Introduction
Although diverse in morphological and clinical
manifestations, neoplasms of the testicles account for a notable
proportion of all male urogenital tumors. They had a relatively
rare overall occurrence, with a peak prevalence rate during the
second and third decades of life, being the most popular solid
tumor in men of this age (1).
According to the American Cancer Society statistics, in 2015
approximately 8,430 new cases of testicular cancer were diagnosed
and more than 380 patients succumbed of the disease in the US
(2). Similarly in China these
figures were estimated to be 4,000 and 1,000, respectively in the
year 2015 (3). With the development
of cisplatin-based chemotherapy and the integration of surgery,
testicular tumors, especially germ cell tumors (GCTs), have been
considered a curable disease, the 5-year relative survival rate of
which has notably increased from 83 to 97% in the past 4 decades
(2). However, there are certain
pathological patterns in which the treatment options are unclear
and remain a clinical challenge. Testis-sparing surgery (TSS),
commonly known as partial orchiectomy in recent years has emerged
as promising treatment option especially in selective patients
including bilateral lesions, monorchide tumors and those facing
psychological stress or with paternity demand.
Traditionally TSS was a controversial surgical
modality, carried out only in selected cases including bilateral
organ-confined small lesions, tumors in a solitary testis with
sufficient androgen production, or suspected benign tumors when
serum tumor markers are normal (4)
with no significant role in GCT patients. It was only by recent
studies that showed equivalent oncological outcomes for
organ-sparing surgery when compared with radical orchiectomy (RO)
in elective groups. Also the functional issues and quality of life
pertaining to treatment also seemed to be promising (5). However, comparative studies between TSS
and traditionally applied RO are still limited both in quantity and
in perspective view. Here we reviewed retrospectively our series of
cases undergoing organ-sparing surgery for testicular tumors, and
share our experiences in patient selection, surgical technics, and
clinical difficulties we faced.
Subjects and methods
The retrospective cohort
The present study performed a retrospective
comparative study only; all TSS and RO procedures were conducted
previously. Ethical approval was obtained from the Institutional
Review Board of Second Military Medical University (Shanghai,
China) and all participants provided written informed consent
during the follow-up procedures. The present study retrospectively
retrieved patients' information from the hospital information
system (HIS) database of Changzheng Hospital from January 1999 to
December 2016. The diagnoses in the HIS system were made and
compiled according to the ICD-9 categories. During data retrieving,
the ambiguous matching strategy was used to maximize the valid
cases. All secondary tumors, such as those with metastasis and
lymphoma testicular infiltration, were excluded from the study. The
cohorts were determined according to surgical procedure, viz. RO
group and TSS group, in order to better assess and compare the
clinical and prognostic features for each surgical group.
Evaluation of clinical
characteristics
After admission detail history taking and physical
examinations were done and marital status as well as prior
paternity was inquired in every patient. The patients usually
presented with a chief complaint of palpable, painless mass within
the scrotum, only few patients had discernable inguinal lymph nodes
during physical examination. Scrotal ultrasound is the most widely
used screening method for discrimination of testis tumors with
extratesticular or epididymis lesions. Contrast enhanced pelvic CT
and MRI scanning are two major diagnostic tools used before
surgery. These two imaging techniques are more helpful to suggest
the tumors' malignant nature and at the same time help assess the
local and retroperitoneal lymph nodes as well as metastasis
status.
When surgery is considered, both ultrasound and
CT/MRI should be referred by the surgeons, for they act
complementary roles in pre-operative assessments. The Doppler
ultrasonography is helpful in the evaluation of the tumors' blood
supply. Ultrasound may tend to overestimate the size of the tumors
and underestimate the residual testis parenchyma (6), therefore a more precise portrait of the
tumor should be obtained by CT/MRI to determine whether a partial
orchiectomy is feasible or not.
Serum testosterone level (T), serum α-fetoprotein
(AFP), human chorionic gonadotrophin (hCG) and lactate
dehydrogenase (LDH) levels were also evaluated before surgery.
Chest X-ray is essential to rule out the possible metastatic foci
and complete the accurate staging in all clinically suspected
cases, because testicular cancers are prone to metastasis
especially to the lungs. Chest CT scan is also recommended if
clinically indicated.
Intraoperative frozen-section examination (FSE) was
sent when the surgeon encountered ambiguous consideration of
whether the tumor was malignant or benign. However, definitive
pathological diagnosis was determined by final pathological
analysis (FPA). After TSS, tumor bed biopsy is mandatory to exclude
positive margins and intratubular germ cell neoplasia (ITGCN),
6-point systemic biopsy is especially recommended in order to
obtain higher positive rate. Normal parenchyma biopsy was not
performed, because it is usually hard to define between tumor bed
and normal parenchyma, since the cutting edge was supposed to be
overriding the margin of the tumor. Therefore, the parenchyma
biopsy is not emphasized in our practice.
Protocol of primary and adjuvant
treatment
Surgery is the mainstay treatment for testicular
tumors and RO is the surgery of choice in majority of the cases.
TSS is recommended when: i) The tumor size is small enough to leave
sufficient normal testicular parenchyma; ii) preoperative imaging
suggestive of benign lesions; iii) monorchide or bilateral tumor
patients who may be virtually castrated if RO is implemented; and
iv) patients have strong psychological and social demand to
preserve the organ, or fear of infertility and life-long androgen
deficiency and substitution.
Adjuvant treatment, including chemotherapy,
radiotherapy, and retroperitoneal lymph nodes dissection (RPLND),
were to be done according to the instruction of the NCCN guideline
for testicular cancer (version 1.2014).
