Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the
fourth leading cause of cancer-related deaths worldwide with an
unfavorable 5-year survival rate of 8% (1). National Comprehensive Cancer Network
(NCCN) recommends chemotherapy or chemo-radiation therapy for the
treatment of locally advanced unresectable (UR-LA) PDAC. However,
these therapies do little to improve disease prognosis. Recently,
surgery after chemo-radiation therapy, i.e., conversion surgery,
for UR PDAC was reported to improve prognosis (2). We demonstrated a case of successful
conversion surgery following treatment of an UR-LA PDAC patient
with interstitial pneumonitis using a gemcitabine and
nab-paclitaxel (GnP) therapy. In addition, we retrospectively
compare clinical efficacy of two chemotherapeutic regimens, GnP
therapy and gemcitabine plus S-1 (GS) therapy for treatment of
UR-LA PDAC.
Case report
A 67-year-old woman suffering from dermatomyositis
and interstitial pneumonitis (IP) controlled by an
immunosuppressive agent, tacrolimus, was referred to the Department
of General Surgery, Chiba University Hospital. Laboratory data
reported a high level of carbohydrate antigen 19-9 (CA19-9; 1,713
U/ml), with no other remarkable laboratory findings. Abdominal
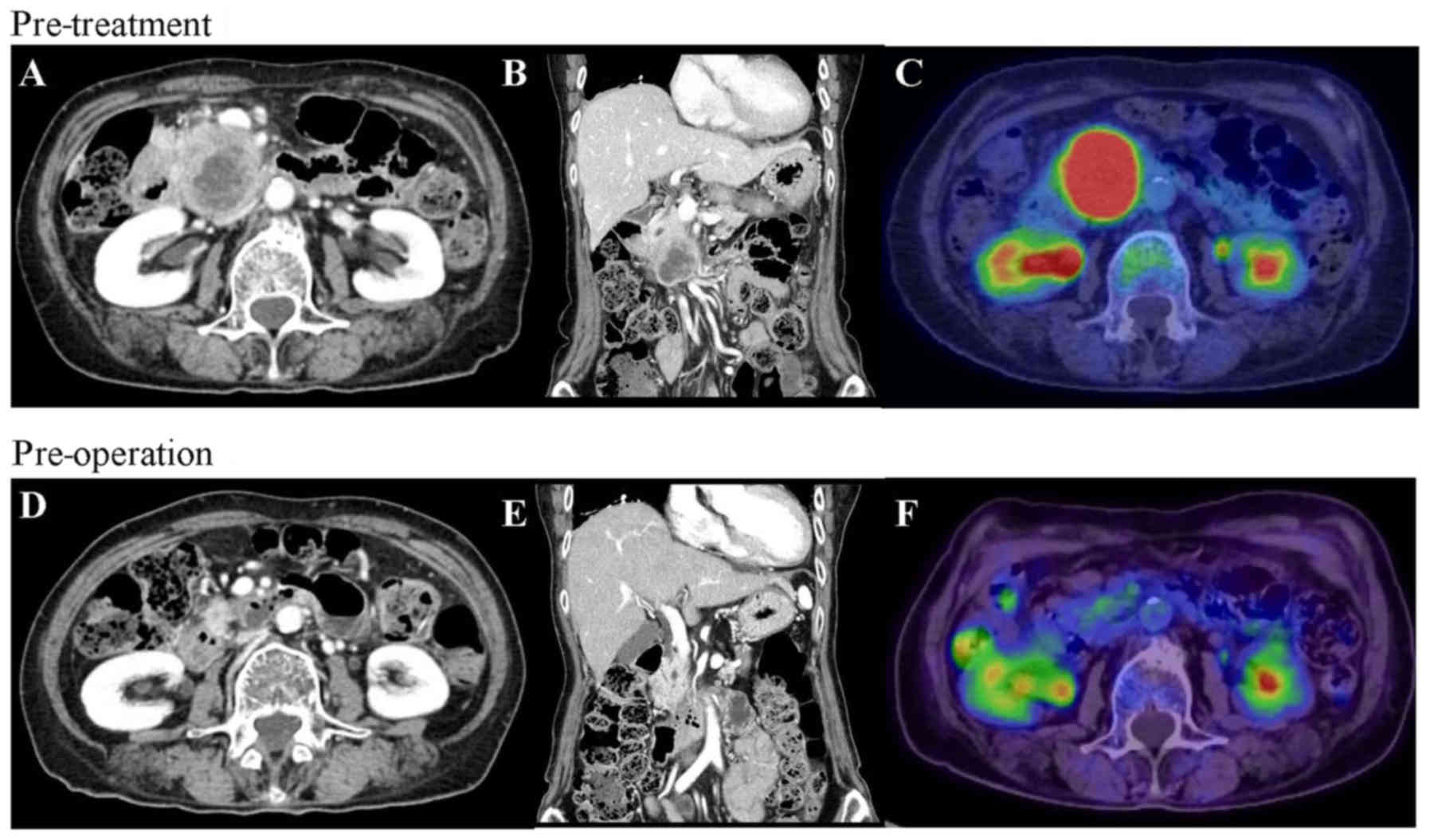
