Introduction
Glioblastomas (GBMs) are highly malignant brain
tumours with a poor prognosis, despite aggressive surgical and
oncological treatments. They are genetically and
histopathologically highly heterogeneous (1,2). In
addition to malignant astrocytes, there are also large numbers of
microglia/macrophages, known as tumour-associated macrophages
(TAMs), which can account for up to 30–50% of the total tumour cell
mass in human GBMs (3). Microglial
proliferation or differentiation of macrophages from blood-borne
precursors contribute to TAM accumulation (4). The influence of TAMs on GBM growth
seems to be multifaceted and dependent on the polarization of TAMs;
thus, TAMs are potential therapeutic targets (5). TAM activation is complex and comprises
two dynamic states: M1 (pro-inflammatory) or M2
(anti-inflammatory), which translates to tumour suppressive (M1) or
tumour supportive (M2). These activities are closely linked to
glioma maintenance and progression (3,6,7). However, this categorization of TAMs is
debatable and based on in vitro studies (5).
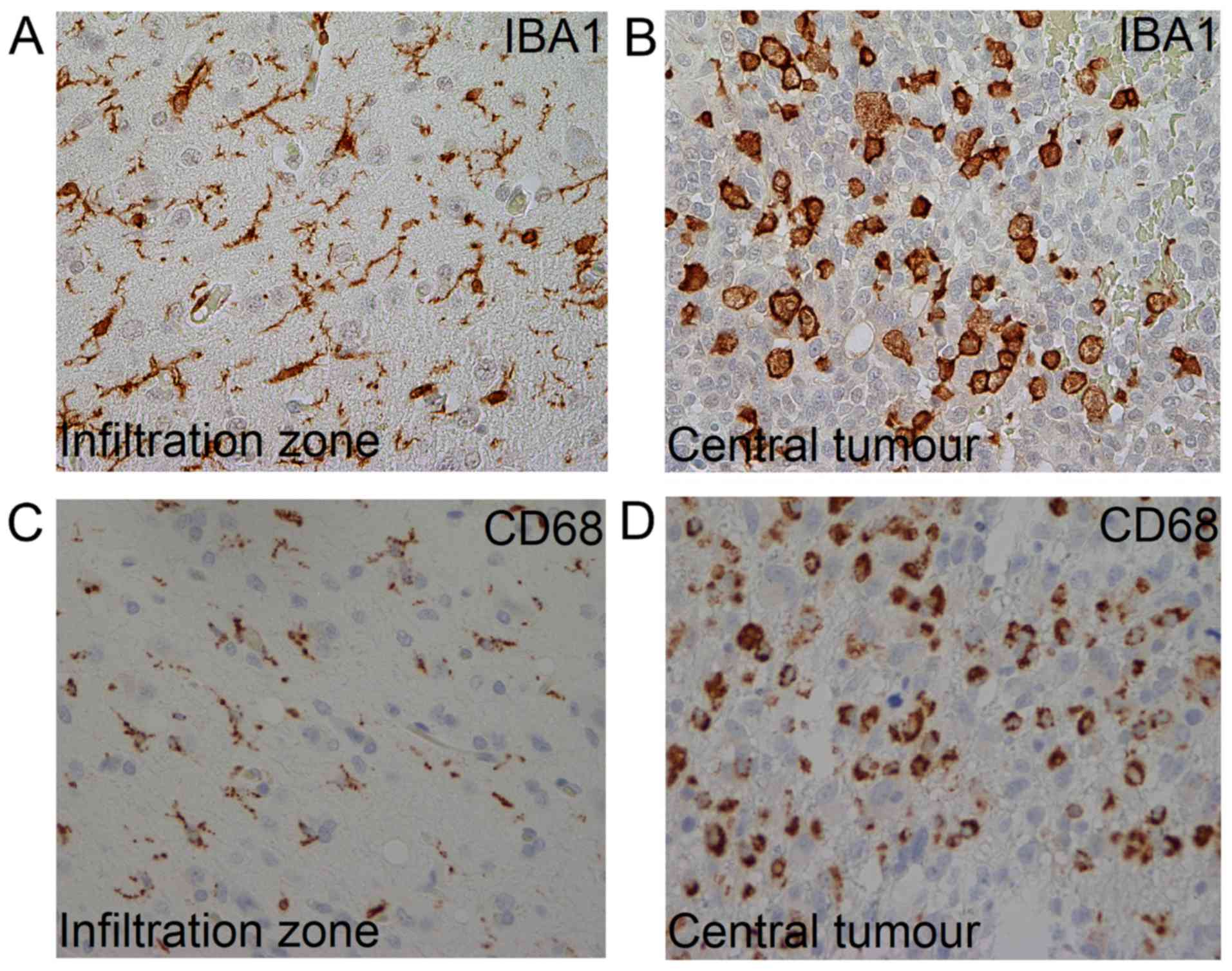
In GBMs, TAMs can assume different morphologies
(8), i.e. ramified and amoeboid
appearances related to various functional states (9). The ramified phenotype, which is only
expressed by microglia, is considered typical for a ‘resting’
phase; whereas, the amoeboid phenotype that is expressed by both
microglia and macrophages, is associated with a more active state
(10). Much research in this field
is based on cell lines and animal studies. Therefore, few studies
have explored the distribution and frequency of TAMs in relation to
the growth of human GBMs.
Recently, the pretreatment growth dynamics of a
cohort of 106 GBMs were estimated using tumour volume segmentations
from two contrast-enhancing T1-weighted MRIs, from diagnostic and
preoperative scans (11). The GBMs
were divided into two groups, slow and fast-growing tumours, and
the former was determined to be a significant predictor of long
term survival (12). From the
original pretreatment 106 GBMs we selected 16 cases that contained
sufficient tissue to study MRI-estimated tumour growth in relation
to histopathological TAM distribution. TAMs were classified using