Protocol of follow-up
Once surgical procedure was determined, follow-up
sessions were also initiated simultaneously. Endpoint information
(disease relapse, survival status, postoperative paternity) were
obtained mainly by telephone, mail, e-mail and instant messaging
(IM) tools (WeChat® and Fetion®). Medical
imaging and serum tumor markers were acquired during outpatient
department (OPD) visits. Measurement of serum tumor markers and
endocrine status indices were implemented, including serum AFP,
LDH, HCG and testosterone levels during the follow-up period.
Childbearing and need for androgen substitution therapy after
surgery were also inquired and recorded. Deaths due to disease and
postoperative paternity by natural conception were considered as
primary endpoints and disease relapse as secondary endpoint during
follow-up.
Statistical analysis
The baseline of all the patients was described by a
cross-sectional survey. The patients were subdivided into the RO
and TSS groups. The differences in rates were tested using
Chi-square or Fisher's exact probability test. The differences in
quantitative values were tested using Student's t-test, after
statistical tests confirmed samples' normal distribution and
homogeneity of variance. When taking postoperative paternity,
disease relapse and disease related deaths to be follow-up
endpoints, survival analysis was applied using product limit
method. Kaplan-Meier curves were plotted with log-rank test to
estimate the difference between the two groups. All statistical
analyses were applied using Stata® software (version
11.0 Special Edition; StataCorp LLC, College Station, TX, USA).
Kaplan-Maier analysis plots were drawn using GraphPad
Prism® 5 (version 5.01; GraphPad Software, Inc., La
Jolla, CA, USA).
Results
Demographic and clinical features of
the patients
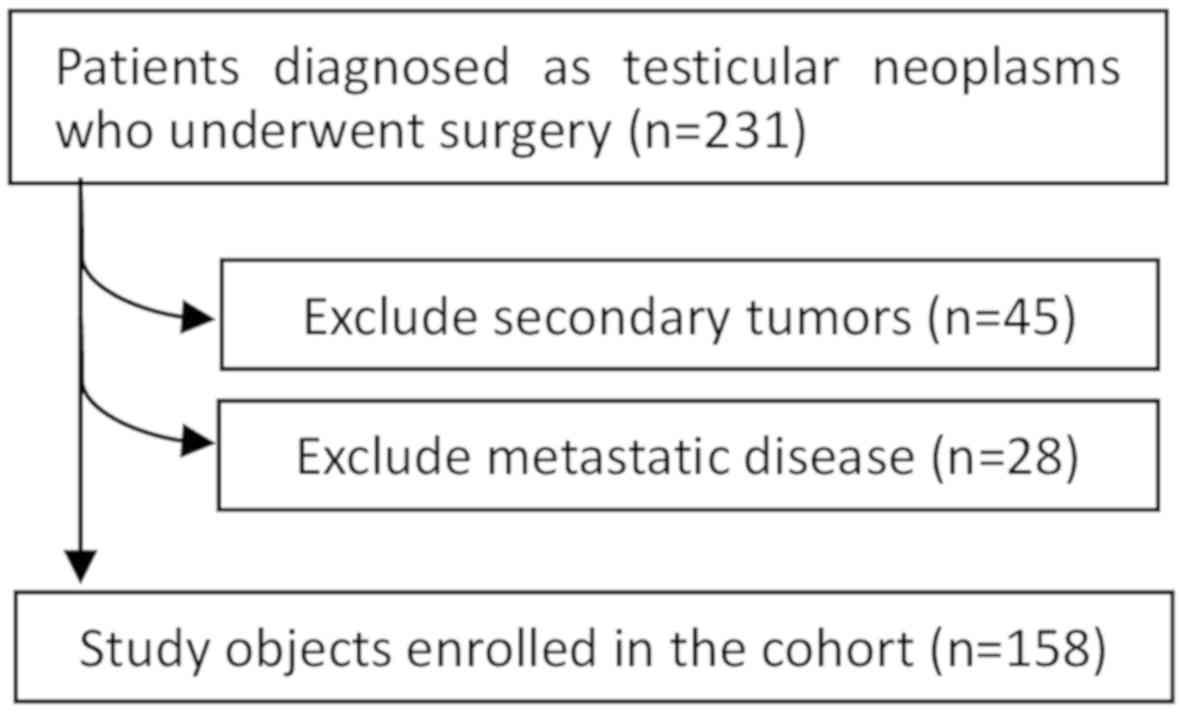
A total of 158 patients were enrolled in this
retrospective study. The enrollment procedure is shown in Fig. 1. Among these 158 patients only 125
completed the follow-up. Follow-up periods range from 8 to 214
months, with a median follow-up of 78 months. The average course of
disease was 33.2 months (31.2 and 45 months in RO group and TSS
group, respectively, no significant difference). The average age at
diagnosis is 45.4 s (median age 44). The average age in TSS group
(29.3 s, standard deviation 13.0) were younger compared to RO group
(47.7 s, standard deviation 17.3, P<0.0001), which showed the
TSS procedure is in favor of younger patients. Here we noticed the
imbalance between the two comparative cohorts, the reason may due
to: i) The significance of TSS in the treatment of testicular
tumors had just been recognized since a few decades ago; and ii)
the selection of TSS candidates has an intrinsic bias towards
younger patients with imperative functional demands. No positive
surgical margin was reported and no ITGCN was detected during
intraoperative biopsy and final pathology analysis in all 23
patients who underwent TSS.
We also carefully recorded patients' chief
complaints, tumor size and other related clinical parameters.