multi-detector row computed tomography (MDCT) revealed a
hypovascular tumor measuring 50 mm in the head of the pancreas. The
tumor was in contact with the superior mesenteric artery (SMA),
with invasion extending to the most proximal draining jejunal
branch into the superior mesenteric vein (SMV) (Fig. 1A and B). Further, the tumor spread
over a third of the duodenum. Endoscopic ultrasonography (EUS) also
indicated that the tumor was in contact with both the SMA and the
SMV, and fine needle aspiration biopsy revealed adenocarcinoma.
Positron emission tomography (PET) exhibited fluorodeoxyglucose
uptake in the primary pancreatic tumor (Fig. 1C); however, both PET and
ethoxybenzyl-magnetic resonance imaging showed no evidence of
distant metastasis.
On the basis of these clinical findings, the patient
was diagnosed with UR-LA PDAC. She understood the risk of
exacerbating her IP, and agreed to treatment with a combined
regimen of gemcitabine (GEM, 1,000 mg/m2) and
nab-paclitaxel (125 mg/m2), with subsequent conversion
surgery. GnP chemotherapy was administered intravenously on days 1
and 8 and was repeated every three weeks. Before the start of GnP
therapy, we consulted a physician specializing in allergy and
collagen diseases and stopped administering tacrolimus. After six