CD68 and Iba1, two common macrophage/microglia markers (13). The aims of this study were to examine
the morphology, distribution, and density of TAMs in GBM tissue by
means of immunohistochemistry using CD68 and Iba1 in relation to
the MRI-estimated slow- and fast-growth properties.
Materials and methods
The GBM tissues used in this paper were from sixteen
patients from a previous study that estimated pretreatment growth
rates in 106 adult GBM patients operated at St. Olavs University
Hospital, Trondheim, Norway between January 2004 and May 2014
(11). From the 106 initial GBMs, 16
had sufficient tissue to assess the distribution of TAMs in various
locations (i.e. central and peripheral areas). All cases were
previously shown to be GFAP-immunoreactive and IDH1 R132H
immunonegative (12). Clinical data
were extracted from electronic medical records at St. Olavs
University Hospital.
Since all patients underwent at least two MRI
examinations (one at the time of diagnosis and one taken before
operation), MRI-estimated, pretreatment GBM growth could be
estimated based on manual segmentation of both tumour volumes
(11). A detailed description of the
segmentation, estimation of growth rates, and the categorization
into two growth groups has been reported elsewhere (11,12).
Seven of the included tumours displayed slow growth and nine
displayed fast growth. Routine haematoxylin and eosin (H&E)
stained 5 µm paraffin tissue sections were revised and classified
according to 2016-WHO-criteria. Sections were incubated with the
polyclonal antibody Iba1 (ab5076, goat polyclonal; Abcam Inc.,
Cambridge MA, USA), dilution 1:500, and CD68 (M 0814, mouse clone
KP1; Dako, Glostrup, Denmark), dilution 1:6,000, for 30 min at room
temperature. Standard immunohistochemical procedures were followed
using an automated staining unit (Dako Techmate 500). Human spleen
served as the positive control, and the primary antibody was
omitted in the negative control.
We distinguished the following areas: The
infiltration zone, defined as the periphery of the tumour
containing both normal brain and tumour cells and central areas
with emphasis on necrosis, pseudopalisades, and perivascular areas.