During the course of the disease we were surprised to note that
local pain and fever, which were considered as a hallmark to
suggest a non-tumorous lesion such as acute infection or torsion,
occurred more than expected in our patients. This indicates that
differential diagnosis based on symptoms and signs may not be quite
reliable. Besides that, as is shown in the table, the average tumor
size is much smaller in TSS group than in RO group, with only a few
cases of scrotum enlargement observed in TSS than in RO group
(Table I).
 | Table I.Demographic and clinical features of
patients. |
Table I.
Demographic and clinical features of
patients.
| Feature | Overall | RO | TSS |
|---|
| Patients, n | 158 | 135 | 23 |
| Course of disease,
months (±SD) | 33.2 (±83.2) | 31.2 (±80.4) | 45 (±99.3) |
| Average age of
diagnosis (±SD)a |
| Median
age | 45.0 (±17.9) | 47.7 (±17.3) | 29.3 (±13.0) |
| Average
age | 44 | 47 | 25 |
| Left/Right, n | 70/79 | 61/68 | 9/11 |
| Bilateral tumors,
n | 9 | 6 | 3 |
| Monorchide tumors,
n | 6 | 4 | 2 |
| Clinical
manifestations |
| Local
pain | 37 (23.4%) | 33 (24.4%) | 4 (17.4%) |
|
Fever | 5 (3.2%) | 5 (3.7%) | 0 (0%) |
| Scrotum
enlargementb | 79 (50.0%) | 77 (57.0%) | 2 (8.7%) |
|
Non-palpable disease | 20 (12.7%) | 15 (11.1%) | 5 (21.7%) |
| Tumor diameter, mm
(±SD)c | 47.2 (±25.3) | 51.5 (±24.4) | 21.7 (±11.4) |
| Simultaneous
hydrocele |
|
Unilateral | 12 | 11 | 1 |
|
Bilateral | 4 | 3 | 1 |
|
Overall | 16 | 14 | 2 |
| Tumor cyst
degeneration | 10 | 7 | 3 |
| Previous or current
cryptorchidism | 18 | 16 | 2 |
| Follow-up period,
months (±SD) |
| Average
follow-up | 82.3 (±48.9) | 86.2 (±48.2) | 59.2 (±43.3) |
| Median
follow-up | 78 (8–214) | 85 (8–214) | 44.5 (9–133) |
| Loss to follow-up
rate (number of dropout) | 20.9% (33) | 20.7% (28) | 21.7% (5) |
After the tumors resection, the specimens were sent
to the hospital's pathology department to obtain a final
pathological diagnosis (Table II).
GCTs were the most commonly seen pathological type (accounts for
81.6% of all patients). The ratio was 86.7% in RO group and only
52.2% in TSS group, which reflected a current prudence of doctors
when considered TSS for possible malignant cases. Among the 28
benign lesions, 5 of them were mature teratomas, 3 dermoid cysts, 3
Leydig cell tumors, 3 Sertoli cell tumors, 5 inflammatory
granulomas, 8 epidermal cysts and 1 vascular anomaly. Leydig cell
tumor is a rare kind of testicular tumor rises from sex
cord-gonadal stroma. These patients came to consultation because of
gynecomastia. In the 3 Sertoli cell tumor cases, 1 were
Peutz-Jeghers syndrome patient transferred from the department of
gastroenterology, 1 with gynecomastia and 1 detected by
self-palpation.
 | Table II.Pathological types of tumors. |
Table II.
Pathological types of tumors.
| Histopathological
types | Overall | RO | TSS |
|---|
| GCTa | 129 | 117 | 12 |
|
Seminoma | 66 | 61 | 5 |
| NSGCT | 63 | 56 | 7 |
|
Embryonal carcinoma | 13 | 12 | 1 |
| Yolk
sac tumor | 3 | 3 | 0 |
| Mature
teratoma | 5 | 3 | 2 |
| Dermoid
cyst | 3 | 3 | 0 |
|
Immature teratoma | 2 | 2 | 0 |
|
Teratoma with malignant
areas | 5 | 5 | 0 |
| Mixed
forms | 32 | 28 | 4 |
| Adenoma of
collecting ducts and rete | 4 | 4 | 0 |
|
Paratesticular sarcoma | 1 | 1 | 0 |
| Adenomatoid
tumor | 4 | 3 | 1 |
| Sex
cord-gonadal stromal tumors | 6 | 4 | 2 |
| Leydig cell
tumor | 3 | 1 | 2 |
| Sertoli cell
tumor | 3 | 3 | 0 |
|
Inflammatory granuloma | 5 | 4 | 1 |
| Epidermal cyst | 8 | 2 | 6 |
|
Vascular anomaly | 1 | 0 | 1 |
| Sum | 158 | 135 | 23 |
Oncological and functional outcome in
bilateral and monorchide tumors
It was estimated that bilateral testicular tumors,
both synchronous and metachronous, accounts only less than 5% of
all testicular tumors. But when considering solitary testicular
tumors together, the treatment-related definitive castration and
the ensuing problems in fertility and virilization is not rare
(7–9). Here we listed the characteristics and
clinical turnover of a selected subgroup: Patients with bilateral
tumors or solitary testicle tumors (Tables III and IV). It may be noticed in the table that
only 2 of 6 monorchide tumor patients and 3 of 9 bilateral tumor
patients received partial orchiectomy, the proportion of which was
supposed to be higher. The main reasons for not choosing TSS were
i) preoperative hypogonadism; ii) fear of disease relapse; and iii)
not in urgent need of future paternity. The average age of the 10
patients undergoing RO (59.3 s) was significantly older than those
who had testis-sparing surgeries (32.8 s). Three of the five TSS
patients were stage IA diseases, whereas in RO group only 1 patient
was stage IA disease. All patients who underwent TSS were
recommended for external irradiation of the remaining testis to
eradicate the possible undetected ITGCN. However, the youngster
(case no. 6, Table III) who
refused this suggestion developed disease relapse after 12 months'
follow-up, and RO was done after careful evaluation. In patients
who were planning to father a child, sperm cryopreservation was
performed for future use. The postoperative paternity status (by
natural conception rather than use of cryopreserved sperm) was also
recorded.
 | Table III.Characteristics and clinical turnover
of bilateral and solitary testis tumors. |
Table III.