courses of GnP administration over five months, MDCT, EUS, and PET
imaging demonstrated a significant response to chemotherapy
(Fig. 1D-F). Tumor size decreased to
18 mm, contact with the SMV was reduced from 180 degrees to 90
degrees and the SMA separated from the tumor. The level of CA19-9
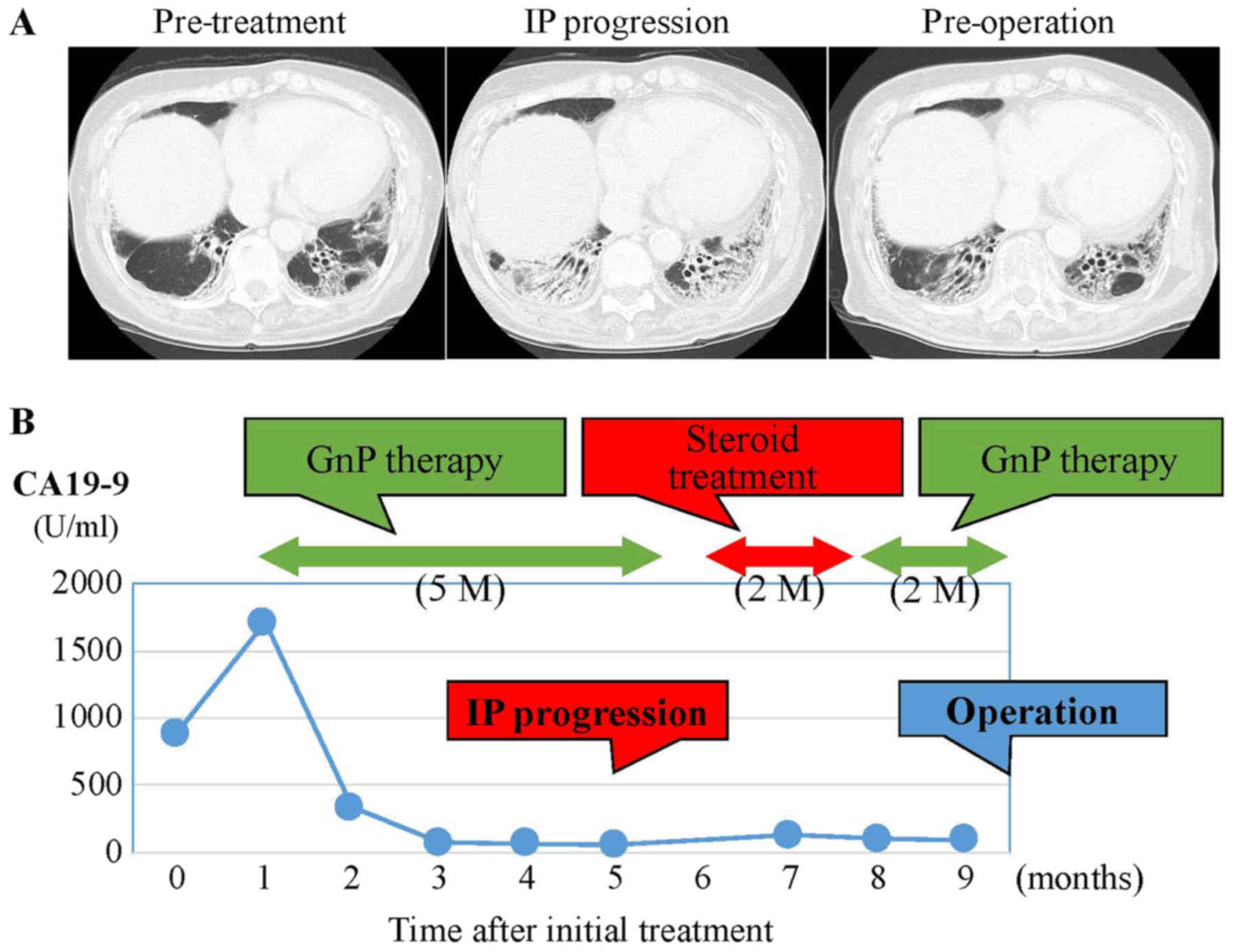
decreased from 1,713 to 60.1 U/ml (Fig.
2B). Despite these positive clinical responses, we could not
schedule conversion surgery because the patient's IP had worsened
(Fig. 2A). For one month after
steroid medication and restart of regular tacrolimus
administration, lung function recovered. Despite the lack of tumor
growth on MDCT, she was treated with three additional courses of
GnP chemotherapy over two months because CA19-9 level had increased
to 132.1 U/ml. After the additional chemotherapy, CA19-9 level
decreased to 99.5 U/ml, and CT and EUS showed a partial response
(PR) to chemotherapy using RECIST criteria with controlled IP.
Based on criteria from the UICC-the 8th edition, TNM staging of
before and after chemotherapy were T4N0M0 stage III and T1cN0M0
stage IA, respectively. After discussion with the patient and her
family, conversion surgery was planned.
Pancreaticoduodenectomy was performed.
