Introduction
Malignant gliomas (MGs) are the most common primary
malignant brain tumours and include anaplastic gliomas (AG) and
glioblastoma multiforme (GBM) (1).
Maximal safe surgical resection followed by radiotherapy with
concomitant and adjuvant temozolomide (TMZ) is the standard
first-line treatment of GBM (2),
leading to a median overall survival (mOS) of 12–15 months
(3). Despite the optimal standard
treatment, the local infield recurrence rate remains high (~90%),
and despite the molecular advances, no standard therapies are
established for recurrent MGs. Different options are under
investigation, including resurgery, reirradiation and chemotherapy,
as well as their combinations (3,4).
A recent review of the literature (5) showed a survival benefit and an improved
functional status after resurgery followed by adjuvant treatments,
with a higher OS in selected patients with favourable clinical and
radiological characteristics at the time of recurrence.
Preoperative Karnofsky Performance Status (KPS>70%) and age
(<60 years) are important predictors of longer survival
(5–7). Multiple studies have also demonstrated
that a greater extent of resection is associated with better
survival outcomes (8–10). However, prospective data are lacking
to confirm resurgery as an independent predictor of survival
(11,12).
Focal radiotherapy is a similarly controversial
option due to the lack of prospective randomised trials and the
risk of toxicity, regarding radionecrosis and neurocognitive
impairment. Recent advances in radiotherapy techniques, including
stereotactic and hypofractionated treatments, allow for more
precise treatment, sparing healthy surrounding tissue and reducing
late toxicity (13). Younger age
(<70 years) and good performance status (PS) (KPS>60%) are
the most important predictors of longer survival for reirradiation
(14). Multiple trials have studied
the combination of radiotherapy and systemic therapy, such as
bevacizumab (BEV) and TMZ (13,15,16).
Proton-beam therapy (PBT), a type of radiation treatment, has the
advantage over photon-therapy of sparing considerable volumes of
previously irradiated healthy tissue (13,15).
Survival and clinical benefits of PBT, alone or in association with
chemotherapy, have been studied in newly diagnosed and recurrent
MGs (17–20).
Many clinical trials on recurrent GBM studied the
efficacy of single and/or combined chemotherapy agents, including
nitrosoureas, and of targeted therapies, such as BEV, alone or
associated with chemotherapy, with encouraging results (6,21,22).
Nitrosoureas, mainly fotemustine (FTM) (23), have been employed either in
monotherapy or in combination with other agents (21), including BEV, showing potential
survival benefit (21,24–27).
Improved outcomes with a multimodality management of
recurrent MGs have been reported in a few trials (28–30), but
no standard treatment algorithm has been defined.
The aim of this study is to analyse the efficacy of
the multimodal treatment as a combination of chemotherapy, as FTM
and BEV in combination or in sequence, and resurgery and/or
reirradiation, including PBT, in MGs patients at first
recurrence.
Patients and methods
Study population
This study was conducted at the Department of
Medical Oncology of Policlinico Umberto I of Rome and Latina, both
of Sapienza University of Rome. The study was approved by the
Institutional Review Board of Latina.
From August 2011 to August 2017, we retrospectively
analysed recurrent MGs patients at first relapse treated with
multimodal therapy as a combination of resurgery and/or
reirradiation, including PBT, followed by chemotherapy or
chemotherapy alone. All patients underwent first-line therapy with
surgery followed by radio-chemotherapy according to Stupp protocol
(2).
The initial diagnosis was established by magnetic
resonance imaging (MRI) and histologically using WHO criteria
(31). Diagnosis of recurrence was
assessed by MRI in all patients and by histological examination
when resurgery was performed. Clinical data included patients'
characteristics, tumour characteristics and treatment information
(Table I).
 | Table I.Patient characteristics and treatment
at recurrence (n=26). |
Table I.
Patient characteristics and treatment
at recurrence (n=26).
| A, Patient
characteristics at recurrence |
|---|
|
|---|
|
Characteristics | n (%) |
|---|
| Sex |
|
|
Male | 16 (62) |
|
Female | 10 (38) |
| Median age, years
(range) | 50 (26–67) |
| Karnofsky
performance status |
|
| Median
(range) | 80 (60–100) |
|
90–100 | 9 (35) |
|
70–80 | 16 (61) |
| 60 | 1 (4) |
| Laterality |
|
|
Right | 11 (42) |
|
Left | 15 (58) |
| Lobe |
|
|
Fronto-temporal | 7 (27) |
|
Parieto-temporal | 5 (19) |
|
Monolobar | 13 (50) |
|
Multilobar | 1 (4) |
| Histotype |
|
| Primary
GBM | 20 (77) |
|
Secondary GBM | 6 (23) |
| MGMT methylation
status at diagnosis |
|
|
Methylated | 16 (62) |
|
Unmethylated | 10 (38) |
| IDH-1 status at
diagnosis |
|
|
Mutated | 5 (19) |
| Non
mutated | 9 (36) |
|
Unknown | 12 (46) |
| First-line
therapy |
|
| Stupp
protocol (RT/TMZ-TMZ) | 26 (100) |
|
| B, Treatment at
recurrence |
|
|
Treatment | n (%) |
|
| Type of treatment
at recurrence |
|
|
Multimodal therapy | 11 (42) |
|
Monotherapy | 15 (58) |
| Surgery at
recurrence |
|
|
Yes | 7 (27) |
| No | 19 (73) |
| Reirradiation at
recurrence |
|
|
Yes | 9 (35) |
| No | 17 (65) |
| Type of
reirradiation |
|
|
Photon-therapy | 4 (15) |
|
Proton-therapy | 5 (19) |
| Chemotherapy at
recurrence |
|
| BEV +
FTM | 12 (46) |
| FTM →
BEV | 14 (54) |
| No. of median
cycles of chemotherapy received (range) |
|
| BEV +
FTM | 8 (1–24) |
| FTM →
BEV | 5 (1–7) |
|
FTM |
|
|
BEV | 8 (2–40) |
Treatment plan
At first recurrence, patients received either a
multimodal therapy consisting of chemotherapy preceded by resurgery
and/or reirradiation or chemotherapy alone. Resurgery consisted of
maximal safe surgical resection. Reirradiation, including
radiotherapy or PBT, was given prior to chemotherapy and after
surgery. Reirradiation consisted of fractionated stereotactic
radiotherapy (total dose of 60 Gy in 1.8 to 2.0 Gy fractions).