Histologic assessment of the localization and distribution of TAMs
and their morphology was performed throughout the tumour tissue, as
well as the number of TAMs. Areas with the highest density of
labelled TAMs were then identified using the 10× objective. The
amount of immunoreactive cells was then semi-quantitatively
categorized in high power fields (40× objective) using the
following rating system for the amounts of immunoreactive cells:
Low (+, ~0–10% of the cells were positive), moderate (++, ~10–50%
of the cells were positive), and high (+++, >50% of the cells
were positive). TAM morphology was classified as a dominating
ramified or amoeboid phenotype (Fig.
1). Cells with a so-called ‘bushy’ phenotype were classified as
ramified TAMs (14). The microscopic
analyses were performed by MK, VEM, and an experienced
neuropathologist (SHT) using a Nikon Eclipse 80i microscope.
Statistical analysis was performed using SPSS
version 22, and comparisons between groups were based on the
Mann-Whitney U test or Wilcoxon signed rank test. Based on
Bonferroni correction for 20 tests, P-values <0.0025 were
considered statistically significant. This project was approved by
the Regional Ethics Committee (Central) as part of a larger project
(references 2011/974 and 2013/1348) and adhered with the
Declaration of Helsinki.
Results
Sixteen adult GBM patients with a median age of 62
years (range 50–79) were included in this study, five females and
11 males. Among these patient's tumours, nine were fast-growing and
seven were slow-growing. The distribution of TAMs identified with
CD68 and Iba1 is shown in Table I;
pseudopalisading regions are not included, as they were observed in
only 12 of the cases. The infiltration zones showed a dispersed
distribution of TAMs compared with central tumour areas.
 | Table I.Amount of TAMsIba1 and