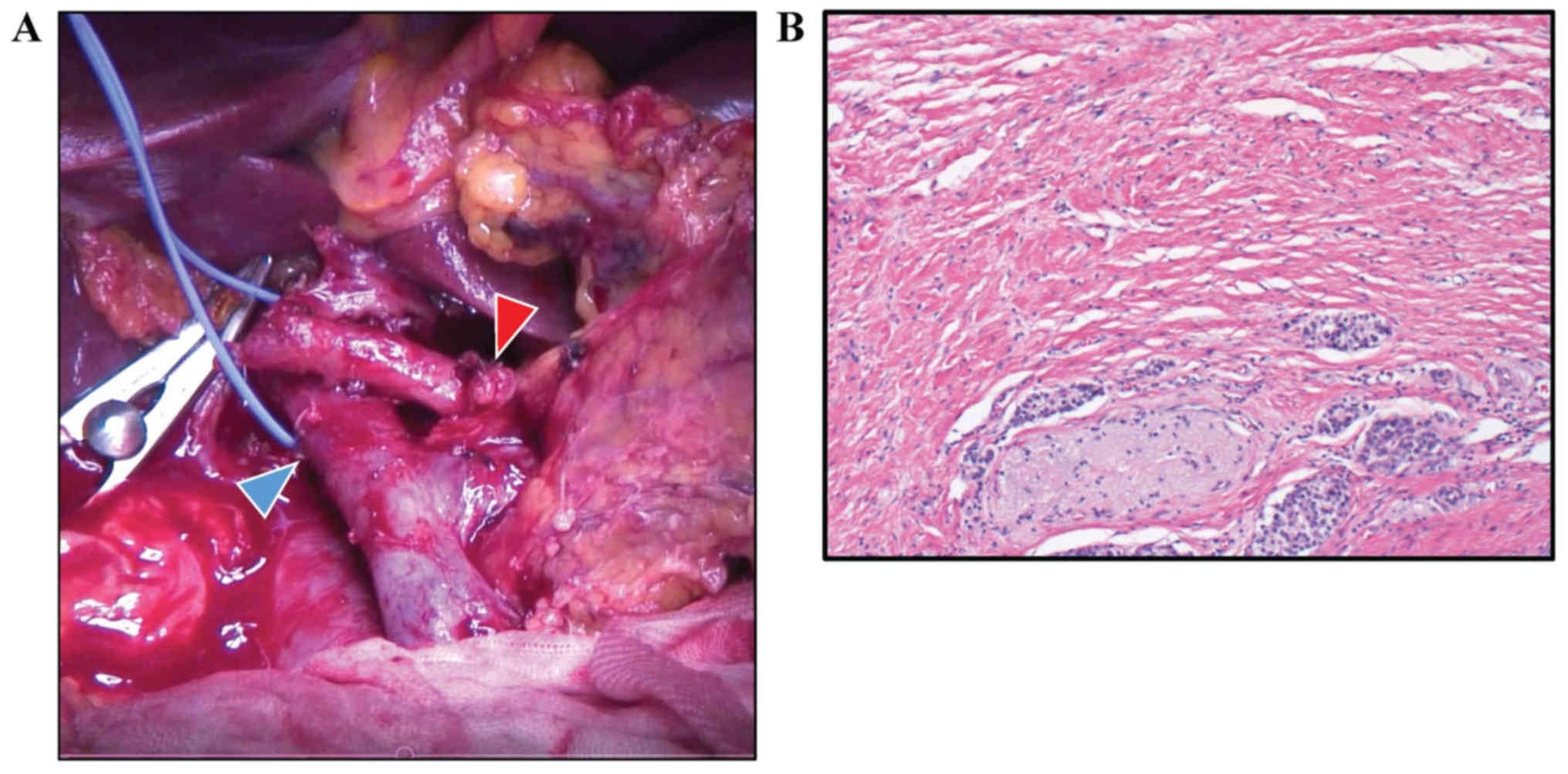
Intraoperative pathological examination of a frozen section showed
that the margins of the bile duct and stump of the pancreas were
negative for cancer tissue (Fig.
3A). Histological examination showed R0 (no residual tumor)
resection, and less than 10% of tumor cells were replaced with
fibrosis (Evans' criteria I; Fig.
3B). From pathological findings (well differentiated tubular
adenocarcinoma, pT2 (20×15 mm), positive for common bile duct,
duodenum, and perineural invasion, pN0 (0/20), pM0), the tumor was
defined as f-stage IB. Although the patient suffered from chylous
ascites after surgery, the patient made a satisfactory recovery and
was discharged on postoperative day 42. After administration of
adjuvant chemotherapy (S-1, 100 mg/day) for 6 months, the patient
is vigorously alive at 23 months after initial treatment (14 months
after surgery), with no recurrence.
Accumulative rates for the duration of chemotherapy
prior to surgery were calculated using the Kaplan-Meier method.