Chemotherapy consisted of FTM as second-line therapy
in combination with BEV (concomitant FTM/BEV; cFTM/BEV) or as
second-line therapy followed by third-line BEV (sequential FTM/BEV;
sFTM/BEV).
The sequential treatment FTM, according to the Addeo
schedule (23), consisted of an
induction phase dose of 80 mg/mq every 2 weeks for 5 consecutive
administrations followed by a 4-week rest period and a maintenance
phase dose of 80 mg/mq every 4 weeks. BEV was administered at 10
mg/kg every 2 weeks, in off-label use. In patients who underwent
resurgery, BEV commenced 4–6 weeks after surgery.
The cFTM/BEV therapy, according to the Soffietti
schedule (24), consisted of an
induction phase with BEV at 10 mg/kg on days 1 and 15 and FTM at 75
mg/mq on days 1 and 8, followed by a 3-week rest period and a
maintenance phase with BEV at 10 mg/kg and FTM at 75 mg/mq every 3
weeks.
Response evaluation
Radiological evaluations consisted of 3-Tesla MRI
scans (contrast-enhanced T1-weighted, T2/FLAIR-weighted,
perfusion-weighted and diffusion-weighted scans and MR
spectroscopy). MRI evaluations were made at baseline, between each
treatment modality, after the first 2 cycles of BEV or after the
induction phase of FTM and then after every two cycles of BEV or
FTM in the maintenance phase. Evaluation response was assessed
according to RANO criteria (32) as
complete (CR) and partial (PR) response, stable (SD) and
progression (PD) disease. Overall response rate (ORR) was defined
as the sum of CR and PR and disease control rate (DCR) was defined
as the sum of CR, PR and SD.
Statistical analysis
Survival analysis was conducted on the efficacy of
multimodal therapy compared to chemotherapy alone in terms of
median progression-free survival (mPFS) and OS (mOS) from diagnosis
of recurrence disease and of cFTM/BEV versus sFTM/BEV in terms of
mPFS and mOS from the start of chemotherapy. Median PFS and OS were
estimated with a 95% confidence interval. Survival curves of PFS
and OS were generated using the Kaplan-Meier method. Differences in
PFS and OS were evaluated using the log-rank test (Mantel-Cox) for
statistical significance, which was defined at the P<0.05 level
(33).
Subgroup analyses according to treatment and
O6-methylguanine-DNA methyltransferase (MGMT) and
isocitrate dehydrogenase 1 (IDH-1) status were performed. Other
subgroup analyses according to surgery, radiotherapy and other
biological markers were not possible to perform due to the low
number of patients.
Toxicity evaluation
All adverse events were graded according to
NCI-CTCAE, version 4.03 (34).
Toxicity assessment was performed at each cycle or, if clinically
indicated, at weekly intervals. Evaluation of quality of life was
not performed due to the lack of questionnaires in clinical
practice.
Results
Patient characteristics
Twenty-six MGs patients treated at first relapse
with multimodal therapy or chemotherapy were included in the
analysis. Patients' characteristics are summarised in Table I. The two treatment groups are
balanced for demographic and clinical characteristics.
Most patients were male (62%), median age was 50
years (range, 26–67 years) and median KPS was 80 (range, 60–100).
All patients had a histological diagnosis of MGs (77% GBM and 23%
grade-III gliomas). At first relapse all grade-III gliomas evolved
into GBM (secondary GBM), a diagnosis that was made radiologically
in 5 patients and histologically after resurgery in 1 patient.
The assessment of MGMT promoter status was conducted
in all patients and resulted methylated in 16 patients (62%) and
unmethylated in 10 patients (38%). The assessment of IDH status was
conducted in 14 patients (54%) and resulted mutated in 5 patients
(19%) and wild-type in 9 patients (35%).
Fifteen patients (58%) received chemotherapy alone
and 11 patients (42%) received multimodal therapy. Of these, 2
patients (8%) underwent surgery followed by chemotherapy, 4
patients (15%) received reirradiation followed by chemotherapy and
5 patients (19%) underwent surgery followed by reirradiation and
then chemotherapy. Twelve patients (46%) were treated with cFTM/BEV
and 14 patients (54%) with sFTM/BEV.
Treatment response evaluation
All patients included in the study were assessable
for response analysis (Table II).
Multimodal therapy showed 1 vs. 0 CR (9 vs. 0%), 7 vs. 7 PR (64 vs.
47%) and 1 vs. 3 SD (9 vs. 20%) compared to chemotherapy alone. ORR
and DCR of multimodal therapy were 73 and 82% compared to 47 and
67% with chemotherapy alone, respectively. Concomitant FTM/BEV
resulted in 1 vs. 0 CR (8 vs. 0%), 9 vs. 5 PR (76 vs. 36%), 1 vs. 3
SD (8 vs. 21%) compared to sFTM/BEV. ORR and DCR of cFTM/BEV were
of 84 and 92% respectively compared to 36 and 57% of sFTM/BEV.
 | Table II.Results for objective response and
survival outcomes according to type of approach and treatment. |
Table II.
Results for objective response and
survival outcomes according to type of approach and treatment.
| Variables | Multimodal therapy
(n=11) | Monotherapy
(n=15) | Concomitant FTM/BEV
(n=12) | Sequential FTM/BEV
(n=14) |
|---|
| Objective response,
n (%) |
| CR | 1 (9%) | 0 (0%) | 1 (8%) | 0 (0%) |
| PR | 7 (64%) | 7 (47%) | 9 (76%) | 5 (36%) |
| SD | 1 (9%) | 3 (20%) | 1 (8%) | 3 (21%) |
| PD | 2 (18%) | 5 (33%) | 1 (8%) | 6 (43%) |
|
ORR | 73 | 47 | 84 | 36 |
| DC | 82 | 67 | 92 | 57 |
| Survival data |
| 6
months-PFS, % | 82 | 67 | 92 | 71 |
| 12
months-PFS, % | 27 | 20 | 25 | 21 |
| Median PFS,
months | 11 | 7 | 10 | 5 |
| 6
months-OS, % | 91 | 73 | 83 | 50 |
| 12
months-OS, % | 55 | 20 | 25 | 21 |
| Median OS,
months | 13 | 8 | 11 | 5.2 |
General survival outcomes
All patients included in the study were assessable
for survival analysis (Table II).