TAMsCD68 in slow- and fast-growing glioblastomas. |
Table I.
Amount of TAMsIba1 and
TAMsCD68 in slow- and fast-growing glioblastomas.
|
| Slow growth
(n=7) | Fast growth
(n=9) |
|---|
|
|
|
|
|---|
| Location | Low amount + (%) | Moderate amount ++
(%) | High amount +++
(%) | Low amount + (%) | Moderate amount ++
(%) | High amount +++
(%) |
|---|
| Infiltration
zone |
|
TAMIba1 | 0/7 (0) | 7/7 (100) | 0/7 (0) | 1/9 (11) | 7/9 (78) | 1/9 (11) |
|
TAMCD68 | 7/7 (100) | 0/7 (0) | 0/7 (0) | 8/9 (89) | 1/9 (11) | 0/9 (0) |
| Infiltration zone
vessels |
|
TAMIba1 | 0/7 (0) | 3/7 (43) | 4/7 (57) | 0/9 (0) | 6/9 (67) | 3/9 (33) |
|
TAMCD68 | 0/7 (0) | 2/7 (29) | 5/7 (71) | 4/9 (44) | 5/9 (56) | 0/9 (0) |
| Central tumour |
|
TAMIba1 | 0/7 (0) | 0/7 (0) | 7/7 (100) | 0/9 (0) | 2/9 (22) | 7/9 (78) |
|
TAMCD68 | 0/7 (0) | 3/7 (43) | 4/7 (57) | 2/9 (22) | 7/9 (78) | 0/9 (0) |
| Central tumour
vessels |
|
TAMIba1 | 0/7 (0) | 2/7 (29) | 5/7 (71) | 0/9 (0) | 2/9 (22) | 7/9 (78) |
|
TAMCD68 | 0/7 (0) | 2/7 (29) | 5/7 (71) | 0/9 (0) | 4/9 (44) | 5/9 (56) |
| Necroses |
|
TAMIba1 | 6/7 (86) | 1/7 (14) | 0/7 (0) | 7/9 (78) | 2/9 (22) | 0/9 (0) |
|
TAMCD68 | 4/7 (57) | 3/7 (43) | 0/7 (0) | 8/9 (89) | 1/9 (11) | 0/9 (0) |
In central parts of the tumour, there were
statistically higher numbers of TAMs compared with the periphery
for both antibodies (P=0.001 for TAMsCD68 and P<0.001
for TAMsIba1) (Table
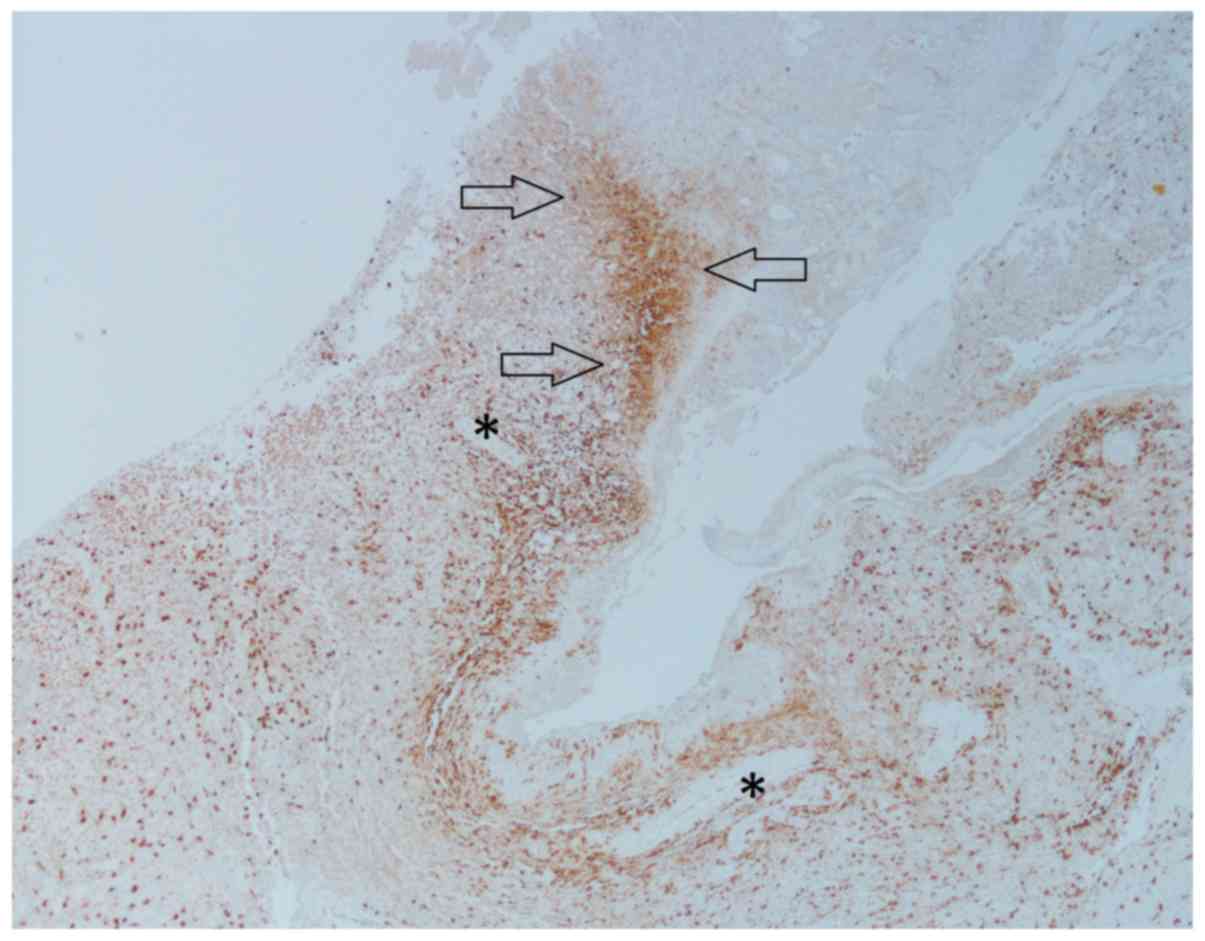
II). There was both diffuse as well as condensed infiltration
of TAMs in various histological compartments, such as the
perivascular spaces, pseudopalisades, and in areas between larger
vessels and necrotic areas (Fig. 2).
However, there were few TAMs within necrotic areas. In central
tumour areas, most TAMs were amoeboid.
 | Table II.Distribution of TAMsIba1
and TAMsCD68 at specific locations. |
Table II.