Statistical significance of the results was determined by
Mann-Whitney U test. P<0.05 was considered to indicate a
statistically significant difference. Statistical calculations were
performed using the JMP® 13 (SAS Institute, Inc., Cary,
NC, USA).
Discussion
Surgical resection offers the only chance for cure
in patients with PDAC. Conversion surgery with multidisciplinary
therapy is an attractive and crucial treatment for UR-LA PDAC.
Satoi et al (3) reported that
conversion surgery for UR-LA significantly prolonged survival
compared to survival without conversion surgery. Median overall
survival (OS) time (MST) was 39.7 and 20.8 months following
conversion surgery compared with a control group (3). Asano et al (4) reported that MST was 3.8 years in the
conversion surgery group. Recently, Okura et al (5) reported that Kaplan-Meier analysis
showed that patients treated with GnP therapy followed by
conversion surgery presented significantly longer OS than those
treated with GnP therapy without conversion surgery (MST: 22.5 vs.
11 months) (5).
The MPACT study demonstrated that GnP therapy is
effective for treatment of metastatic PDAC (6). This therapy draws attention as standard
chemotherapy for UR PDAC. Saito et al (7) showed that, after GnP therapy for UR-LA,
tumor reduction was 37%, response rate was 71%, and conversion rate
was 29%. Another chemotherapeutic regimen used to treat UR PDAC,
FOLFIRINOX (5-Fluorouracil/leucovorin combined with irinotecan and
oxaliplatin), is also used for chemotherapy preceding conversion
surgery (8). Giovanni showed that
response rate was 49% and conversion rate was 72.9% for UR-LA and
borderline resectable PDAC (9).
Furthermore, the percentage of UR-LA PDAC patients was reduced from
78 to 32% by use of FOLFIRINOX. Muranaka et al (10) have described that GnP (40.9%) showed
higher response rate than that of FOLFIRINOX (6.3%) in UR PDAC;
however, the superiority of including conversion surgery with
either of these two regimes is still under debate. GS therapy is
also a useful PDAC treatment, especially for Asian patients. The
GEST study reported that response rates for GEM, S-1, and GS
therapies were 12.9, 21.0 and 28.9%, respectively with a low
incidence of adverse side effects (11). Thus, GS therapy is also considered an
appropriate chemotherapeutic regimen for UR PDAC.
CA19-9 level is a reasonably reliable indicator of
whether conversion surgery should be considered. Previous studies
show that CA19-9 response to neoadjuvant therapy is closely related
to high R0 resection rate and prolongation of survival (12,13). In
the present case, we decided to continue GnP treatment until CA19-9
level dropped because CA19-9 level in the patient increased
slighted after steroid medication despite lack of progression of
the tumor in radiological imaging.
A total of 29 cases treated with conversion surgery
after chemotherapy for UR-LA PDAC between July 2009 and September
2017 (18 cases: GS therapy and 11 cases: GnP therapy) were
identified (according to the NCCN guideline 2018.2) from records
from the Department of General Surgery, Chiba University Hospital.