Median PFS and OS from diagnosis of recurrence were 9 months (95%
CI 6.5–11.5) and 11 months (95% CI 9.1–12.9) respectively, whereas
mPFS and mOS from the start of chemotherapy were 7.1 months (95% CI
5.6–8.6) and 9.5 months (95% CI 5.1–13.9), respectively.
Survival outcomes according to
treatment
Multimodal therapy reported better survival outcomes
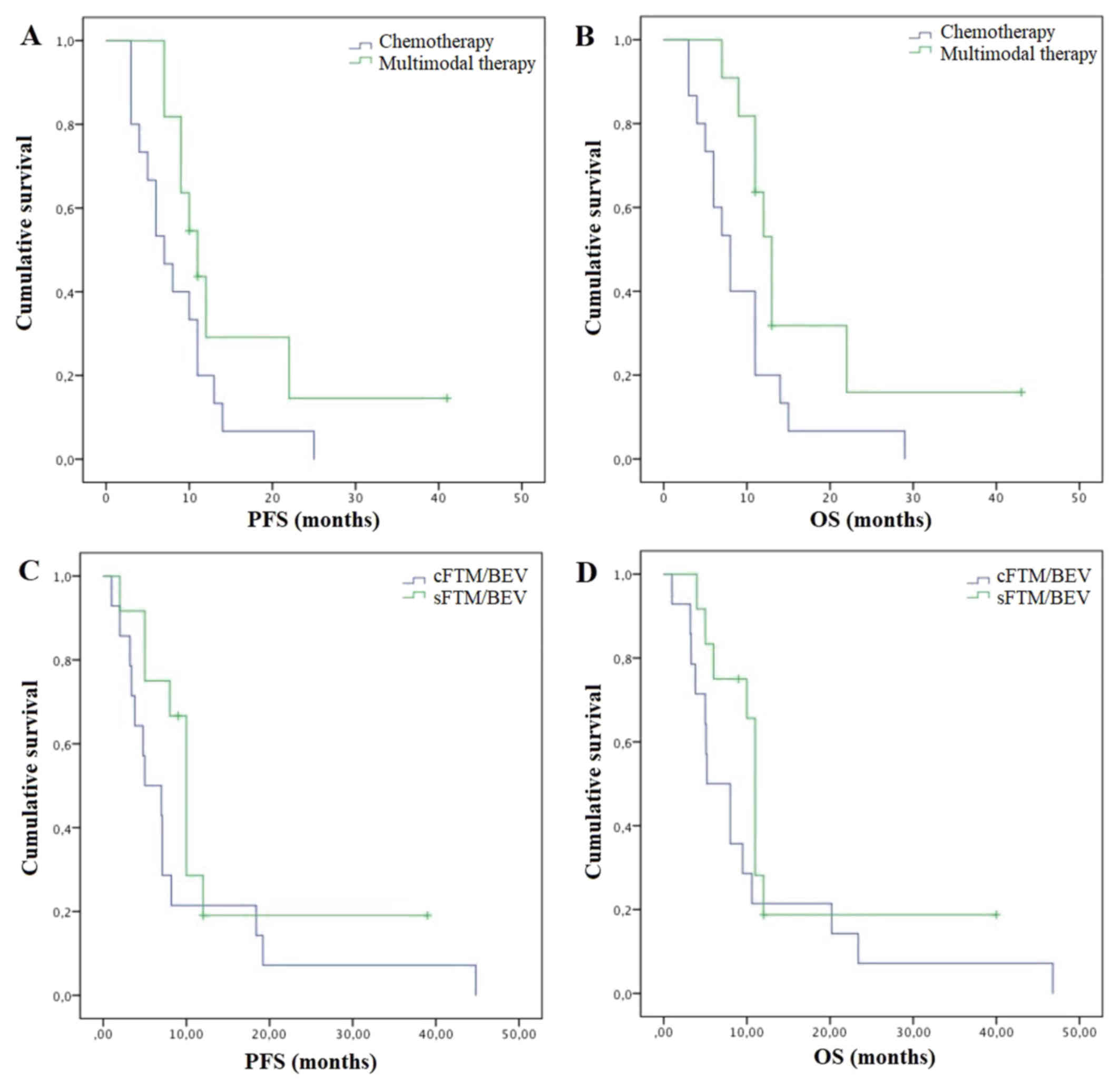
in terms of mPFS and mOS compared to chemotherapy alone. Median PFS
was 11 months (95% CI 8–14) vs. 7 months (95% CI 4.2–9.8) (P=0.08)
and mOS was 13 months (95% CI 11.2–14.8) vs. 8 months (95% CI
5.5–10.5) (P=0.04) (Fig. 1A and B).
Concomitant FTM/BEV was associated with better survival outcomes in
terms of mPFS of 10 months (95% CI 8.6–11.4) versus 5 months (95%
CI 1–9) and mOS of 11 (95% CI 10.3–11.7) vs. 5.2 months (95% CI
1.7–8.7) compared to sFTM/BEV (P=0.22 and P=0.15, respectively)
(Fig.1C and D).
