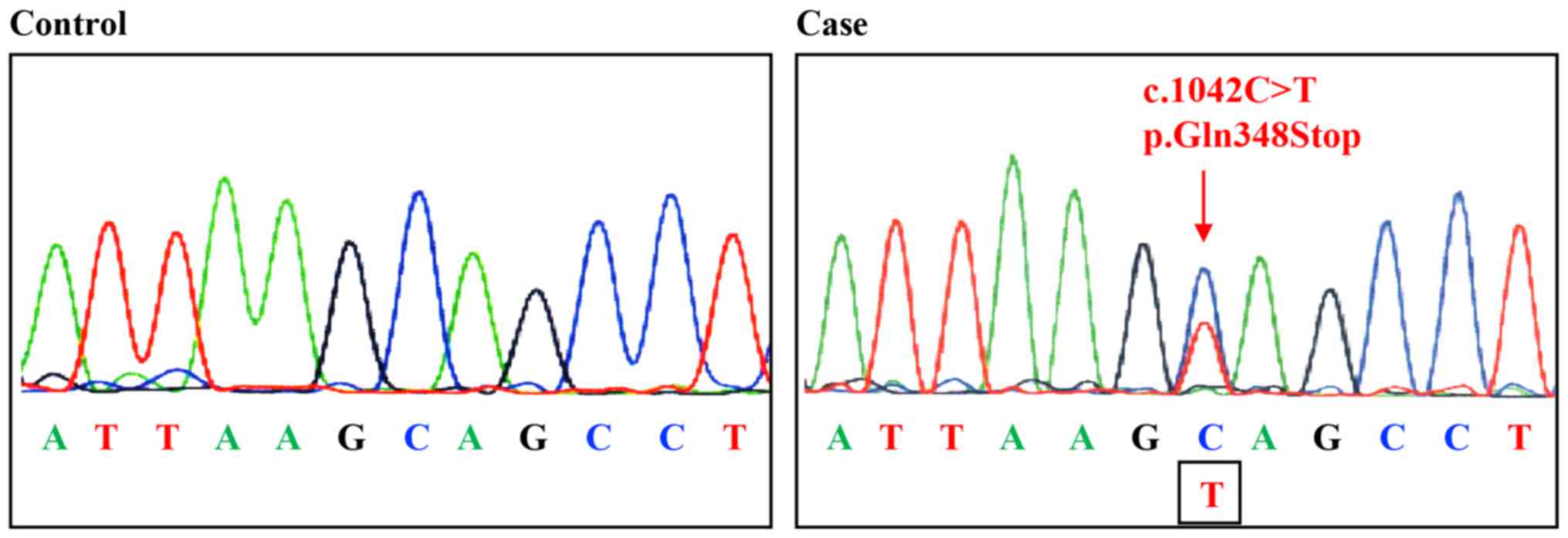
and direct sequencing revealed a germline mutation in exon 6 of
MSH2 (c.1042C>T, p.Gln348*), confirming the diagnosis of
Lynch syndrome (Fig. 5).
Discussion
Lynch syndrome accounts for 2–6% of endometrial
cancers and 0.4–1.0% of ovarian cancers (14,15). The
cumulative lifetime risks of endometrial and ovarian cancer are
~28–60 and 6–14%, respectively, in female patients with Lynch
syndrome (16–18). However, the risk of SEOC in the
context of this syndrome is uncertain, although it has been
suggested that ~3–14% of SEOC cases exhibit a causative association
with Lynch syndrome (8–10). Synchronous or metachronous
Lynch-associated cancer with a family history shows a high
prevalence of Lynch syndrome (13),
and our patient had SEOC with a family history of Lynch-associated
cancers. The calculated risk of Lynch syndrome by the PREMM5
prediction model was 26.3%. The patient in the present case had an
endometrioid carcinoma component in the endometrial and ovarian
cancers, consistent with a previous report that SEOC tends to
include endometrioid components in both cancers (2). The majority of clinically diagnosed
SEOC cases also have clonally related cancers, which probably
reflects dissemination from one site to the other (19,20).
However, our patient had a clear cell component in the left ovarian
cancer and a right ovarian borderline clear cell adenofibroma,
which made it easy to diagnose the case as SEOC, rather than
metastatic ovarian cancer.
Endometrial cancer with Lynch syndrome is mainly
caused by a MSH2 or MSH6 mutation, whereas ovarian
cancer with Lynch syndrome is mainly caused by a MSH2
mutation (21,22). It is also likely that MSH2 and
MSH6 mutations may be the main cause of SEOC based on the
frequency of these mutations in endometrial or ovarian cancer. Some
reports have linked MLH1, MSH2 and MSH6 to SEOC, but
the available data are limited (9,10,23). In
addition, the only entries in the InSiGHT database registered for
synchronous or metachronous endometrial and ovarian cancer are two
MLH1 variants, two MLH3 variants, and one MSH6
variant (MLH1: c.1162dup, c.1852_1854del; MLH3:
c.1939C>T, c.2449A>G; and MSH6: c.3632T>C)
(https://www.insight-group.org/variants/databases/).