Characteristics and clinical turnover
of bilateral and solitary testis tumors.
| No. | Diagnosis | Surgical
procedure | Age (years) | Reason of
monorchide/Chief complaints for consultation | Histological
types | AJCC's TNMS
staging | Adjuvant
treatment | Turnover/Follow up
(months) | Subsequent
treatment following relapse |
|---|
| 1 | Monorchide | RO | 37 | Previous
cryptorchidism | Seminoma | pT2N0M0S0/IB | Surveillance | -/182 | − |
| 2 | Monorchide | RO | 58 | Prior history of
GCT | Seminoma | pT2N0M0S0/IB | Surveillance | -/139 | − |
| 3 | Monorchide | TSS | 11 | Prior history of
parotitis, right testicle atrophy | Mature
teratoma | pT1N0M0S0/IA | RT, total dose 20Gy
in 10 days | -/117 | − |
| 4 | Monorchide | RO | 25 | Previous
cryptorchidism | Seminoma | pT2N1M0S0/IIA | BEP 3 cycles | -/70 | − |
| 5 | Monorchide | RO | 57 | Previous
cryptorchidism | Seminoma | pT2N1M0S0/IIA | EP 4 cycles | -/51 | − |
| 6 | Monorchide | TSS | 19 | Previous
cryptorchidism | Mixed forms
GCT | pT1N0M0S0/IA | Surveillance | Local
relapse/12 | RO, EP 4
cycles |
| 7 | Bilateral | RO | 59 | Scrotum enlargement
and palpable masses | Seminoma | pT2N1M0S0/IIA | RT/BEP 3
cycles | -/99 | − |
| 8 | Bilateral | TSS | 65 | Palpable
masses | Mixed forms
GCT | pT2N1M0S0/IIA | RPLND/EP 4
cycles | -/92 | − |
| 9 | Bilateral | RO | 65 | Scrotum enlargement
and palpable masses | Yolk sac tumor | pT2N1M0S1/IIA | EP 4 cycles | Metastasis/86 | RT, EP 4
cycles |
| 10 | Bilateral | TSS | 34 | Scrotum enlargement
and palpable masses | Mixed forms
GCT | pT1N0M0S0/IA | RPLND/RT, total
dose 20Gy in 10 days | -/76 | − |
| 11 | Bilateral | RO | 72 | Scrotum
enlargement | Mixed forms
GCT | pT1N1M0S0/IIA | BEP 3 cycles | Metastasis/4;
Died/12 | EP 2 cycles |
| 12 | Bilateral | RO | 76 | Scrotum
enlargement | Immature
teratoma | pT1N0M0S0/IA | Surveillance | Loss to follow
up | − |
| 13 | Bilateral | RO | 66 | Palpable
masses | Mixed forms
GCT | pT2N0M0S0/IB | BEP 2 cycles | -/20 |
|
| 14 | Bilateral | RO | 78 | Palpable
masses | Seminoma | pT1N0M0S0/IA | Surveillance | -/19 |
|
| 15 | Bilateral | TSS | 35 | Scrotum enlargement
and palpable masses | Embryonal
carcinoma | pT2N0M0S1/IS | BEP 3 cycles | -/12 |
|
 | Table IV.Functional and paternity results of
bilateral and solitary testis tumors. |
Table IV.
Functional and paternity results of
bilateral and solitary testis tumors.
| No. | Diagnosis | Surgical
procedure | Age (years) | Marital/Paternity
status (no. of children) | Preoperative sperm
cryopreservation | Postoperative
paternity by natural conception | Postoperative serum
androgen level | Postoperative
hormone substitution |
|---|
| 1 | Monorchide | RO | 37 | Unmarried/- | No | - (Unmarried) | Low | Yes |
| 2 | Monorchide | RO | 58 | Married/2 | No | − | NA | No |
| 3 | Monorchide | TSS | 11 | Unmarried/- | No | - (Unmarried) | Normal | No |
| 4 | Monorchide | RO | 25 | Married/- | Yes | − | Low | Yes |
| 5 | Monorchide | RO | 57 | Married/- | No | − | NA | No |
| 6 | Monorchide | TSS | 19 | Unmarried/- | Yes | -(Unmarried) | Low | Yes |
| 7 | Bilateral | RO | 59 | Married/1 | No | − | NA | No |
| 8 | Bilateral | TSS | 65 | Married/1 | No | − | NA | No |
| 9 | Bilateral | RO | 65 | Married/1 | No | − | NA | No |
| 10 | Bilateral | TSS | 34 | Married/1 | No | − | Normal | No |
| 11 | Bilateral | RO | 72 | Married/2 | No | − | NA | No |
| 12 | Bilateral | RO | 76 | Married/2 | No | Loss to follow
up | − | − |
| 13 | Bilateral | RO | 66 | Married/1 | No | − | Low | Yes |
| 14 | Bilateral | RO | 78 | Married/2 | No | − | NA | No |
| 15 | Bilateral | TSS | 35 | Married/- | Yes | − | Normal | No |
Oncological and functional outcome in
TSS for unilateral malignant tumors
Although currently not recommended, in selected
cases of unilateral GCTs depending on tumor size and other clinical
conditions, TSS is considered as treatment of choice. Here we
summarized patients who had undergone TSS with a pathological
diagnosis of GCTs. The reason for choosing TSS instead of standard
RO were: i) Cannot accept the loss of genital organ; ii) resent of
possible hypogonadism as well as life-long androgen substitution;
and iii) early clinical staging and tumor size small enough to
allow a TSS procedure. Of the 7 unilateral GCT patients who had
TSS, 5 cases were seminomas, 1 mature teratoma and 1 mixed forms
GCT. They were all staged IA phase of disease according to AJCC's
TNMS staging system. All, except the mature teratoma patient, were
recommended for adjuvant radiotherapy after surgery, including 1
seminoma patient who had adjuvant radiotherapy after successful
child bearing. 4 of the 7 patients completed the follow-up process,
and none of them had postoperative hypogonadism or needed androgen
replacement therapy. No disease recurrence and cancer related
deaths were observed during the follow-up.