Activity according to MGMT status
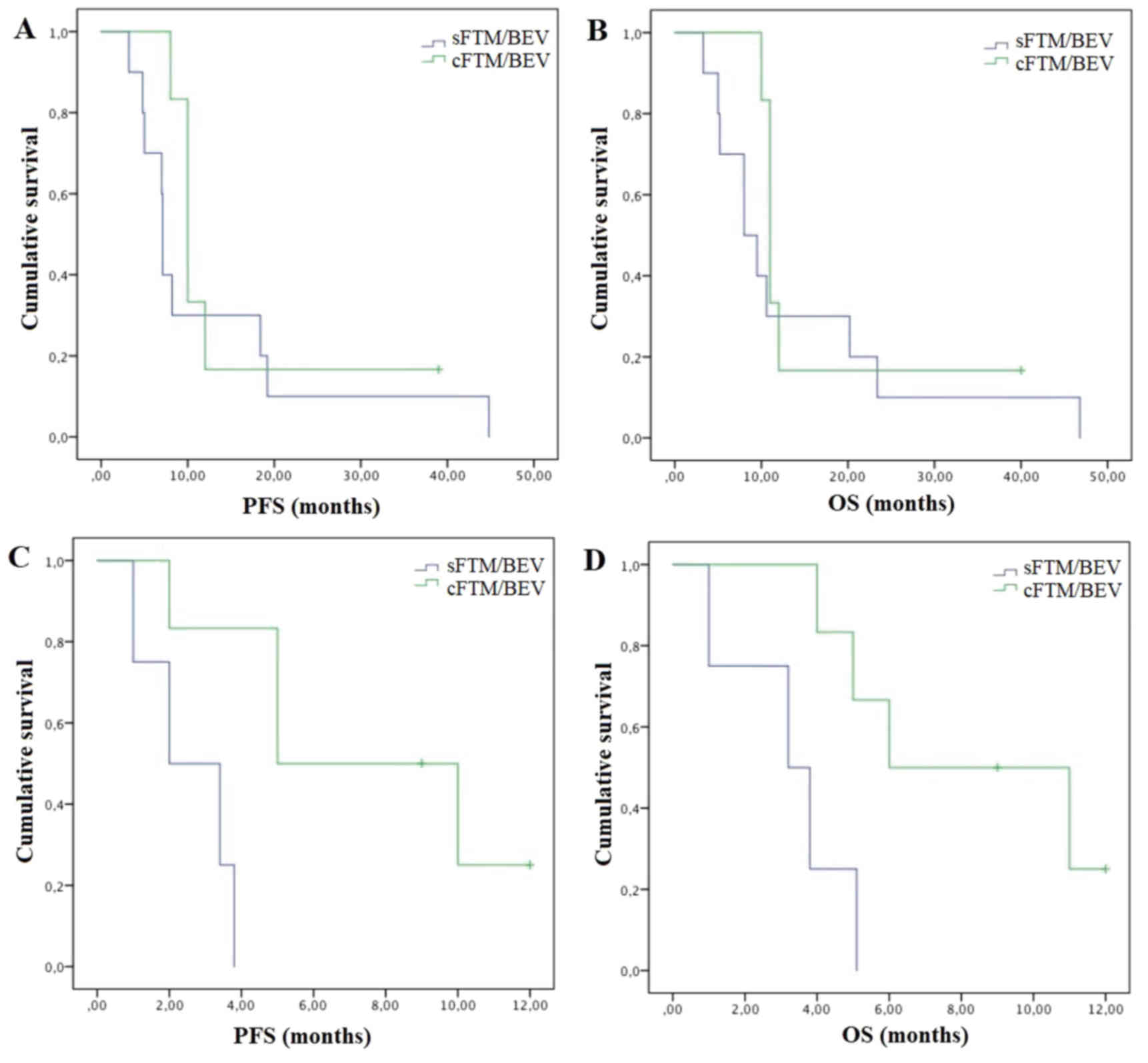
Methylated patients (n=16) experienced longer
survival from the diagnosis of recurrence (both mPFS and mOS of 11
vs. 6 months; P=0.03 and P=0.05, respectively) and from the start
of chemotherapy (mPFS: 8.2 vs. 3.8 months, P=0.11; mOS: 10.6 vs. 5
months, P=0.08), independently of the type of treatment. In
methylated patients, multimodal treatment (n=8) was associated with
similar mPFS (both 11 months) and mOS (12 vs. 11 months) compared
to chemotherapy alone (n=8). Methylated patients experienced
greater mPFS (10 vs. 7.1 months; P=0.33) and mOS (11 vs. 8 months;
P=0.33) with cFTM/BEV (n=6) compared to sFTM/BEV (n=10) (Fig. 2A and B).
The greatest benefit was observed in unmethylated
patients who experienced statistically significant longer survival
with multimodal therapy and cFTM/BEV. Unmethylated patients
experienced higher mPFS (10 vs. 5 months; P=0.02) and mOS (11 vs. 6
months; P=0.02) with multimodal therapy (n=3) compared to
chemotherapy alone (n=7) and greater mPFS (5 vs. 2 months; P=0.01)
and mOS (6 vs. 3.2 months; P=0.01) with cFTM/BEV (n=6) compared to
sFTM/BEV (n=4) (Fig. 2C and D).
Toxicity evaluation
All patients were evaluated for safety. Concomitant
FTM/BEV was well-tolerated with grade 1–2 myelotoxicities in 62 vs.
70% of patients, grade 3 myelotoxicity in 8 vs. 15% of patients and
grade 1–2 hypertransaminasemia in 23 vs. 38% of patients compared
to sFTM/BEV. Grade 1–2 fatigue was present in 30% of patients in
both treatments. Grade 1–2 hypertension and proteinuria developed
in 10 and 15% of patients in cFTM/BEV vs. 20% and 40% of patients
in sFTM/BEV. No grade 4 adverse events were observed. None of the
patients discontinued for toxicity.
Discussion
For recurrent MGs, different treatment strategies
are available, such as resurgery, reirradiation and systemic
chemotherapy, as well as their combinations, depending on clinical
status, tumour location and extension and time interval since last
treatment. Nonetheless, the optimal management of recurrent MGs has
not yet been established, which represents a marked clinical
challenge.
Local recurrence within 2 cm of the resection bed of
the primary tumour is the most common pattern of failure.
Therefore, local strategies such as surgical resection and/or
radiotherapy in combination with systemic chemotherapy, in a
multidisciplinary approach, may offer an advantage in local control
and may improve survival outcomes.