The mutation detected in the present case (MSH2 exon6,
c.1042C>T, p.Gln348*) is registered only for one case in
InSiGHT, and this report did not mention the cancer origin
(24). This mutation is not
classified as either pathogenic or non-pathogenic in InSiGHT, but
is classified as class 5 (pathogenic) in the original report
(24). The mutation stops
translation at position 348 of the 934 amino acids of MSH2, and
this region serves as the MSH3/MSH6 interaction domain. Therefore,
it appears to be appropriate to classify this mutation as
pathogenic.
To the best of our knowledge, this is the first
reported case of the c.1042C>T MSH2 mutation in a
gynecological tumor or SEOC. Lynch syndrome in SEOC is not common,
but is more often detected in SEOC compared with general
endometrial cancer cases. Given that universal screening for
endometrial cancer is becoming a standard practice and SEOC would
be a high risk of Lynch syndrome, as stated in our previous report
on screening for Lynch syndrome in ovarian cancer, it is necessary
to perform MSI or IHC analysis for all SEOC cases (25,26).
Detecting MSI-H in SEOC may be helpful for the diagnosis of Lynch
syndrome, as well as for the use of precision medicine for ovarian
or endometrial cancer, including targeted therapy of anti-PD1/PDL1
for MSI-H cancer. Furthermore, since the frequency and tendency for
MMR gene mutation are not clear in SEOC, use of IHC for examination
of loss of MMR protein expression may be informative in identifying
the gene carrying the mutation.
Acknowledgements
The authors gratefully acknowledge the financial
support and the support from the patient for publication of this
report.
Funding
The present study was supported by the Keio Gijuku
Academic Development Fund, the Keio University Grant-in-Aid for
Encouragement of Young Medical Scientists and JSPS KAKENHI
Grant-in-Aid for Young Scientists (JP18K16812).
Availability of data and materials
The datasets used during the present study are
included in this published article and are also available from the
corresponding author on reasonable request.
Authors' contributions
TakasT wrote the manuscript, K.B. checked the
manuscript, MY performed tumor tests, MA, AK, AS and TakayT
collected clinical data, MA, YK and AH contributed to genetic
counseling, and HN, ET and DA supervised the study. All authors
have read and approved the final version of this manuscript.
Ethics approval and consent to
participate
The study was performed in accordance with the
Declaration of Helsinki and principles of Good Clinical Practice.
Samples and clinical data were obtained after approval of the
Institutional Ethics Committee of Keio University (ID: 2007-0081).
The patient provided written informed consent.
Patient consent for publication
This patient provided informed consent for the
publication of the study details, including use of data and
images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SEOC
|
synchronous endometrial and ovarian
cancer
|
|
MMR
|
mismatch repair
|
|
MSI
|
microsatellite instability
|
|
IHC
|
immunohistochemistry
|
|
MLPA
|
multiplex ligation-dependent probe
amplification
|
References
|
1
|
Kurman RJ, Carcangiu ML, Herrington CS and
Young RH: WHO Classification of Tumours of Female Reproductive
OrgansIARC WHO Classification of Tumours. International Agency for
Research on Cancer; Lyon: 2014
|
|
2
|
Zaino R, Whitney C, Brady MF, DeGeest K,
Burger RA and Buller RE: Simultaneously detected endometrial and
ovarian carcinomas-a prospective clinicopathologic study of 74
cases: A gynecologic oncology group study. Gynecol Oncol.
83:355–362. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tong SY, Lee YS, Park JS, Bae SN, Lee JM
and Namkoong SE: Clinical analysis of synchronous primary neoplasms
of the female reproductive tract. Eur J Obstet Gynecol Reprod Biol.
136:78–82. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singh N: Synchronous tumours of the female
genital tract. Histopathology. 56:277–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sozen H, Vatansever D, Iyibozkurt AC,
Topuz S, Ozsurmeli M, Salihoglu Y, Guzelbey B and Berkman S:
Clinicopathologic and survival analyses of synchronous primary
endometrial and epithelial ovarian cancers. J Obstet Gynaecol Res.