Postoperative paternity, tumor relapse
and survival
Kaplan-Maier plots based on product-limit method
were applied in this section of analysis. We first observed
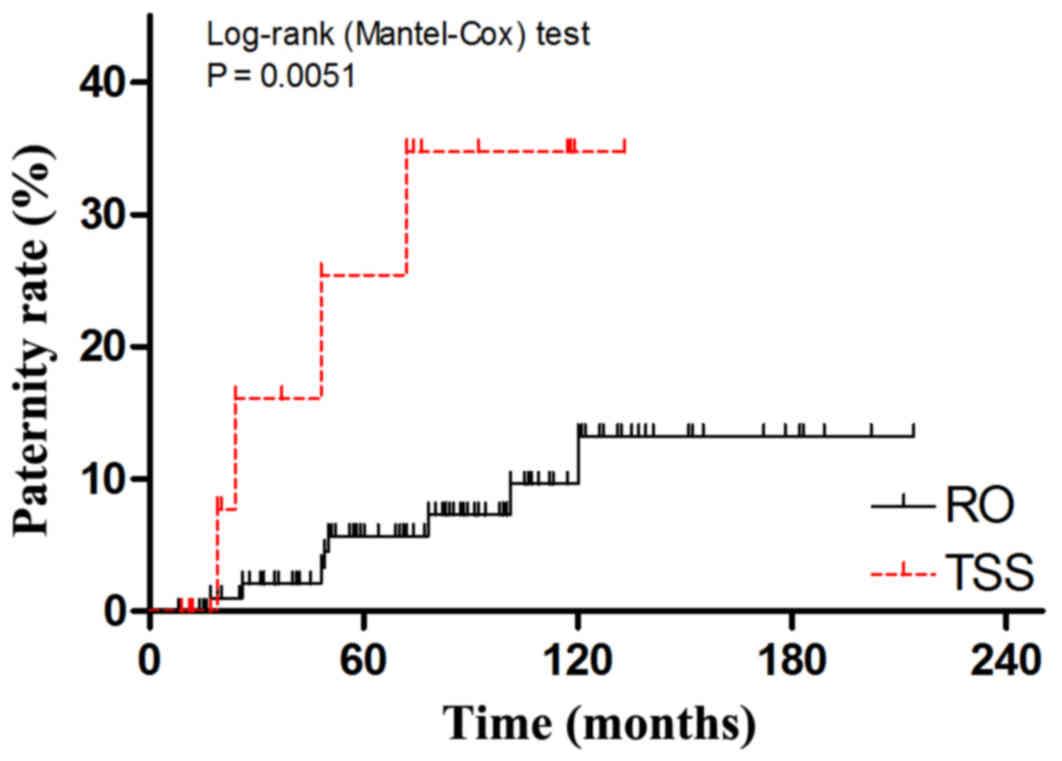
postoperative paternity status. A cumulative hazard curve was
plotted to show the postoperative paternity by natural conception
rather than use of cryopreserved sperm. As is shown in Fig. 2, the cumulative paternity rate in TSS
group is significantly higher than in RO group (log-rank test,
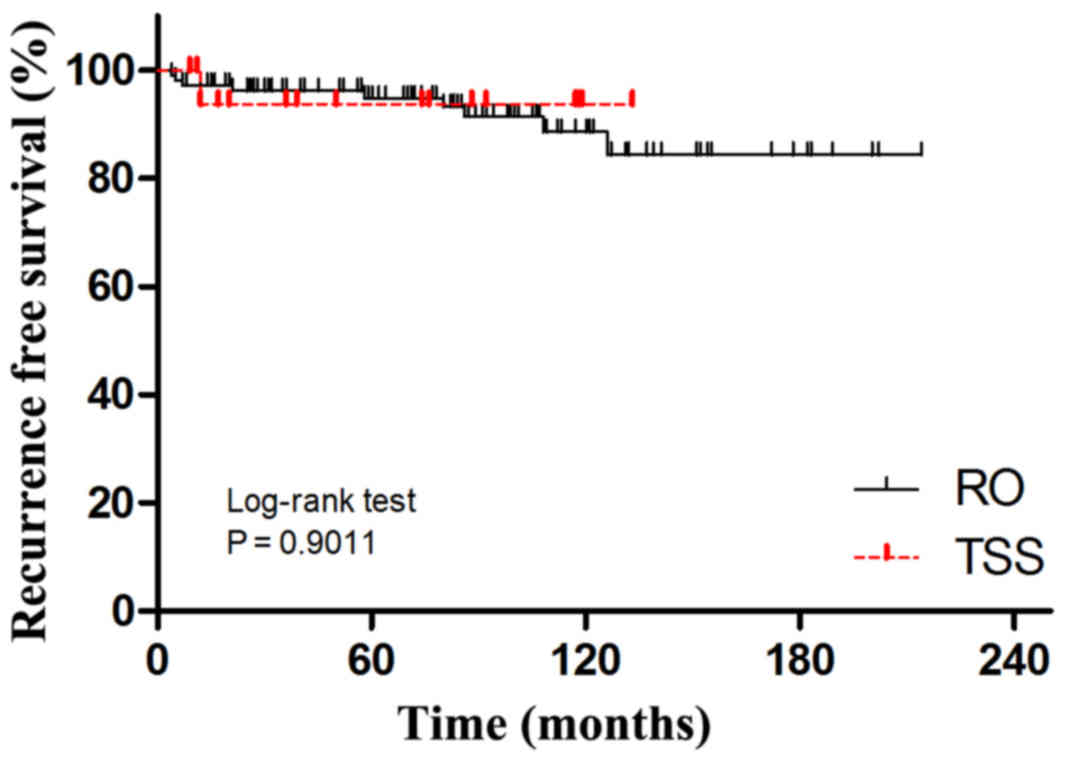
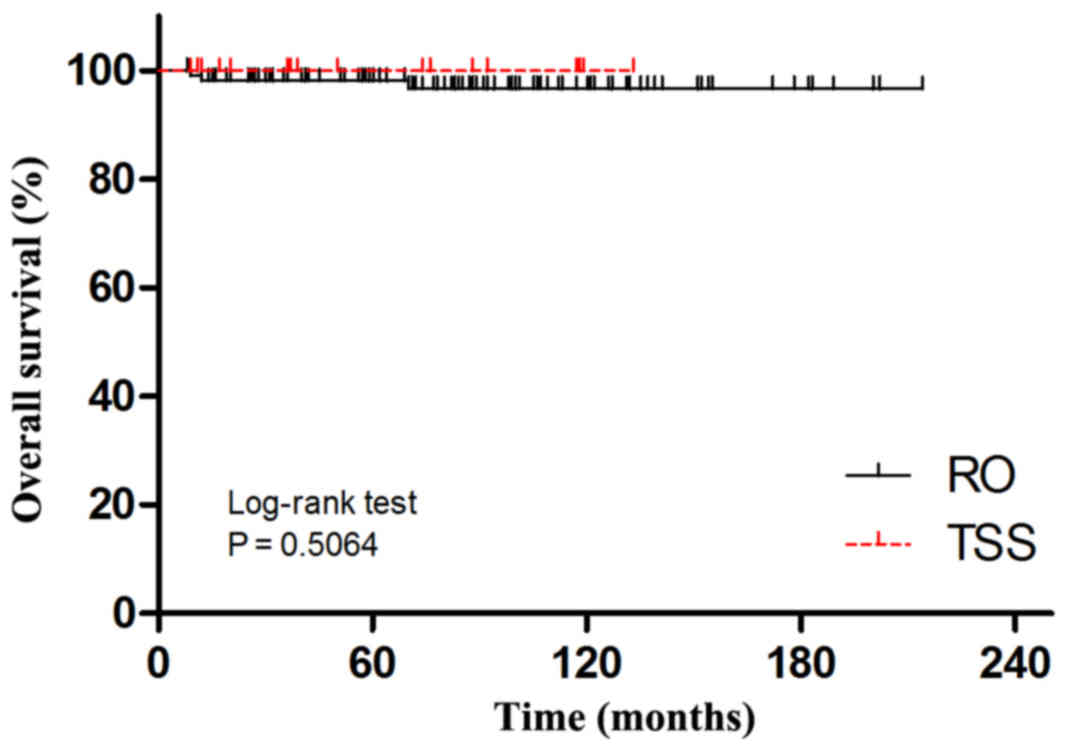
P=0.0051). Kaplan-Maier analyses for disease relapse and cancer
related deaths (Figs. 3 and 4) showed no significant difference between
RO and TSS groups.
Discussion
The improvement in disease control of testicular
tumors and the increase in therapy related long-term survival has
enabled us to focus more on treatment-related side effects and
preservation of quality of life. The germinal epithelium is
exquisitely sensitive to platin-based chemotherapy and
radiotherapy, which provides the opportunity to concentrate our
research from life-saving procedure to function-saving novel
therapies. In testicular tumor patients, impaired reproductive
function (such as oligospermia and azoospermia) is not only the
reason for seeking medical consultation (10), but also the undesirable treatment
consequence related especially to RO (11), which is even more troublesome.
Hypogonadism in testicular cancer survivors is a major concern for
both patients and surgeons.
However, when considering the pros and cons of the
testis-sparing surgery, the notorious nature of testicular cancers'
fast growth and progression has always casted a shadow over the
procedure's optimistic perspective. For many years discretion and
conservatism had been the keynote of testicular cancer treatment.
Despite the deleterious effect of radiotherapy or chemotherapy on
both fertility and virility, radical measures including
‘desperation surgery (salvage surgery)’ and high-dose chemotherapy
were thought to be beneficial for testicular cancer patients
(12,13). Moreover, because of the organ's small
size, acquisition of both a tumor-free surgical margin and well
preserved normal parenchyma will be a great challenge. Finally,
adjuvant radiotherapy and chemotherapy following TSS not only
exhibit their toxic effect within therapeutic doses, but also may
induce secondary malignant neoplasms (SMNs). Thus striking a
balance between the merits and demerits of this controversial
procedure is easier than a dilemma.