Recent literature reviews and several retrospective
studies suggest a survival benefit with reoperation at the time of
recurrence. Favourable PS and extent of resection (gross total
resection vs. partial surgery) are the main predictors of survival
(5–10) and the addition of adjuvant treatments
(chemotherapy and radiosurgery) prolongs survival (5,35,36).
Several trials suggest an improvement in survival
and functional status with local reirradiation in younger patients
with good PS, tumour size <4 cm and progression more than 6
months from first irradiation (14,37,38).
Retrospective and prospective trials have investigated the benefits
of reirradiation as adjuvant therapy after resurgery (36) or as part of a combined approach with
chemotherapy (39). There is no
consensus on one particular radiation regimen, but higher doses per
fraction with modern precision radiotherapy (PBT, fractionated
stereotactic radiotherapy or stereotactic radiosurgery), are
associated with smaller recurrences and clinical efficacy with low
toxicity rates. Systemic agents used as radiosensitizers in
combination with radiotherapy are cytotoxic and targeted systemic
agents, such as TMZ and BEV (38).
Systemic therapy, consisting of chemotherapeutic and
anti-angiogenetic drugs, is the main treatment employed and
investigated for recurrent gliomas as single agents or as
combination regimens (21,30), but the optimal combination and
sequencing have not yet been established. The most used drugs are
rechallenge TMZ, nitrosoureas and BEV (40).
According to several systematic reviews and
meta-analyses, BEV as a single agent and in combination with
chemotherapy, both as first or second-line treatments, has been
shown to be effective in terms of ORR, PFS and reducing symptoms,
but not in terms of OS (5,41).
Interesting results were shown with the combination
of BEV and nitrosoureas (41,42),
such as lomustine and FTM. The BELOB phase II trial (42), the subsequent phase III trial
EORTC-26101 (43) and two recent
trials (44,45) showed that the combination
BEV/lomustine at first recurrence was superior to BEV or lomustine
monotherapy (41). Other interesting
results were obtained by retrospective and prospective trials on
the combination of BEV and FTM (24–27)
(Table III). Soffietti et al.
showed the efficacy of the association of BEV/FTM at first
recurrence in recurrent grade-III gliomas (24) and GBM patients, in terms of survival
outcome and response rate (26).
Similar results were reported by a retrospective analysis conducted
by Liu et al (27) and an
observational prospective study by Vaccaro et al (25).
 | Table III.Clinical trials on concomitant
FTM/BEV as second-line therapy in recurrent MGs. |
Table III.
Clinical trials on concomitant
FTM/BEV as second-line therapy in recurrent MGs.
|
|
|
|
|
|
|
|
| mPFS in patients
(months) |
|
| mOS in patients
(months) |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Authors
(years) | Type of study | No. of
patients | Hystotype | RR (%) | DCR (%) | mPFS (months) | PFS-6 (%) | Methylated | Unmethylated | mOS (months) | 1-year OS (%) | Methylated | Unmethylated | (Refs.) |
|---|
| Soffietti et
al (2012) | Prospective phase
II study | 32 | Grade III
gliomas | 50.0 | 94.0 | 5.0 | 31.0 | NA | NA | 8.6 | 37.8 | NA | NA | (24) |
| Soffietti et
al (2014) | Prospective phase
II study | 54 | GBM | 52.0 | 89.0 | 5.2 | 42.6 | NA | NA | 9.1 | 29.7 | NA | NA | (26) |
| Vaccaro et
al (2014) | Observational
prospective study | 26 | MGs (50% GBM) | 31.0 | 92.5 | 4.0 | 23.1 | NA | NA | 6.0 | 20.5 | NA | NA | (25) |
| Liu et al
(2015) | Retrospective
analysis | 176 | GBM | 46.6 | 90.9 | 5.0 | 33.3 | 6 | 5 | 8.0 | 22.0 | NA | NA | (27) |
|
|
|
|
|
|
|
|
|
|
|
|
| NA | NA |
|
| Present study | Retrospective
analysis | 12 | MGs (77% GBM) | 84.0 | 92.0 | 10.0 | 92.0 | 10 | 5 | 11.0 | 25.0 | 11 | 6 |
|
Several studies showed that the efficacy of systemic
chemotherapy in terms of disease control and survival is improved
by the combination with local treatments such as surgery and/or
irradiation (28–30,39,46)
(Table IV). In 2015 Scorsetti et
al (30) evaluated 43 GBM
patients treated by chemotherapy plus local treatment or
chemotherapy alone, showing that the combined treatment achieved
better survival results in terms of PFS (15 vs. 5 months) and OS
(17 vs. 6 months).
 | Table IV.Clinical trials on multimodal
treatment as second-line therapy in recurrent MGs. |
Table IV.
Clinical trials on multimodal
treatment as second-line therapy in recurrent MGs.
| Authors
(years) | Type of study | No. of
patients | Hystotype | mPFS (months) | mPFS in methylated
patients (months) | 1-year PFS | mOS | mOS in methylated
patients (months) | 1-year OS | (Refs.) |
|---|
| Archavlis et
al (2014) | Prospective
study | 17 | GBM | 7 | NA | NA | 7 | NA | NA | (28) |
| Archavlis et
al (2014) | Prospective
study | 66 | GBM | 7 | NA | NA | 8 | NA | NA | (29) |
| Scorsetti et
al (2015) | Retrospective
study | 21 | GBM | 15 | NA | 65% | 17 | NA | 69% | (30) |
| Azoulay et
al (2017) | Retrospective
study | 41 | GBM | NA | NA | NA | 10 | NA | NA | (39) |
| Archavlis et
al (2017) | Retrospective
study | 15 | GBM | 3 | NA | NA | 6 | NA | NA | (46) |
| Present study | Retrospective
study | 11 | MGs (77% GBM) | 11 | 11 | 27% | 13 | 12 | 55% |
|
Azoulay et al (39) conducted a retrospective study to
assess the benefits of resurgery followed by chemotherapy and/or
reirradiation compared to resurgery alone and chemotherapy and/or
reirradiation. Median survival was superior in the multimodal
treatment compared to the other treatment approaches (10 vs. 6.8
vs. 6.6 months).