41:1813–1819. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsuo K, Machida H, Frimer M, Marcus JZ,
Pejovic T, Roman LD and Wright JD: Prognosis of women with stage I
endometrioid endometrial cancer and synchronous stage I
endometrioid ovarian cancer. Gynecol Oncol. 147:558–564. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ulbright TM and Roth LM: Metastatic and
independent cancers of the endometrium and ovary: A
clinicopathologic study of 34 cases. Hum Pathol. 16:28–34. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kobayashi Y, Nakamura K, Nomura H, Banno
K, Irie H, Adachi M, Iida M, Umene K, Nogami Y, Masuda K, et al:
Clinicopathologic analysis with immunohistochemistry for DNA
mismatch repair protein expression in synchronous primary
endometrial and ovarian cancers. Int J Gynecol Cancer. 25:440–446.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim MK, Song SY, Do IG, Kim SH, Choi CH,
Kim TJ, Lee JW, Bae DS and Kim BG: Synchronous gynecologic
malignancy and preliminary results of Lynch syndrome. J Gynecol
Oncol. 22:233–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Soliman PT, Broaddus RR, Schmeler KM,
Daniels MS, Gonzalez D, Slomovitz BM, Gershenson DM and Lu KH:
Women with synchronous primary cancers of the endometrium and
ovary: Do they have Lynch syndrome? J Clin Oncol. 23:9344–9350.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Millar AL, Pal T, Madlensky L, Sherman C,
Temple L, Mitri A, Cheng H, Marcus V, Gallinger S, Redston M, et
al: Mismatch repair gene defects contribute to the genetic basis of
double primary cancers of the colorectum and endometrium. Hum Mol
Genet. 8:823–829. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Planck M, Rambech E, Möslein G, Müller W,
Olsson H and Nilbert M: High frequency of microsatellite
instability and loss of mismatch-repair protein expression in
patients with double primary tumors of the endometrium and
colorectum. Cancer. 94:2502–2510. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hirai Y, Banno K, Suzuki M, Ichikawa Y,
Udagawa Y, Sugano K and Miki Y: Molecular epidemiological and
mutational analysis of DNA mismatch repair (MMR) genes in
endometrial cancer patients with HNPCC-associated familial
predisposition to cancer. Cancer Sci. 99:1715–1719. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Norquist BM, Harrell MI, Brady MF, Walsh
T, Lee MK, Gulsuner S, Bernards SS, Casadei S, Yi Q, Burger RA, et
al: Inherited mutations in women with ovarian carcinoma. JAMA
Oncol. 2:482–490. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pal T, Permuth-Wey J, Kumar A and Sellers
TA: Systematic review and meta-analysis of ovarian cancers:
Estimation of microsatellite-high frequency and characterization of
mismatch repair deficient tumor histology. Clin Cancer Res.
14:6847–6854. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aarnio M, Sankila R, Pukkala E, Salovaara
R, Aaltonen LA, de la Chapelle A, Peltomäki P, Mecklin JP and
Järvinen HJ: Cancer risk in mutation carriers of
DNA-mismatch-repair genes. Int J Cancer. 81:214–218. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bonadona V, Bonaïti B, Olschwang S,
Grandjouan S, Huiart L, Longy M, Guimbaud R, Buecher B, Bignon YJ,
Caron O, et al: Cancer risks associated with germline mutations in
MLH1, MSH2 and MSH6 genes in Lynch syndrome. JAMA. 305:2304–2310.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Watson P, Vasen HFA, Mecklin JP, Bernstein
I, Aarnio M, Järvinen HJ, Myrhøj T, Sunde L, Wijnen JT and Lynch
HT: The risk of extra-colonic, extra-endometrial cancer in the
Lynch syndrome. Int J Cancer. 123:444–449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chao A, Wu RC, Jung SM, Lee YS, Chen SJ,
Lu YL, Tsai CL, Lin CY, Tang YH, Chen MY, et al: Implication of
genomic characterization in synchronous endometrial and ovarian
cancers of endometrioid histology. Gynecol Oncol. 143:60–67. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schultheis AM, Ng CK, De Filippo MR,
Piscuoglio S, Macedo GS, Gatius S, Mies Perez B, Soslow RA, Lim RS,
Viale A, et al: Massively parallel sequencing-based clonality
analysis of synchronous endometrioid endometrial and ovarian
carcinomas. J Natl Cancer Inst. 108:djv4272016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schweizer P, Moisio AL, Kuismanen SA,
Truninger K, Vierumäki R, Salovaara R, Arola J, Butzow R, Jiricny
J, Peltomäki P and Nyström-Lahti M: Lack of MSH2 and MSH6
characterizes endometrial but not colon carcinomas in hereditary
nonpolyposis colorectal cancer. Cancer Res. 61:2813–2815.
2001.PubMed/NCBI
|
|
22
|
Helder-Woolderink JM, Blok EA, Vasen HF,
Hollema H, Mourits MJ and De Bock GH: Ovarian cancer in Lynch
syndrome; a systematic review. Eur J Cancer. 55:65–73. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dogan A, Schultheis B, Rezniczek GA, Hilal
Z, Cetin C, Häusler G and Tempfer CB: Synchronous endometrial and
ovarian cancer in young women: Case report and review of the
literature. Anticancer Res. 37:969–978. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sjursen W, McPhillips M, Scott RJ and
Talseth-Palmer BA: Lynch syndrome mutation spectrum in New South
Wales, Australia, including 55 novel mutations. Mol Genet Genomic
Med. 4:223–231. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gupta S, Provenzale D, Regenbogen SE,
Hampel H, Slavin P Jr, Hall MJ, Llor X, Chung DC, Ahnen DJ, Bray T,
et al: NCCN Guidelines Insights: Genetic/Familial High-Risk
assessment: Colorectal, Version 3.2017. J Natl Compr Canc Netw.
15:1465–1475. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takeda T, Tsuji K, Banno K, Yanokura M,
Kobayashi Y, Tominaga E and Aoki D: Screening for Lynch syndrome
using risk assessment criteria in patients with ovarian cancer. J
Gynecol Oncol. 29:e292018. View Article : Google Scholar : PubMed/NCBI
|