Distribution of TAMsIba1
and TAMsCD68 at specific locations.
| Comparisons of the
amounts of TAMs | P-value |
|---|
| TAMsIba1
vs. TAMsCD68 in the infiltration zone |
<0.001a |
| TAMsIba1
vs. TAMsCD68 in central tumour | 0.001a |
| TAMsIba1
vs. TAMsCD68 surrounding infiltration zone vessels | 0.096 |
| TAMsIba1
vs. TAMsCD68 surrounding central vessels | 0.414 |
| TAMsIba1
in the infiltration zone vs. central areas |
<0.001a |
| TAMsIba1
surrounding vessels in infiltration and central areas | 0.058 |
| TAMsCD68
in the infiltration zone vs. central areas | 0.001a |
| TAMsCD68
surrounding vessels in infiltration and central areas | 0.038 |
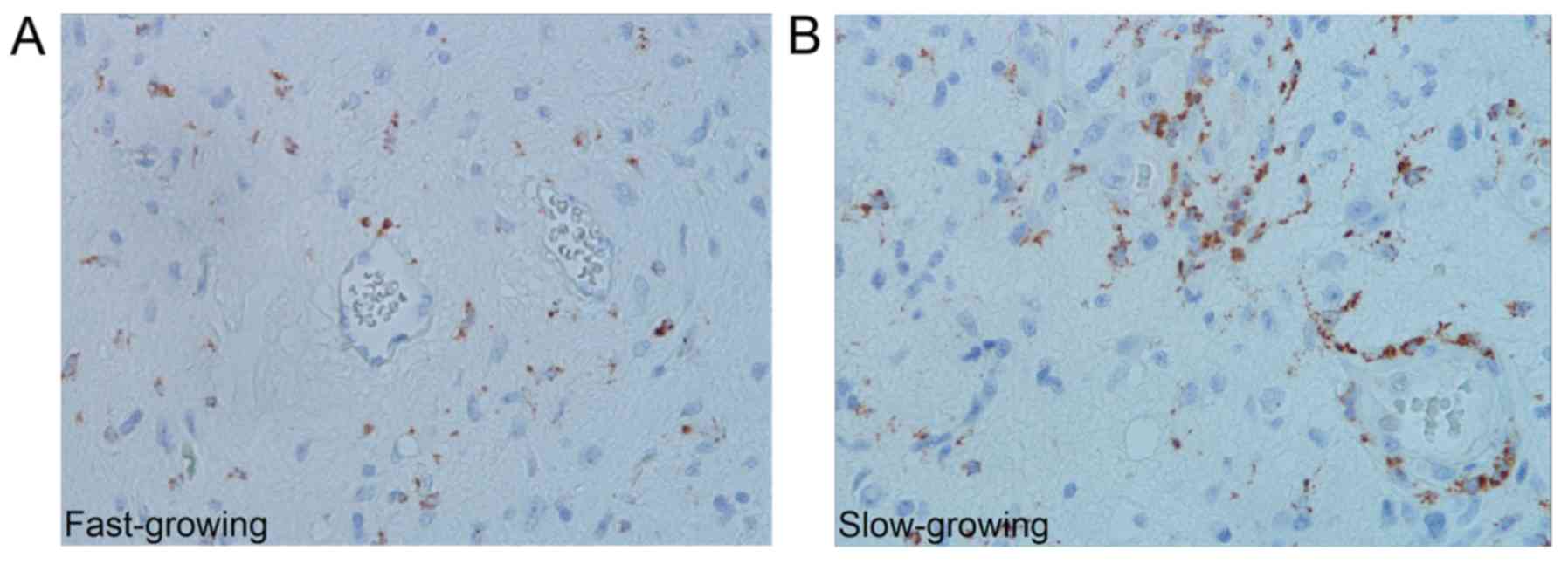
Slow- and fast-growing tumours contained different
quantities of TAMsCD68, whereas no such difference was
seen with TAMsIba1. Slow-growing tumours contained more
perivascular TAMsCD68 in the infiltration zone and more
TAMsCD68 in central areas (P=0.003 and P=0.025
respectively) (Fig. 3); however,
these results did not reach the significance level set by the
Bonferroni correction (P<0.0025). Comparisons between slow- and
fast-growing tumours are summarized in Table III.
 | Table III.Comparison of amount of TAMs in
various locations in slow- and fast-growing tumours. |
Table III.