In our study, we illustrated the feasibility of the
surgical procedure and the unique advantage that TSS possesses. One
of the many advantages is the good potential of preserving
fertility. The cumulative paternity rate shown in our results also
supported the benefits of TSS, although this result has to be
considered with caution of some inevitable biases. The
postoperative child-bearing is a complex course which may be
influenced by many confounders. The desire of paternity may vary
depending on one's previous paternity status and social-economical
ability. The surgical history on genital organ is an unfavorable
factor to a youngster seeking to get married which apparently
cannot be conclusively related to infertility. Contraceptive
measures may also significantly influence the postoperative
paternity rate, thus impairing the estimation of postoperative
fertility preservation. Besides, the child birth in China has
always been regulated by laws and policies to avoid overpopulation,
which may also impact the result. It is worthwhile to note that the
post-TSS patients were advised to father a child prior to
radiotherapy as soon as possible to avoid possible reproductive
toxicity (14). But even if
conditions do not permit, TSS allows a short window period to
recover from such harmful exposure (15–18).
This is beneficial especially to those who do not have access to
sperm cryopreservation or artificial insemination technology.
Another advantage of TSS compared with RO is the
potential of preserving patients' virility. Hypogonadism occurred
in approximately 10–20% of patients who underwent RO (19). The TSS procedure allows preservation
of an essential amount of Leydig cells for normal endocrine
functioning. It has already been well accepted that the TSS
procedure with a good preservation of normal testicular parenchyma
is particularly important in monorchide and bilateral testicular
tumor patients. In our study, hypogonadism did not occur in
post-TSS patients, which also supported this point of view.
TSS has already been accepted as a treatment
modality for bilateral and monorchide tumors with normal
preoperative testosterone levels. But elective TSS is currently
still not advised in patients with a normal contralateral testis.
However, RO survivors are at high risk of contralateral relapse
when compared to average population, thus leaving with fewer
therapeutic options. Besides, previous observation overestimated
the proportion of malignant cases in all testicular tumors, while
recent studies have proved a much lower constituent ratio (20). When considering children and
adolescent patients, the registry-based epidemiology studies
formerly conducted had conspicuous bias. The inaccurate assessment
of pathological types and their degree of malignancy in children
and adolescent patients' would definitely impact the therapeutic
strategy as well as the functional outcome (21,22). It
is reasonable to infer that previous treatment modality of
testicular tumors should not be extrapolated precipitately to
current status, especially in youngsters and other possible
long-survivors who are more concerned about better quality-of-life
(21). Therefore, recent studies on
testicular cancer have emerged with promising results. Galosi et
al (23) applied a
‘diagnostic-therapeutic pathway’ in small, non-palpable single
testis lesions to minimize the overtreatment in benign tumors. Ye
et al (24) retrospectively
reviewed the trend of TSS for pediatric testicular tumors in South
China, and advocated the potential benefits of this procedure.
Keske et al (25) showed a
significant decrease in neighboring testis ITGCN but increase in
multifocality, hence advised caution and safety rim of normal
tissue within the resection margin. Bojanic et al (26) even made a big step forward by
evaluating the feasibility of TSS in GCTs alone. With a median
follow-up time of 45 months, only 1 out of 9 GCT patients developed
local recurrence after 39 months. None of the studies above had set
definite criterion excluding TSS for malignant patients such as
GCTs, but the results were still far from conclusive. In our study
GCT patients underwent successful TSS and were observed with
satisfactory outcomes. By extending the inclusion criteria of TSS
we hope these results will provide supporting evidences to TSS
procedure in oncological and functional efficacy.
Still there are some limitations within our study.
Except the above-mentioned imbalance between the two comparative
cohorts, the follow-up of functional results has not always been
executed in a consistent manner. Some of the laboratory tests and
radiological examinations were not listed as routine re-examination
items since the introduction of TSS procedure in our department. It
was only after around the year 2010 that we gradually established a
set of follow-up protocols, including history taking, physical
examination, medical image and laboratory tests. Also we noticed a
very low detection rate of ITGCN, which is a considerable
discrepancy between our result and previous reports. Analysis and
retrospection were performed, and the following reasons had been
summed up to be responsible for the unreasonable results: i) Small
sample size (23 TSS cases, only 12 of them were GCTs) resulted in
the lack of statistical power; ii) selection bias during the
initial phase of the conduction of TSS (we usually preferred small,
confined lesions which were easier to perform the surgery); and
iii) the biopsy of tumor bed in the first few cases were performed
in a more or less careless style, though (i.e., 1 point random
biopsy and 2 points). A better-defined follow-up and biopsy
protocol would be mandatory in the future to achieve better study
results.
In summary, we believe TSS may be a suitable option
for patients subgroup including: patients who are undecided or
planning to father children in the near future, especially when
they have hesitation in sperm cryopreservation/insist on natural
insemination; patients who cannot accept loss of testis or the
possibility of life-long androgen substitution. Once the tumor
volume allows an organ-sparing attempt, especially if a benign
tumor is assumed on preoperative examinations, TSS should be
offered to patients as a potential option against the traditionally
practiced RO.
Acknowledgements
The authors would like to sincerely thank Professor
Wang Tao and Professor Liu Xiaming from the Urology and Andrology
Department of HUST Tongji Hospital (Wuhan, China), for their advice
and editing the language of the manuscript.
Funding
Shanghai Municipal Commission of Health and Family
Planning Science Research Fund (grant no. 20154Y0085).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to individual privacy
and ethical considerations. However, a minimal amount of the
dataset used during the current study is available from the
corresponding author on reasonable request.
Authors' contributions
FX, JZS, JKW and LHW designed the present study. FX
and JZS performed statistical analysis and wrote the manuscript.
JKW and LHW reviewed and edited the manuscript. YSL performed
statistical analysis and reviewed the manuscript. YL, TL and JW
executed the follow-up procedures. All authors read and approved
the manuscript.
