Introduction
Uterine leiomyosarcomas (ULMS) are rare malignant
tumors. They represent approximately 1% of overall uterine tumors,
and have a very poor prognosis. The 5-year overall survival (OS) is
between 30 and 42% (1). Less than
20% of metastatic ULMS showed complete or partial responses with
doxorubicin, gemcitabine, or dacarbazine treatment. The median
progression-free survival (PFS) was 5 months and OS 12 months
(2,3). By contrast, a systematic review has
confirmed the potential activity of the combination of gemcitabine
with docetaxel in second line chemotherapy. Benefits of the
Trabectedin (Yondelis) are yet to be confirmed (4).
Trabectedin inhibits cell proliferation by
specifically binding to the guanine residue within the minor groove
of DNA causing a bend in the major groove, which interferes with
the DNA binding proteins and transcription factors of the cancer
cell. This drug has been shown to have minimal toxicity with a
promising PFS and OS (4).
We report two cases demonstrating a durable partial
response of Trabectedin in advanced line treatment of patients with
advanced and metastatic uterine leiomyosarcoma.
Case report
Case 1
In July 2010, a 62-year-old female presented pelvic
pain. Pelvic echography showed a right tissular latero-uterine mass
with cystic component. Pelvic MRI showed a 16 cm mass, compatible
with a necrobiotic myoma. In August 2010, a PET CT-scan performed
showed no hypermetabolism and thoracic-abdominal-pelvic CT-Scan
(TAP CT-Scan) did not show sign of metastasis. A total hysterectomy
associated with omentectomy and appendicectomy was performed on
August 2010. Histological analysis revealed the tumor to be a grade
3 uterine leiomyosarcoma. The tumor was 12 cm long, with high
mitotic index (>20 mitotic figures/10HPF). The tumor infiltrated
2/3 of the posterior part of the uterus, and 1/3 of the anterior
part. Tumoral invasion was found in the uterine serous, on the
surface of the left ovary and in the omentectomy. Tumor necrosis
was below 50%. No other local or regional invasion was found. In
October 2010, adjuvant radiotherapy was performed. In November
2010, another TAP CT-Scan showed 10 pulmonary micronodules, 2
pulmonary nodules and a right pulmonary embolism. A first line
chemotherapy with gemcitabine/docetaxel was initiated in December
2010. Six cycles were performed; however, the dose of docetaxel had
to be reduced by 20% at the second cycle consequence of skin
toxicity (skin rash), and then interrupted for the 3 last cycles.
TAP CT-Scan performed in June 2011 showed increase in size of the
pulmonary nodules with no other progression elsewhere. A second
line of chemotherapy with adriamycin/ifosfamide was introduced. Six
cycles between July 2011 and February 2012 were administered. TAP
CT-Scan was performed after 3 cycles and at the end of the six
cycles morphological partial response (RECIST criteria) was
evident. A therapeutic break was done between February and May
2012. In May 2012, TAP CT-Scan showed progression: increase in size
of pulmonary lesions and detection of peritoneal lesions.
Therefore, in May 2012, a third line chemotherapy with Trabectedin
(1,3 mg/m2) was introduced. After 3 cycles stability in
size of the pulmonary and peritoneal nodules was evident. After the
sixth cycle, in October 2012, TAP CT-Scan showed partial regression
of the peritoneal nodules with stability of the pulmonary lesions.