Comparison of amount of TAMs in
various locations in slow- and fast-growing tumours.
| Amount of TAMs in
slow-growing vs. fast-growing tumours at the given locations | P-value |
|---|
| TAMsIba1
infiltration zone | 1.000 |
| TAMsIba1
central area | 0.470 |
| TAMsCD68
infiltration zone | 0.758 |
| TAMsCD68
central area | 0.025 |
| TAMsCD68
infiltration zone vessels | 0.003 |
| TAMsIba1
infiltration zone vessels | 0.252 |
| TAMsIba1
central vessels | 0.837 |
| TAMsCD68
central vessels | 0.606 |
| TAMsCD68
necrosis | 0.299 |
| TAMsIba1
necrosis | 0.837 |
| TAMsCD68
pseudopalisades | 0.114 |
| TAMsIba1
pseudopalisades | 0.918 |
Discussion
In this study, we investigated the histopathological
aspects of TAMs in human GBMs with emphasis on the number,
distribution and morphology of Iba1 and CD68 immunoreactive TAMs,
as well as the relationship with tumour growth estimated from MRI
scans. In specific tumour regions, there were more
TAMsCD68 in the slow-growing GBMs, while the quantities
of TAMsIba1 were similar in slow- and fast-growing
tumours. There were significantly more TAMsIba1 than
TAMsCD68. The lba1 antibody reacts with an ionized
calcium-binding protein typical for both resting and activated
microglia/macrophages, whereas anti-CD68 labels lysosomal membranes
commonly found in these cells (9).
In general, these two antibodies are regarded as pan-markers of
TAMs (14, 15). There are, however, certain differences between
them, as Iba1 seems to stain more widely (including more of the
activation states) than CD68 (16).
As there were no differences in TAMsIba1 between slow
and fast-growing tumours, it seems that the absolute number of TAMs
is not an essential factor in GBM growth.
Accordingly, the phenotypes and activation states of
TAMs may be more relevant. Ramified TAMs dominated the peripheral
parts of the tumour, in contrast to the central areas where TAMs
mainly had an amoeboid phenotype. TAMs can be simply categorized as
classically activated M1 (pro-inflammation/anti-tumour) and
alternatively activated M2 (anti-inflammatory/pro-tumour) (5). Actually, there are various antibodies
reactive against different epitopes and functional states of TAMs
(9, 15), and currently no single reliable immunohistochemical
marker exists for TAMs. For that reason, standardized markers for
histological immunophenotyping of TAMs are needed. Nevertheless, as
CD68 is highly expressed among TAMs in the M1 state (16,17), our
finding of many TAMsCD68 in the periphery of
slow-growing GBMs suggests there are growth inhibitory effects of
these cells in this part of the tumour that may serve as a
potential target for therapy.
To study the TAM morphology, we selected sections of
GBM cases with sufficient tumour tissue to display the whole
transition from central tumour to the infiltration zone. We
observed a gradual increase in the number of TAMs toward the tumour
centre. Further, there was a transition, via hybrid forms, of TAMs
with a ramified morphology in the periphery to the dominant
amoeboid phenotype in central areas. Such a pattern has been
described by others (9,16), however, not in a setting as our
study, and supports that TAMs can have various phenotypes and
activation states throughout the tumour (10,18,19).
Since ramified and amoeboid forms of TAMs are linked to low- and
high activation states, respectively (9), these observations support a gradual
increase in activated TAMs toward the tumour centre. Accordingly,
this variable and dynamic TAM population in human GBMs may have
various impacts on the biology in different parts of the tumour,
and may explain the discrepant effects on TAMs on survival in human
GBMs (20).