In general, the indication for conversion surgery of these cases
were determined on the basis of the significant reduction of serum
CA19-9 level and loss of arterial invasion of tumor in MDCT
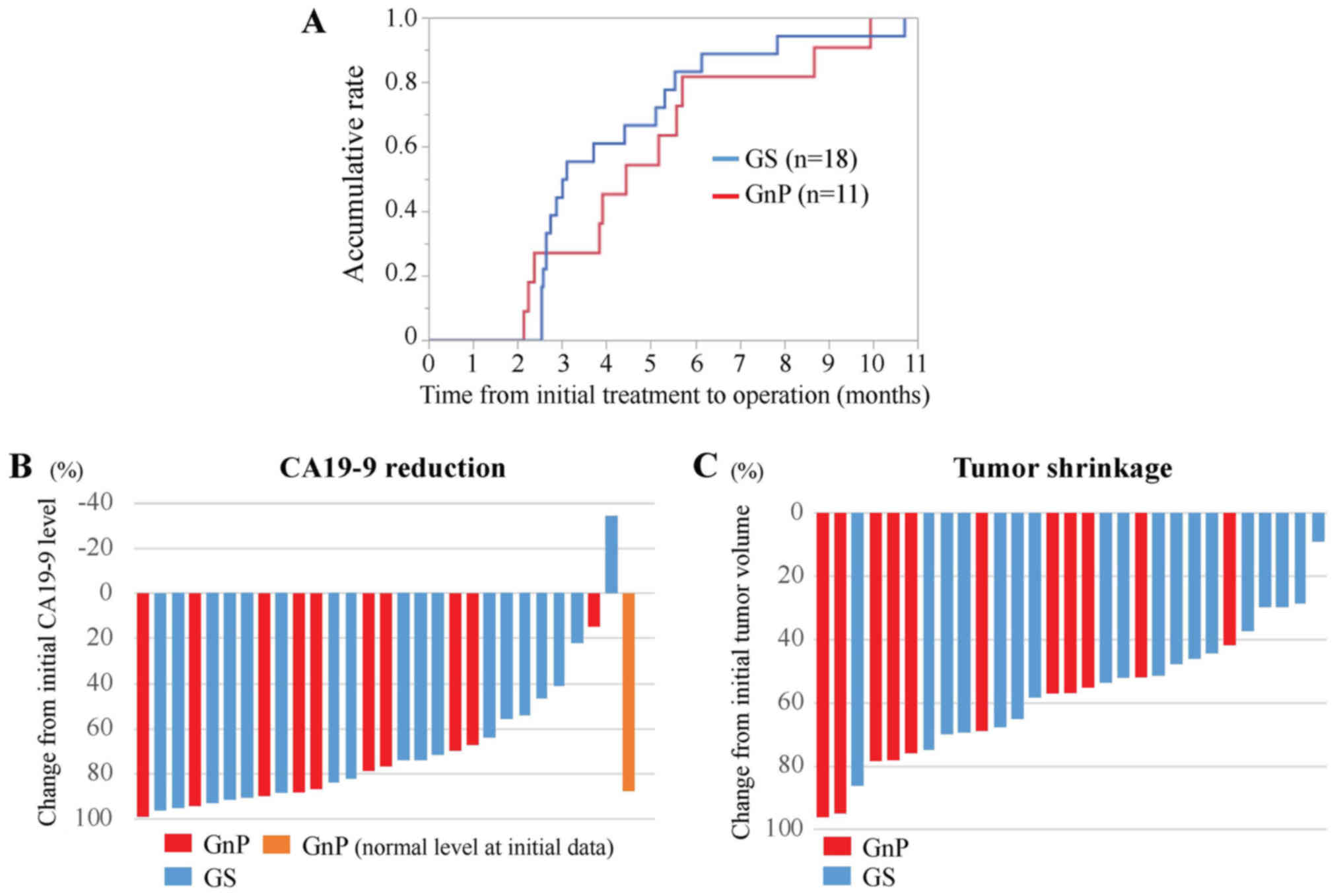
imaging. The median duration of chemotherapy followed by surgery
was 3.1 months after GS therapy, and 4.4 months after GnP therapy
(Fig. 4A). To compare
chemotherapeutic efficacy, median reduction rates in CA19-9 levels
were 86.7 and 73.7% after GnP and GS therapy, respectively
(Fig. 4B).
As shown in the present case, GnP therapy showed a
greater reduction of tumor volume. Tumor reduction rates were
significantly higher following GnP than after GS therapy, medians:
68.8 and 51.6%, respectively in this retrospective cohort (P=0.02,
Mann-Whitney Wilcoxon test) (Fig.
4C). The mechanism for this difference may involve stroma
reduction induced by GnP therapy (14). GnP therapy may be suitable for
conversion surgery because of this additional biological effect to
the tumor volume reduction.