A therapeutic break after the six cycles was performed. Multiple
morphological evaluations between October 2012 and June 2013 were
performed with persistence of stabile disease RECIST criteria. In
June 2013, the TAP CT-Scan showed morphological progression of the
pulmonary and peritoneal lesions, resulting in reintroduction of
Trabectedin. Nine cycles were performed between June 2013 and May
2014 with morphological stability. Despite the stability in size of
the peritoneal lesions the increase in the size of the pulmonary
nodules forced us to stop Trabectedin and started a fourth line
with weekly Paclitaxel. In November 2014, after progression with
Paclitaxel, Pazopanib (Votrien) was started as sixth line. After 3
months of pazopanib, progression led to the introduction of
Trabectedin based on the previous relatively good response. In
accordance with this long survival of the patient, a new biopsy of
pulmonary lesion was made before reintroduction of Trabectedin,
which confirmed it was ULMS. Ki-67 that was carried out on this new
biopsy was strongly high (70%), whereas the Ki-67 of the initial
biopsy was less important (30%). Lesions were stable for 6 months
with Trabectedin, before presenting another progression and the
introduction of best palliative support care. The patient
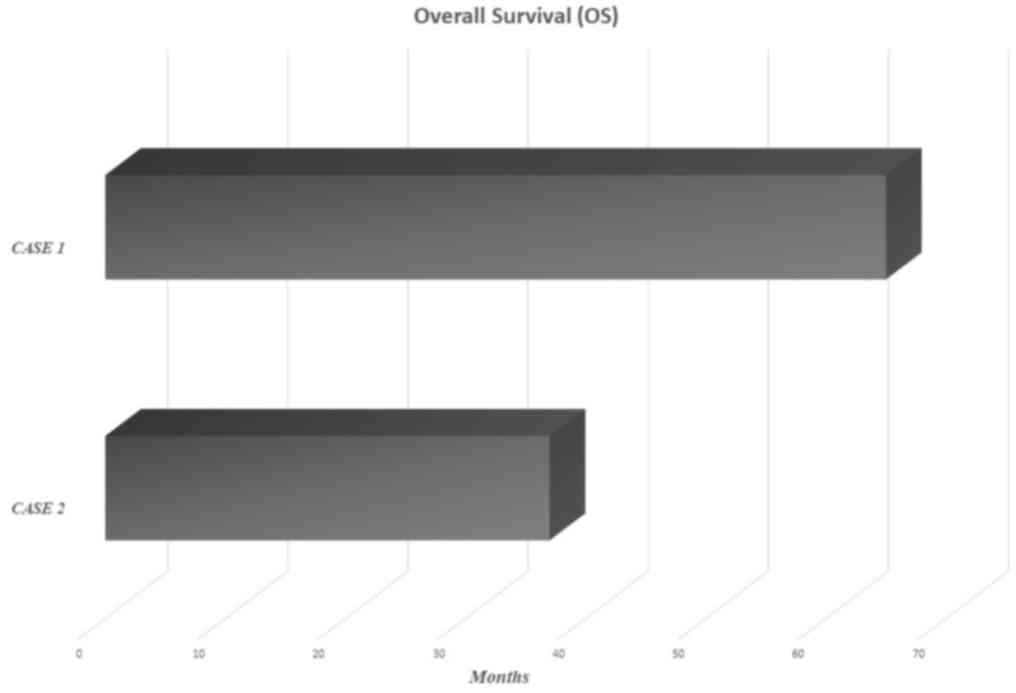
succumbed, in June 2015 (Fig.
1).
Case 2
In September 2012, a 55-year-old female was
hospitalized due to asthenia. An abdominal CT-scan was performed
and showed multiple suspected malignant hepatic lesions. An already
known myomatous uterus with necrobiosis was also described. A
biopsy of one of the hepatic lesions allowed us to diagnose a
well-differentiated uterine leiomyosarcoma. A PET CT-scan showed
multiple hypermetabolic hepatic lesions on the right and left
liver, and massive hypermetabolism on the uterine
(SUVmax12). Therefore, a hysterectomy with bilateral
annexectomy was performed. Histological analysis revealed a ULMS
with Ki-67 30–40%. Annexes were free from tumor, as was the
peritoneal cytology. From October to December 2012, 3 cycles of
Gemcitabine/Docetaxel were performed, followed by tumoral
progression according to RECIST criteria. In fact, TAP CT-Scan
showed respectively a 20 and 15 mm increase of two of the hepatic
lesions, and an increase of a peritoneal nodule. From December 2012
to October 2013, 11 cycles of Trabectedin (1.3 mg/m2)
were performed. The treatment was not well tolerated, so the
patient underwent two major side effects: i) After the first cycle,
grade 2 neutropenia was treated by introduction of G-CSF injection;
and ii) after the third cycle, an increase of transaminases (grade
II), which made us delay the fourth cycle and reduce the
chemotherapy dose by 30%. The morphological analyses, performed
with TAP CT-Scan, showed partial response. In February 2013, TAP
CT-Scan concluded to a 37% decrease according to RECIST criteria.