Archavlis et al. showed in three clinical studies
(28,29,46) that
a combined therapy of resurgery, brachytherapy and chemotherapy
achieved better survival outcomes compared to a historical control
group of patients treated with TMZ.
We report our experience with the multimodal
management of recurrent MGs, as the combination of resurgery and/or
radiotherapy and chemotherapy, compared to chemotherapy alone. In
regards to chemotherapy, we studied the efficacy of cFTM/BEV
compared to sFTM/BEV, an idea born from the study of Piccioni et
al (47), which demonstrated
equal efficacy of BEV monotherapy on first, second or third
recurrence in recurrent GBM.
We observed that multimodal therapy was associated
with 25% higher response rates, 15% higher DCR and a survival
improvement of 4 months in PFS and 5 months in OS compared to
chemotherapy alone. Our results are in line with those reported by
other retrospective and prospective trials on multimodal treatment
(Table IV), showing the possibility
of combining systemic chemotherapy with local treatment to improve
local control of the disease and survival outcomes.
According to the type of chemotherapy, we observed
~50% higher response rates, 35% higher DCR and better survival
outcomes with cFTM/BEV compared to sFTM/BEV. Our results seem to be
stronger than those reported by other trials regarding cFTM/BEV
(Table III), which was probably
due to the addition of reirradiation, alone or after surgery.
Only recently, the DIRECTOR and the BELOB trials
demonstrated the prognostic value of the MGMT methylation also in
recurrent GBM (42,48,49). The
AVAREG trial (50) demonstrated also
that MGMT methylation status was predictive of efficacy of FTM in
the recurrence setting. We observed an association between MGMT
methylation and longer survival independent of the type of
treatment. Methylated patients appear not to benefit from a
multimodal approach, but a survival benefit was observed with the
combination therapy compared to FTM alone, whereas unmethylated
patients appear to benefit from both multimodal therapy and
concomitant systemic therapy better than methylated patients.
Subgroup analyses on MGMT methylation in this setting were not
reported in other similar clinical trials (Table III–IV). Similar to the other trials on
cFTM/BEV, the combination therapy was well-tolerated, with most
frequent grade 3–4 toxicities related to chemotherapy.
The main limitations of this study are the low
number of patients with small subgroups resulting in a lack of
statistically significant results, the heterogeneity and
non-standardisation in the therapeutic approach used, and the
retrospective and non-randomised nature, resulting in possible
selection biases for each treatment modality.
Despite these limitations our encouraging survival
and local control results underlined that the management of
recurrent MGs patients, especially those with a poorer survival
such as unmethylated patients, should involve a multidisciplinary
approach, associating local treatments (surgery and/or
radiotherapy) to chemotherapy, or a combination of chemotherapies,
whenever possible. Moreover, in this multimodal view of the
treatment of MGs patients, molecular characteristics play a
relevant role in the decision making to determine the best choice
of treatment and the highest survival benefit possible.
Considering that no optimal treatment combinations
and sequencing have been established, our results could be a
starting point for further larger prospective studies.
In conclusion, our experience showed that in MG
patients at first recurrence, multimodal treatment (chemotherapy
plus surgery and/or radiotherapy) achieves better survival and
response results compared to chemotherapy alone. Moreover,
concomitant BEV/FTM provides higher survival benefit and response
rates, without adding higher toxicity, compared to the sequential
approach. Better survival outcomes were observed in MGMT methylated
patients but MGMT unmethylated patients have shown a greater
survival benefit with both multimodal therapy and cFTM/BEV.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AP and SER were the major contributors in writing
the manuscript, analysing and interpreting the patient data. MG,
JRGB, SP and CF were involved in acquisition, analysis and
interpretation of patient data. MS, ST, VB were involved in writing
the manuscript and revising it critically for important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards. Informed consent was obtained from all individual
participants included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ostrom QT, Gittleman H, Farah P, Ondracek
A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C and Barnholtz-Sloan JS:
CBTRUS statistical report: primary brain and central nervous system
tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 15
Sup 6:ii1–ii56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: European Organisation for Research and Treatment of
Cancer Brain Tumor and Radiotherapy Groups; National Cancer
Institute of Canada Clinical Trials Group: Radiotherapy plus
concomitant and adjuvant temozolomide for glioblastoma. N Engl J
Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Delgado-López PD and Corrales-García EM:
Survival in glioblastoma: A review on the impact of treatment
modalities. Clin Transl Oncol. 18:1062–1071. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weller M, van den Bent M, Hopkins K, Tonn
JC, Stupp R, Falini A, Cohen-Jonathan-Moyal E, Frappaz D,
Henriksson R, Balana C, et al: European Association for
Neuro-Oncology (EANO) Task Force on Malignant Glioma: EANO
guideline for the diagnosis and treatment of anaplastic gliomas and
glioblastoma. Lancet Oncol. 15:e395–e403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Montemurro N, Perrini P, Blanco MO and
Vannozzi R: Second surgery for recurrent glioblastoma: A concise
overview of the current literature. Clin Neurol Neurosurg.
142:60–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tosoni A, Franceschi E, Poggi R and
Brandes AA: Relapsed Glioblastoma: Treatment Strategies for Initial
and Subsequent Recurrences. Curr Treat Options Oncol. 17:492016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hervey-Jumper SL and Berger MS:
Reoperation for recurrent high-grade glioma: A current perspective
of the literature. Neurosurgery. 75:491–499; discussion 498–499.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Davis ME: Glioblastoma: Overview of
Disease and Treatment. Clin J Oncol Nurs. 20:S2–S8. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brandes AA, Bartolotti M, Tosoni A, Poggi
R, Bartolini S, Paccapelo A, Bacci A, Ghimenton C, Pession A,
Bortolotti C, et al: Patient outcomes following second surgery for
recurrent glioblastoma. Future Oncol. 12:1039–1044. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suchorska B, Weller M, Tabatabai G, Senft
C, Hau P, Sabel MC, Herrlinger U, Ketter R, Schlegel U, Marosi C,
et al: Complete resection of contrast-enhancing tumor volume is
associated with improved survival in recurrent glioblastoma-results
from the DIRECTOR trial. Neuro-oncol. 18:549–556. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gorlia T, Stupp R, Brandes AA, Rampling
RR, Fumoleau P, Dittrich C, Campone MM, Twelves CC, Raymond E, Hegi
ME, et al: New prognostic factors and calculators for outcome
prediction in patients with recurrent glioblastoma: A pooled
analysis of EORTC Brain Tumour Group phase I and II clinical
trials. Eur J Cancer. 48:1176–1184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nava F, Tramacere I, Fittipaldo A,
Bruzzone MG, Dimeco F, Fariselli L, Finocchiaro G, Pollo B,
Salmaggi A, Silvani A, et al: Survival effect of first- and
second-line treatments for patients with primary glioblastoma: A
cohort study from a prospective registry, 1997–2010. Neuro-oncol.