Chemotherapy causes a variety of adverse
side-effects. In the present case, the patient had progressive IP
during GnP therapy. Studies have shown that severe interstitial
lung disease (ILD) has occurred following gemcitabine-based
chemotherapy (15,16). These studies reported an occurrence
rate from 1.7 to 7.6%, and risk factors were prior thoracic
radiotherapy, pre-existing pulmonary fibrosis, age of over 80
years, and lung cancer. The occurrence rate of ILD induced by
nab-paclitaxel is reported as 6% (17). In this case, FOLFIRINOX was not
selected because reported adverse events were more frequent in
Japanese patients when compared with GnP therapy (18). IP did progress after GnP therapy;
however cessation of immunosuppressive treatment for
dermatomyositis and IP may be the underlying factor. Thus,
collaboration between medical specialists for allergy and oncology
is necessary to prevent progression of IP during GnP treatment for
PDAC patients with ILD.
In conclusion, we describe a case of successful
conversion surgery following gemcitabine plus nab-paclitaxel
treatment of UR-LA PDAC. GnP therapy followed by conversion surgery
is a suitable regimen to shrink UR PDAC tumors; however, long-term
clinical outcomes need further investigation. Of particular note is
that careful management of systemic conditions is needed to treat
PDAC patients with IP using GnP therapy. Conversion surgery should
be considered recognizing radiological responses (tumor shrinkage
adjacent to major arteries) and reductions in CA19-9 levels.
Further evidence and prospective cohort studies are necessary to
establish an optimal strategy for treatment of UR-LA PDAC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Grant-in-Aid
for Scientific Research, The Challenge Exploratory Research (grant
no. 16K15607). This study was also supported by Kashiwado Memorial
Foundation and Japanese Foundation for Multidisciplinary Treatment
of Cancer.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TY obtained the patient's data and wrote the
manuscript. ST treated the patient with preoperative and adjuvant
chemotherapy. MO and ST performed the surgery. TM and MO were
responsible for the pathological diagnosis of the case. HY, KF, TT,
SaK, DS, NS, ShK, HN, EN, and MO discussed, analyzed and
interpreted the data with TY and ST, and assisted in writing the
manuscript. All authors approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committees of Chiba University approved
the content of this manuscript (#2732), and written informed
consent was obtained from each patient prior to surgery.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CA19-9
|
carbohydrate antigen 19-9
|
|
EUS
|
endoscopic ultrasonography
|
|
GS
|
gemcitabine plus S-1
|
|
GnP
|
gemcitabine plus nab-paclitaxel
|
|
IP
|
interstitial pneumonitis
|
|
MDCT
|
multi-detector row computed
tomography
|
|
MST
|
median survival time
|
|
OS
|
overall survival
|
|
PET
|
positron emission tomography
|
|
PDAC
|
pancreatic ductal adenocarcinoma
|
|
PV
|
portal vein
|
|
SMA
|
superior mesenteric artery
|
|
SMV
|
superior mesenteric vein
|
|
UR-LA
|
locally advanced unresectable
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tempero MA, Malafa MP, Al-Hawary M, Asbun
H, Bain A, Behrman SW, Benson AB III, Binder E, Cardin DB, Cha C,
et al National Comprehensive Cancer Network Clinical Practice
Guidelines in Oncology, : Pancreatic Adenocarcinoma, Version
2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr
Canc Netw. 15:1028–1061. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Satoi S, Yamaue H, Kato K, Takahashi S,
Hirono S, Takeda S, Eguchi H, Sho M, Wada K, Shinchi H, et al: Role
of adjuvant surgery for patients with initially unresectable
pancreatic cancer with a long-term favorable response to
non-surgical anti-cancer treatments: Results of a project study for
pancreatic surgery by the Japanese Society of
Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci.
20:590–600. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Asano T, Hirano S, Nakamura T, Okamura K,
Tsuchikawa T, Noji T, Nakanishi Y, Tanaka K and Shichinohe T:
Survival benefit of conversion surgery for patients with initially
unresectable pancreatic cancer who responded favorably to
nonsurgical treatment. J Hepatobiliary Pancreat Sci. 25:342–350.