Even better, in May 2013, TAP CT-Scan concluded to a subtotal
response, where the hepatic lesions were too small to be described,
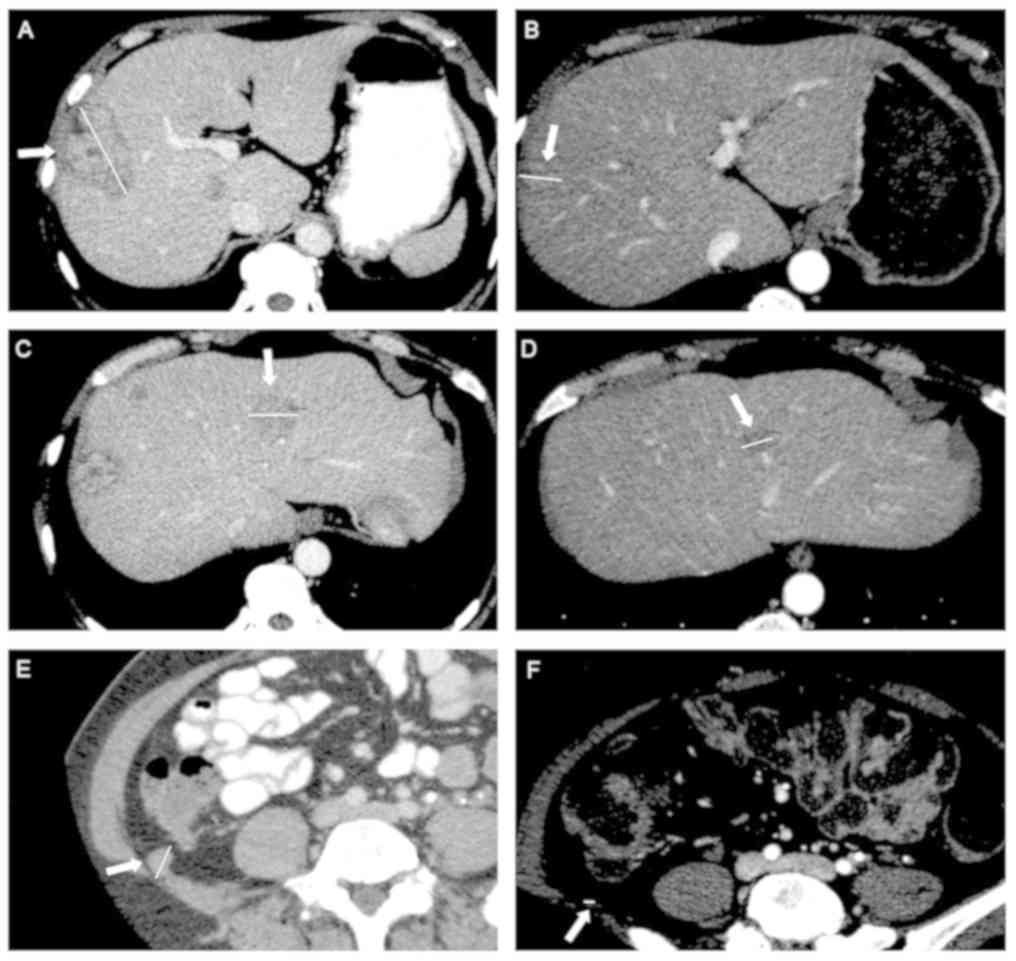
and pulmonary and peritoneal lesions had disappeared (Fig. 2). In October 2013, TAP CT-Scan get a
stabilization morphological results obtained to May 2013. A PET-CT
scan showed only the hypermetabolic of hepatic lesion in segment
VII–VIII (SUVmax6.7) with no other hypermetabolism.
In October 2013, after the thirteenth cycle of
Trabectedin, a therapeutic break was started in consequence of very
good partial response according to RECIST criteria. In June 2014,
after 9 months of therapeutic break morphological progression led
to reintroduction of Trabectedin. Partial response was obtained
after 3 cycles of treatment, followed by relapse after the sixth
cycle. Monotherapy of Ifosfamide was introduced for 3 months
without benefit, following 3 months of Pazopanib (Votrien). The
continuous progression decreases associated with an alteration of
the general state led to introduction of best palliative support
care. The patient succumbed, at the end of July 2015 (Fig. 1).
Discussion
The diagnostic mode employed in the two cases
included in this study is noteworthy. It is interesting to see that
the discovery of the illness was similar for both of these
patients. The findings seemed to be necrotic myoma, whereas they
were malignant tumors. New MRI techniques such as
diffusion-weighted imaging and apparent diffusion coefficient seem
promising to discriminate malignant and non-malignant tumors before
surgery (4).
These two cases also show the difficulty involved in
the evaluation of the prognosis of uterine leiomyosarcoma. Indeed,
the first patient exhibited two major negative prognostic criteria,
which is size over 10 cm and mitotic figures above 20 per HPF
(2). According to a previous study,
the median survival for grade 3 tumor is 3.1 years (3–5).
Nevertheless, the patient of case report 1 is now alive 6 years
after her diagnosis.
Adjuvant radiotherapy was performed on the first
patient (case 1), who presented a local advanced disease.
Nevertheless, studies have failed to confirm the benefit of such a
treatment. Its benefit could be increased with subseries of
patients with locally advanced disease, and through its combination
with chemotherapy to treat metastatic lesions (6,7).
Additionally, adjuvant chemotherapy has yet to be proven as
beneficial for operating on leiomyosarcoma (5).