Most TAMs were located in perivascular regions and
were best visualized in CD68 stained sections, especially in the
infiltration zone. This may reflect the presence of TAMs in so
called perivascular niches, in which there is collaboration between
different cell types (15,21,22). The
impact of this microenvironment on the tumour biology in the
periphery is unclear. However, as CD68 is highly expressed in M1
TAMs, these perivascular macrophages may provide an inhibitory
effect on the infiltration process.
In the central part of the tumour, we observed a
much higher number of TAMs compared with the infiltration zone, in
accordance with others (9). This
illustrates that these cells constitute a large part of the tumour
mass, up to 30% (3,5). The TAMs were mostly diffusely
dispersed; however, there was a concentration in microanatomical
compartments consistent with perivascular and perinecrotic niches
(21,23). The rather few TAMs within necrosis
are in agreement with the observation that these cells are more
common in radiation-induced necrosis. This is an important feature
in the differential diagnosis between tumour and radiation-induced
necrosis (24), and it seems
counterintuitive as this process represents a tissue stress that
normally attracts TAMs (23). On the
other hand, the microenvironment in these areas drive TAMs towards
the M2 phenotype so they lose their ability to remove necrotic
debris (21).
Although this study is retrospective and based on
relatively few cases, it describes histological aspects of TAMs in
a series of untreated GBMs in vivo with MRI estimated tumour
growth. Even though steroid treatment was administrated for many
patients, this has been shown not to affect tumour growth (11). A strength of this study is that
sufficient tumour tissue was available to cover the peripheral and
central areas. We also used two antibodies regarded as pan-markers
of macrophages/microglia to get a more complete description of TAM
infiltration in the GBM tissue.
In conclusion, there is a heavy infiltration of TAMs
in human GBMs, and they are commonly located in distinct
microanatomical compartments, most likely constituting parts of
cellular niches important for tumour biology. Both ramified and
amoeboid TAMs were observed, consistent with a dynamic range of
activation states throughout the tumour tissue. The number of TAMs
seems less critical for tumour growth whereas the phenotype appears
more relevant, pointing to a potential target for therapy that
needs further investigation.
Acknowledgements
We appreciate the technical support of Unn Sophie
Granli and Camilla Bjørk Setsaas. We thank Turid Follestad for
statistical support.
Funding
This study was funded by the Faculty of Medicine and
Health Sciences, Norwegian University of Science and Technology
(grant no. 70367040).
Availability of data and materials
All data and materials used in this study is present
in this manuscript.
Authors' contributions
MK performed microscopical analysis, statistical
analysis and wrote the manuscript. VEM was a major contributor in
writing the manuscript and in acquisition of the data. ALS and OS
performed the MRI investigations and contributed in writing the
manuscript. JW was involved in analysis of the data and reviewing
the manuscript. SHT designed the study, supervised the results and
co-wrote the manuscript.
Ethics approval and consent to
participate
This project was approved by the Regional Ethics
Committee (Central) as part of a larger project (references
2011/974 and 2013/1348) and adhered with the Declaration of
Helsinki. The majority of patients provided written informed
consent to be included in a related glioma outcome study (reference
2011/974). The regional ethics committee waived informed consent
for retrospective evaluation of patient data for the remaining
patients, and did not require written informed consent from a
relative or guardian.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GBM
|
glioblastoma multiforme
|
|
TAMs
|
tumour-associated macrophages
|
|
TAMsCD68
|
tumour-associated macrophages
immunoreactive for CD68 antibody
|
|
TAMsIba1
|
tumour-associated macrophages
immunoreactive for Iba1 antibody
|
References
|
1
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
Classification of Tumors of the Central Nervous System: A summary.
Acta Neuropathol131. 803–820. 2016. View Article : Google Scholar
|
|
2
|
Aldape K, Zadeh G, Mansouri S,
Reifenberger G and von Deimling A: Glioblastoma: Pathology,
molecular mechanisms and markers. Acta Neuropathol. 129:829–848.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hambardzumyan D, Gutmann DH and Kettenmann
H: The role of microglia and macrophages in glioma maintenance and
progression. Nat Neurosci. 19:20–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perdiguero EG and Geissmann F: The
development and maintenance of resident macrophages. Nat Immunol.