16:719–727. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Taunk NK, Moraes FY, Escorcia FE, Mendez
LC, Beal K and Marta GN: External beam re-irradiation, combination
chemoradiotherapy, and particle therapy for the treatment of
recurrent glioblastoma. Expert Rev Anticancer Ther. 16:347–358.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sulman EP, Ismaila N, Armstrong TS, Tsien
C, Batchelor TT, Cloughesy T, Galanis E, Gilbert M, Gondi V, Lovely
M, et al: Radiation Therapy for Glioblastoma: American Society of
Clinical Oncology Clinical Practice Guideline Endorsement of the
American Society for Radiation Oncology Guideline. J Clin Oncol.
35:361–369. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mizumoto M, Yamamoto T, Ishikawa E,
Matsuda M, Takano S, Ishikawa H, Okumura T, Sakurai H, Matsumura A
and Tsuboi K: Proton beam therapy with concurrent chemotherapy for
glioblastoma multiforme: Comparison of nimustine hydrochloride and
temozolomide. J Neurooncol. 130:165–170. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Minniti G, Armosini V, Salvati M, Lanzetta
G, Caporello P, Mei M, Osti MF and Maurizi RE: Fractionated
stereotactic reirradiation and concurrent temozolomide in patients
with recurrent glioblastoma. J Neurooncol. 103:683–691. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Adeberg S, Harrabi SB, Bougatf N,
Bernhardt D, Rieber J, Koerber SA, Syed M, Sprave T, Mohr A,
Abdollahi A, et al: Intensity-modulated proton therapy,
volumetric-modulated arc therapy, and 3D conformal radiotherapy in
anaplastic astrocytoma and glioblastoma: A dosimetric comparison.
Strahlenther Onkol. 192:770–779. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsuda M, Yamamoto T, Ishikawa E, Nakai
K, Zaboronok A, Takano S and Matsumura A: Prognostic factors in
glioblastoma multiforme patients receiving high-dose particle
radiotherapy or conventional radiotherapy. Br J Radiol. 84:S54–S60.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mizumoto M, Okumura T, Ishikawa E,
Yamamoto T, Takano S, Matsumura A, Oshiro Y, Ishikawa H, Sakurai H
and Tsuboi K: Reirradiation for recurrent malignant brain tumor
with radiotherapy or proton beam therapy. Technical considerations
based on experience at a single institution. Strahlenther Onkol.
189:656–663. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Galle JO, McDonald MW, Simoneaux V and
Buchsbaum JC: Reirradiation with proton therapy for recurrent
gliomas. Int J Part Ther. 2:11–18. 2015. View Article : Google Scholar
|
|
21
|
Seystahl K, Wick W and Weller M:
Therapeutic options in recurrent glioblastoma-An update. Crit Rev
Oncol Hematol. 99:389–408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Xing D, Zhao M, Wang J and Yang Y:
The role of a single angiogenesis inhibitor in the treatment of
recurrent glioblastoma multiforme: A meta-analysis and systematic
review. PLoS One. 11:e01521702016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Addeo R, Caraglia M, De Santi MS, Montella
L, Abbruzzese A, Parlato C, Vincenzi B, Carraturo M, Faiola V,
Genovese M, et al: A new schedule of fotemustine in
temozolomide-pretreated patients with relapsing glioblastoma. J
Neurooncol. 102:417–424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Soffietti R, Trevisan E, Bosa C, Bertero L
and Ruda R: Phase II trial of bevacizumab and fotemustine in
recurrent grade III gliomas. J Clin Oncol. 30:Abstract 2075.
2012.
|
|
25
|
Vaccaro V, Fabi A, Vidiri A, Giannarelli
D, Metro G, Telera S, Vari S, Piludu F, Carosi MA, Villani V, et
al: Activity and safety of bevacizumab plus fotemustine for
recurrent malignant gliomas. BioMed Res Int. 2014:3512522014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Soffietti R, Trevisan E, Bertero L,
Cassoni P, Morra I, Fabrini MG, Pasqualetti F, Lolli I, Castiglione
A, Ciccone G, et al: Bevacizumab and fotemustine for recurrent
glioblastoma: A phase II study of AINO (Italian Association of
Neuro-Oncology). J Neurooncol. 116:533–541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Z, Zhang G, Zhu L, Wang J, Liu D, Lian
L, Liu J, Lai T and Zhuang X: Retrospective analysis of bevacizumab
in combination with fotemustine in chinese patients with recurrent
glioblastoma multiforme. Biomed Res Int. 2015:7236122015.PubMed/NCBI
|
|
28
|
Archavlis E, Tselis N, Birn G, Ulrich P
and Zamboglou N: Salvage therapy for recurrent glioblastoma
multiforme: A multimodal approach combining fluorescence-guided
resurgery, interstitial irradiation, and chemotherapy. Neurol Res.