It is difficult, especially with only two cases, to
determine whether PFS of those patients is linked to natural
progrssion of the disease or effect of the chemotherapy. However,
Trabectedin was the most well-tolerated drug over a long period of
administration for patient case report 1. After 9 months of
therapeutic break, disease progression was reduced and the
reintroduction of Trabectedin led to approximately 2 years of
non-progression. Despite the relatively fast control of the disease
obtained with Trabectedin, it has been suggested that it should be
administered at least during the first year of therapy, since
retarded positive effect has been shown for some patients. The two
cases of the current study clearly indicate the potential of this
drug for progressing tumor under first line of chemotherapy.
Indeed, findings have shown that despite the fact that Trabectedin
has failed to prove its effectiveness in tumor reduction, it seems
to have a great stabilization effect, with PFS well above all other
types of chemotherapy used for ULMS (8).
In case report 2, even after heavy dose reduction of
Trabectedin, this drug was positively maintained for almost one
year and allowed us to obtain a subtotal response. More studies are
needed to confirm this stabilization potential; and given the very
good tolerance profile of Trabectedin, it is valid to explore
concomitant administration with other types of chemotherapy, such
as gemcitabine, docetaxel, or doxorubicin. The LMS-02 study, a
phase 2 trial, showed promising results for the
doxorubicin-trabectedin combination, suggesting synergic effects of
these drugs in first-line treatment of metastatic ULM (9).
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
TH, ML and EF conceived and designed the manuscript,
and collected, analyzed, interpreted and presented the data. AC
collected, analyzed and presented the data. LSB contributed to the
analysis of all imaging data. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patients and their families provided consent for
the publication of the present case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
D'Angelo E, Espinosa I, Ali R, Gilks CB,
Rijn Mv, Lee CH and Prat J: Uterine leiomyosarcomas: Tumor size,
mitotic index and biomarkers Ki67 and Bcl-2 identify two groups
with different prognosis. Gynecol Oncol. 121:328–333. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Italiano A, Mathoulin-Pelissier S, Cesne
AL, Terrier P, Bonvalot S, Collin F, Michels JJ, Blay JY, Coindre
JM and Bui B: Trends in survival for patients with metastatic
soft-tissue sarcoma. Cancer. 117:1049–1054. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Penel N, Van Glabbeke M, Marreaud S, Ouali
M, Blay JY and Hohenberger P: Testing new regimens in patients with
advanced soft tissue sarcoma: Analysis of publications from the
last 10 years. Ann Oncol. 22:1266–1272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin G, Yang LY, Huang YT, Ng KK, Ng SH,
Ueng SH, Chao A, Yen TC, Chang TC and Lai CH: Comparison of the
diagnostic accuracy of contrast-enhanced MRI and diffusion-weighted
MRI in the differentiation between uterine leiomyosarcoma/smooth
muscle tumor with uncertain malignant potential and benign
leiomyoma. J Magn Reson Imaging. 43:333–342. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giuntoli RL II, Metzinger DS, DiMarco CS,
Cha SS, Sloan JA, Keeney G and Gostout BS: Retrospective review of
208 patients with leiomyosarcoma of the uterus: Prognostic
indicators, surgical management and adjuvant therapy. Gynecol
Oncol. 89:460–469. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gupta AA, Yao X, Verma S, Mackay H and
Hopkins L: Sarcoma Disease Site Group and the Gynecology Cancer
Disease Site Group: Systematic chemotherapy for inoperable, locally
advanced, recurrent, or metastatic uterine leiomyosarcoma: A
systematic review. Clin Oncol (R Coll Radiol). 25:346–355. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gupta AA, Yao X, Verma S, Mackay H and
Hopkins L: Chemotherapy (gemcitabine, docetaxel plus gemcitabine,
doxorubicin, or trabectedin) in inoperable, locally advanced,
recurrent, or metastatic uterine leiomyosarcoma: A clinical
practice guideline. Curr Oncol. 20:e448–e454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Monk BJ, Blessing JA, Street DG, Muller
CY, Burke JJ and Hensley ML: A phase II evaluation of trabectedin
in the treatment of advanced, persistent, or recurrent uterine
leiomyosarcoma: A gynecologic oncology group study. Gynecol Oncol.
124:48–52. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pautier P, Floquet A, Chevreau C, Penel N,
Guillemet C, Delcambre C, Cupissol D, Selle F, Isambert N,
Piperno-Neumann S, et al: Trabectedin in combination with
doxorubicin for first-line treatment of advanced uterine or
soft-tissue leiomyosarcoma (LMS-02): A non-randomised, multicentre,
phase 2 trial. Lancet Oncol. 16:457–464. 2015. View Article : Google Scholar : PubMed/NCBI
|