17:2–8. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tremble LF, Forde PF and Soden DM:
Clinical evaluation of macrophages in cancer: Role in treatment,
modulation and challenges. Cancer Immunol Immunother. 66:1509–1527.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Domingues P, González-Tablas M, Otero Á,
Pascual D, Miranda D, Ruiz L, Sousa P, Ciudad J, Gonçalves JM,
Lopes MC, et al: Tumor infiltrating immune cells in gliomas and
meningiomas. Brain Behav Immun. 53:1–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li W and Graeber MB: The molecular profile
of microglia under the influence of glioma. Neuro Oncol.
14:958–978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ricard C, Tchoghandjian A, Luche H, Grenot
P, Figarella-Branger D, Rougon G, Malissen M and Debarbieux F:
Phenotypic dynamics of microglial and monocyte-derived cells in
glioblastoma-bearing mice. Sci Rep. 6:263812016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boche D, Perry VH and Nicoll JA: Review:
Activation patterns of microglia and their identification in the
human brain. Neuropathol Appl Neurobiol. 39:3–18. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Colonna M and Butovsky O: Microglia
function in the central nervous system during health and
neurodegeneration. Annu Rev Immunol. 35:441–468. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stensjøen AL, Solheim O, Kvistad KA,
Håberg AK, Salvesen Ø and Berntsen EM: Growth dynamics of untreated
glioblastomas invivo. Neuro Oncol. 17:1402–1411. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stensjøen AL, Berntsen EM, Mikkelsen VE,
Torp SH, Jakola AS, Salvesen Ø and Solheim O: Does pretreatment
tumor growth hold prognostic information for patients with
glioblastoma? World Neurosurg. 101:686–694.e4. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Malley JT, Nadol JB Jr and McKenna MJ:
Anti CD163+, Iba1+, and CD68+
cells in the adult human inner ear: Normal distribution of an
unappreciated class of macrophages/microglia and implications for
inflammatory otopathology in humans. Otol Neurotol. 37:99–108.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou W, Ke SQ, Huang Z, Flavahan W, Fang
X, Paul J, Wu L, Sloan AE, McLendon RE, Li X, et al: Periostin
secreted by glioblastoma stem cells recruits M2 tumour-associated
macrophages and promotes malignant growth. Nat Cell Biol.
17:170–182. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murray PJ and Wynn TA: Protective and
pathogenic functions of macrophage subsets. Nat Rev Immunol.
11:723–737. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sørensen MD, Dahlrot RH, Boldt HB, Hansen
S and Kristensen BW: Tumour-associated microglia/macrophages
predict poor prognosis in high-grade gliomas and correlate with an
aggressive tumour subtype. Neuropathol Appl Neurobiol. 44:185–206.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chávez-Galán L, Olleros ML, Vesin D and
Garcia I: Much more than M1 and M2 macrophages, there are also
CD169(+) and TCR(+) macrophages. Front Immunol.
6:2632015.PubMed/NCBI
|
|
18
|
Glass R and Synowitz M: CNS macrophages
and peripheral myeloid cells in brain tumours. Acta Neuropathol.
128:347–362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mosser DM and Edwards JP: Exploring the
full spectrum of macrophage activation. Nat Rev Immunol. 8:958–969.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bieńkowski M and Preusser M: Prognostic
role of tumour-infiltrating inflammatory cells in brain tumours:
Literature review. Curr Opin Neurol. 28:647–658. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hambardzumyan D and Bergers G:
Glioblastoma: Defining tumor niches. Trends Cancer. 1:252–265.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schiffer D, Annovazzi L, Mazzucco M and
Mellai M: The microenvironment in gliomas: Phenotypic expressions.
Cancers (Basel). 7:2352–2359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang L and Zhang Y: Tumor-associated
macrophages: From basic research to clinical application. J Hematol
Oncol. 10:582017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Prayson RA and Cohen ML: Practical
Differential Diagnosis in Surgical Neuropathology. Humana Press;
Totowa, NJ: pp. 1772000
|