Ethics approval and consent to
participate
Ethical approval was obtained from the Institutional
Review Board of Second Military Medical University (Shanghai,
China). Written informed consent was obtained from all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bazzi WM, Raheem OA, Stroup SP, Kane CJ,
Derweesh IH and Downs TM: Partial orchiectomy and testis
intratubular germ cell neoplasia: World literature review. Urol
Ann. 3:115–118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heidenreich A and Angerer-Shpilenya M:
Organ-preserving surgery for testicular tumours. BJU Int.
109:474–490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heidenreich A, Weissbach L, Höltl W,
Albers P, Kliesch S, Köhrmann KU and DIeckmann KP; German
Testicular Cancer Study Group, : Organ sparing surgery for
malignant germ cell tumor of the testis. J Urol. 166:2161–2165.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Patel AS, Coley BD and Jayanthi VR:
Ultrasonography underestimates the volume of normal parenchyma in
benign testicular masses. J Urol. 178:1730–1732. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferretti L, Sargos P, Gross-Goupil M,
Izard V, Wallerand H, Huyghe E, Rigot JM, Durand X, Benoit G,
Ferriere JM, et al: Testicular-sparing surgery for bilateral or
monorchide testicular tumours: A multicenter study of long-term
oncological and functional results. BJU Int. 114:860–864. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sabanegh ES Jr and Ragheb AM: Male
fertility after cancer. Urology. 73:225–231. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fosså SD, Opjordsmoen S and Haug E:
Androgen replacement and quality of life in patients treated for
bilateral testicular cancer. Eur J Cancer. 35:1220–1225. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Williams DH IV, Karpman E, Sander JC,
Spiess PE, Pisters LL and Lipshultz LI: Pretreatment semen
parameters in men with cancer. J Urol. 181:736–740. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jacobsen KD, Theodorsen L and Fossa SD:
Spermatogenesis after unilateral orchiectomy for testicular cancer
in patients following surveillance policy. J Urol. 165:93–96. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heidenreich A, Thüer D and Polyakov S:
Postchemotherapy retroperitoneal lymph node dissection in advanced
germ cell tumours of the testis. Eur Urol. 53:260–272. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Allen JC, Kirschner A, Scarpato KR and
Morgans AK: Current management of refractory germ cell tumors and
future directions. Curr Oncol Rep. 19:82017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giannarini G, Dieckmann KP, Albers P,
Heidenreich A and Pizzocaro G: Organ-sparing surgery for adult
testicular tumours: A systematic review of the literature. Eur
Urol. 57:780–790. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Petersen PM, Giwercman A, Daugaard G,
Rørth M, Petersen JH, Skakkeaek NE, Hansen SW and von der Maase H:
Effect of graded testicular doses of radiotherapy in patients
treated for carcinoma-in-situ in the testis. J Clin Oncol.
20:1537–1543. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feldman DR, Bosl GJ, Sheinfeld J and
Motzer RJ: Medical treatment of advanced testicular cancer. JAMA.
299:672–684. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fosså SD, Horwich A, Russell JM, Roberts
JT, Cullen MH, Hodson NJ, Jones WG, Yosef H, Duchesne GM, Owen JR,
et al Medical Research Council Testicular Tumor Working Group, :
Optimal planning target volume for stage I testicular seminoma: A
Medical Research Council randomized trial. J Clin Oncol.
17:11461999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brydøy M, Fosså SD, Klepp O, Bremnes RM,
Wist EA, Wentzel-Larsen T and Dahl O: Paternity following treatment
for testicular cancer. J Natl Cancer Inst. 97:1580–1588. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lackner JE, Koller A, Schatzl G, Marberger
M and Kratzik C: Androgen deficiency symptoms in testicular cancer
survivors are associated with sexual problems but not with serum
testosterone or therapy. Urology. 74:825–829. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Krege S, Beyer J, Souchon R, Albers P,
Albrecht W, Algaba F, Bamberg M, Bodrogi I, Bokemeyer C,
Cavallin-Ståhl E, et al: European consensus conference on diagnosis
and treatment of germ cell cancer: A report of the second meeting
of the European Germ Cell Cancer Consensus group (EGCCCG): part I.
Eur Urol. 53:478–496. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Woo LL and Ross JH: The role of
testis-sparing surgery in children and adolescents with testicular
tumors. Urol Oncol. 34:76–83. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pohl HG, Shukla AR, Metcalf PD, Cilento
BG, Retik AB, Bagli DJ, Huff DS and Rushton HG: Prepubertal testis
tumors: Actual prevalence rate of histological types. J Urol.
172:2370–2372. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Galosi AB, Fulvi P, Fabiani A, Servi L,
Filosa A, Leone L, Marronaro A, Caraceni E and Montironi R:
Testicular sparing surgery in small testis masses: A
multinstitutional experience. Arch Ital Urol Androl. 88:320–324.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ye YL, He QM, Zheng FF, Guo SJ, Zhou FJ
and Qin ZK: Trends of testis-sparing surgery for pediatric
testicular tumors in South China. BMC Surg. 17:312017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Keske M, Canda AE, Yalcin S, Kilicarslan
A, Kibar Y, Tuygun C, Onder E, Atmaca AF, Yildirim A, Ozkanli SS,
et al: Is testis-sparing surgery safe in small testicular masses?
Results of a multicentre study. Can Urol Assoc J. 11:E100–100E104.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bojanic N, Bumbasirevic U, Bojanic G,
Vukovic I, Milojevic B and Pekmezovic T: Testis sparing surgery for
treatment of small testicular lesions: Is it feasible even in germ
cell tumors? J Surg Oncol. 115:287–290. 2017. View Article : Google Scholar : PubMed/NCBI
|