36:1047–1055. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Archavlis E, Tselis N, Birn G, Ulrich P
and Zamboglou N: Combined salvage therapies for recurrent
glioblastoma multiforme: Evaluation of an interdisciplinary
treatment algorithm. J Neurooncol. 119:387–395. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Scorsetti M, Navarria P, Pessina F,
Ascolese AM, D'Agostino G, Tomatis S, De Rose F, Villa E, Maggi G,
Simonelli M, et al: Multimodality therapy approaches, local and
systemic treatment, compared with chemotherapy alone in recurrent
glioblastoma. BMC Cancer. 15:4862015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
Classification of Tumors of the Central Nervous System: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wen PY, Macdonald DR, Reardon DA,
Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert
MR, Lassman AB, et al: Updated response assessment criteria for
high-grade gliomas: Response assessment in neuro-oncology working
group. J Clin Oncol. 28:1963–1972. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kaplan E and Meier P: Nonparametric
estimation from incomplete observations. J Am Stat Assoc.
53:457–481. 1958. View Article : Google Scholar
|
|
34
|
Common Terminology Criteria for Adverse
Events v4.03 (CTCAE). http://ctep.cancer.govJuly 22–2017
|
|
35
|
Mandl ES, Dirven CM, Buis DR, Postma TJ
and Vandertop WP: Repeated surgery for glioblastoma multiforme:
Only in combination with other salvage therapy. Surg Neurol.
69:506–509; discussion 509. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Straube C, Elpula G, Gempt J, Gerhardt J,
Bette S, Zimmer C, Schmidt-Graf F, Meyer B and Combs SE:
Re-irradiation after gross total resection of recurrent
glioblastoma: Spatial pattern of recurrence and a review of the
literature as a basis for target volume definition. Strahlenther
Onkol. 193:897–909. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cabrera AR, Kirkpatrick JP, Fiveash JB,
Shih HA, Koay EJ, Lutz S, Petit J, Chao ST, Brown PD, Vogelbaum M,
et al: Radiation therapy for glioblastoma: Executive summary of an
American Society for Radiation Oncology Evidence-Based Clinical
Practice Guideline. Pract Radiat Oncol. 6:217–225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Howard SP, Krauze A, Chan MD, Tsien C and
Tomé WA: The evolving role for re-irradiation in the management of
recurrent grade 4 glioma. J Neurooncol. 134:523–530. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Azoulay M, Santos F, Shenouda G, Petrecca
K, Oweida A, Guiot MC, Owen S, Panet-Raymond V, Souhami L and
Abdulkarim BS: Benefit of re-operation and salvage therapies for
recurrent glioblastoma multiforme: Results from a single
institution. J Neurooncol. 132:419–426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
National Comprehensive Cancer Network:
NCCN Clinical Practice Guidelines in Oncology (NCCN
Guidelines®): Central Nervous System Cancers. NCCN
Evidence Blocks. Version 1.2017. https://www.nccn.org/professionals/physician_gls/pdf/cns_blocks.pdfDecember
5–2017
|
|
41
|
Lombardi G, Pambuku A, Bellu L, Farina M,
Della Puppa A, Denaro L and Zagonel V: Effectiveness of
antiangiogenic drugs in glioblastoma patients: A systematic review
and meta-analysis of randomized clinical trials. Crit Rev Oncol
Hematol. 111:94–102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Taal W, Oosterkamp HM, Walenkamp AM,
Dubbink HJ, Beerepoot LV, Hanse MC, Buter J, Honkoop AH, Boerman D,
de Vos FY, et al: Single-agent bevacizumab or lomustine versus a
combination of bevacizumab plus lomustine in patients with
recurrent glioblastoma (BELOB trial): A randomised controlled phase
2 trial. Lancet Oncol. 15:943–953. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wick W, Brandes AA, Gorlia T, Bendszus M,
Sahm F, Taal W, Taphoorn M, Domont J, Idbaih A, Campone M, et al:
Phase III trial exploring the combination of bevacizumab and
lomustine in patients with first recurrence of a glioblastoma: The
EORTC 26101 trial. Neuro-oncol. 17 Suppl 5:LB052015. View Article : Google Scholar
|
|
44
|
Weathers SP, Han X, Liu DD, Conrad CA,
Gilbert MR, Loghin ME, O'Brien BJ, Penas-Prado M, Puduvalli VK,
Tremont-Lukats I, et al: A randomized phase II trial of standard
dose bevacizumab versus low dose bevacizumab plus lomustine (CCNU)
in adults with recurrent glioblastoma. J Neurooncol. 129:487–494.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Heiland DH, Masalha W, Franco P, Machein
MR and Weyerbrock A: Progression-free and overall survival in
patients with recurrent Glioblastoma multiforme treated with
last-line bevacizumab versus bevacizumab/lomustine. J Neurooncol.
126:567–575. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Archavlis E: Combined Salvage Therapies
for Recurrent Glioblastoma Multiforme: Treatment Options in
Multifocal and Multicentric Patterns of Recurrence. J Cancer Prev
Curr Res. 7:002222017. View Article : Google Scholar
|
|
47
|
Piccioni DE, Selfridge J, Mody RR,
Chowdhury R, Li S, Lalezari S, Wawrzynski J, Quan J, Zurayk M, Chou
AP, et al: Deferred use of bevacizumab for recurrent glioblastoma
is not associated with diminished efficacy. Neuro-oncol.
16:815–822. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Weller M, Tabatabai G, Kästner B, Felsberg
J, Steinbach JP, Wick A, Schnell O, Hau P, Herrlinger U, Sabel MC,
et al: DIRECTOR Study Group: MGMT promoter methylation is a strong
prognostic biomarker for benefit from dose-intensified temozolomide
rechallenge in progressive glioblastoma: The DIRECTOR Trial. Clin
Cancer Res. 21:2057–2064. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Szopa W, Burley TA, Kramer-Marek G and
Kaspera W: Diagnostic and Therapeutic Biomarkers in Glioblastoma:
Current Status and Future Perspectives. BioMed Res Int.
2017:80135752017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Brandes AA, Finocchiaro G, Zagonel V, Reni
M, Caserta C, Fabi A, Clavarezza M, Maiello E, Eoli M, Lombardi G,
et al: AVAREG: A phase II, randomized, noncomparative study of
fotemustine or bevacizumab for patients with recurrent
glioblastoma. Neuro-oncol. 18:1304–1312. 2016. View Article : Google Scholar : PubMed/NCBI
|