2018. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okura R, Takano S, Yokota T, Yoshitomi H,
Kagawa S, Furukawa K, Takayashiki T, Kuboki S, Suzuki D, Sakai N,
et al: Conversion surgery with gemcitabine plus nab-paclitaxel for
locally advanced unresectable pancreatic cancer: A case report. Mol
Clin Oncol. 9:389–393. 2018.PubMed/NCBI
|
|
6
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saito T, Ishido K, Kudo D, Kimura N,
Wakiya T, Nakayama Y and Hakamada K: Combination therapy with
gemcitabine and nab-paclitaxel for locally advanced unresectable
pancreatic cancer. Mol Clin Oncol. 6:963–967. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Blazer M, Wu C, Goldberg RM, Phillips G,
Schmidt C, Muscarella P, Wuthrick E, Williams TM, Reardon J,
Ellison EC, et al: Neoadjuvant modified (m) FOLFIRINOX for locally
advanced unresectable (LAPC) and borderline resectable (BRPC)
adenocarcinoma of the pancreas. Ann Surg Oncol. 22:1153–1159. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marchegiani G, Todaro V, Boninsegna E,
Negrelli R, Sureka B, Bonamini D, Salvia R, Manfredi R, Pozzi
Mucelli R and Bassi C: Surgery after FOLFIRINOX treatment for
locally advanced and borderline resectable pancreatic cancer:
Increase in tumour attenuation on CT correlates with R0 resection.
Eur Radiol. 28:4265–4273. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Muranaka T, Kuwatani M, Komatsu Y, Sawada
K, Nakatsumi H, Kawamoto Y, Yuki S, Kubota Y, Kubo K, Kawahata S,
et al: Comparison of efficacy and toxicity of FOLFIRINOX and
gemcitabine with nab-paclitaxel in unresectable pancreatic cancer.
J Gastrointest Oncol. 8:566–571. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ueno H, Ioka T, Ikeda M, Ohkawa S,
Yanagimoto H, Boku N, Fukutomi A, Sugimori K, Baba H, Yamao K, et
al: Randomized phase III study of gemcitabine plus S-1, S-1 alone,
or gemcitabine alone in patients with locally advanced and
metastatic pancreatic cancer in Japan and Taiwan: GEST study. J
Clin Oncol. 31:1640–1648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van Veldhuisen E, Vogel JA, Klompmaker S,
Busch OR, van Laarhoven HWM, van Lienden KP, Wilmink JW, Marsman HA
and Besselink MG: Added value of CA19-9 response in predicting
resectability of locally advanced pancreatic cancer following
induction chemotherapy. HPB (Oxford). 20:605–611. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boone BA, Steve J, Zenati MS, Hogg ME,
Singhi AD, Bartlett DL, Zureikat AH, Bahary N and Zeh HJ III: Serum
CA 19-9 response to neoadjuvant therapy is associated with outcome
in pancreatic adenocarcinoma. Ann Surg Oncol. 21:4351–4358. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alvarez R, Musteanu M, Garcia-Garcia E,
Lopez-Casas PP, Megias D, Guerra C, Muñoz M, Quijano Y, Cubillo A,
Rodriguez-Pascual J, et al: Stromal disrupting effects of
nab-paclitaxel in pancreatic cancer. Br J Cancer. 109:926–933.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hamada T, Yasunaga H, Nakai Y, Isayama H,
Matsui H, Fushimi K and Koike K: Interstitial lung disease
associated with gemcitabine: A Japanese retrospective cohort study.
Respirology. 21:338–343. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Umemura S, Yamane H, Suwaki T, Katoh T,
Yano T, Shiote Y, Takigawa N, Kiura K and Kamei H: Interstitial
lung disease associated with gemcitabine treatment in patients with
non-small-cell lung cancer and pancreatic cancer. J Cancer Res Clin
Oncol. 137:1469–1475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakaya A, Kurata T, Yokoi T, Takeyasu Y,
Niki M, Kibata K, Satsutani N, Torii Y, Katashiba Y, Ogata M, et
al: Retrospective analysis of single-agent nab-paclitaxel in
patients with platinum-resistant non-small cell lung cancer. Mol
Clin Oncol. 7:803–807. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okusaka T, Ikeda M, Fukutomi A, Ioka T,
Furuse J, Ohkawa S, Isayama H and Boku N: Phase II study of
FOLFIRINOX for chemotherapy-naïve Japanese patients with metastatic
pancreatic cancer. Cancer Sci. 105:1321–1326. 2014. View Article : Google Scholar : PubMed/NCBI
|