Introduction
Liposarcoma (LPS) is one of the most common soft
tissue neoplasms of adults, and accounts for approximately 20% of
all sarcomas (1,2). This tumor of mesenchymal origin is
usually found in the retroperitoneum, trunk, and extremities as a
slow-growing and painless mass developing during the middle decades
of life (1). Furthermore, there are
often multiple satellite lesions extending beyond the boundary of
the primary tumor (3).
In the head and neck region, LPS is rare and
accounts for only 2% of all cases (4). Rarer still is the presence of LPS in
the thyroid, and only 11 cases of primary LPS (2,5–11) and 4 cases of metastatic LPS in the
thyroid gland have been reported (3,12–14).
Herein we report the first case of dedifferentiated
LPS (DDLPS) in the thyroid gland of a 66-year-old male, and discuss
its clinical course; additionally, we performed a literature
review.
Case report
Written informed consent to report the
pathology of this case was obtained from the patient
A 66-year-old male was referred to our hospital with
a one-month history of dysphagia. He also noticed an enlarging
anterior neck mass a few days before presentation. The patient had
undergone resection of a well-differentiated LPS (WDLPS) arising in
the thymus 5 years previously.
At the time of consultation, physical examination
demonstrated a 40 mm firm, rubbery, and fixed mass in the right
lobe of the thyroid gland, and screening laboratory tests
demonstrated an euthyroid state and a normal serum thyroglobulin
level. Ultrasound was performed and revealed a 42×30×27 mm
heterogeneous nodule with poorly defined margins occupying the
right lobe of the thyroid gland. Ultrasound-guided fine needle
aspiration cytology identified spindle cells. While nuclear
enlargement and a clear nuclear body were observed, cytological
atypia was not prominent, and there was low suspicion for
WDLPS.
The vocal folds were initially unaffected; however,
the patient started to complain of hoarseness a few weeks after the
first visit, and a right recurrent laryngeal nerve paralysis was
observed. While the membranous portion of the trachea was
compressed anteriorly by the mass, there was no apparent tumor
invasion into the trachea or esophagus by endoscopic
examination.
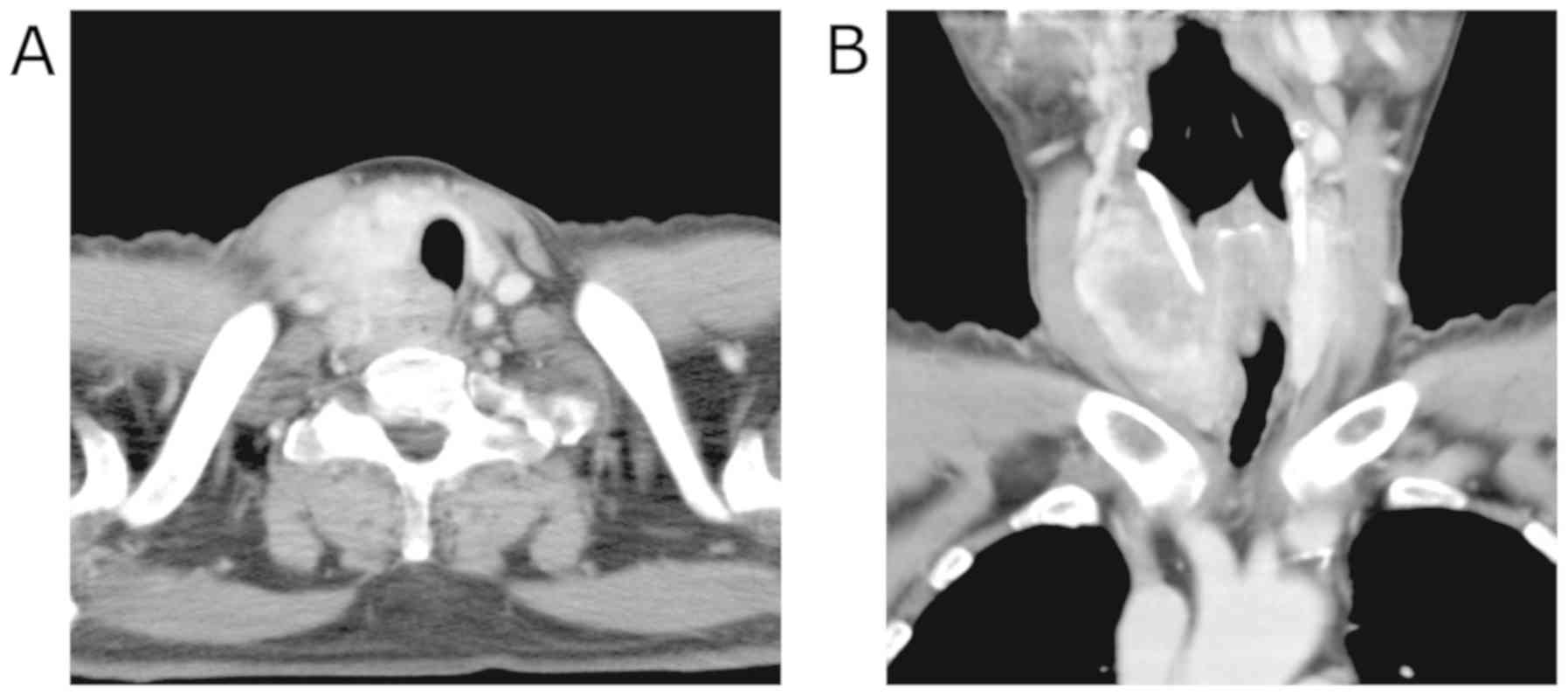
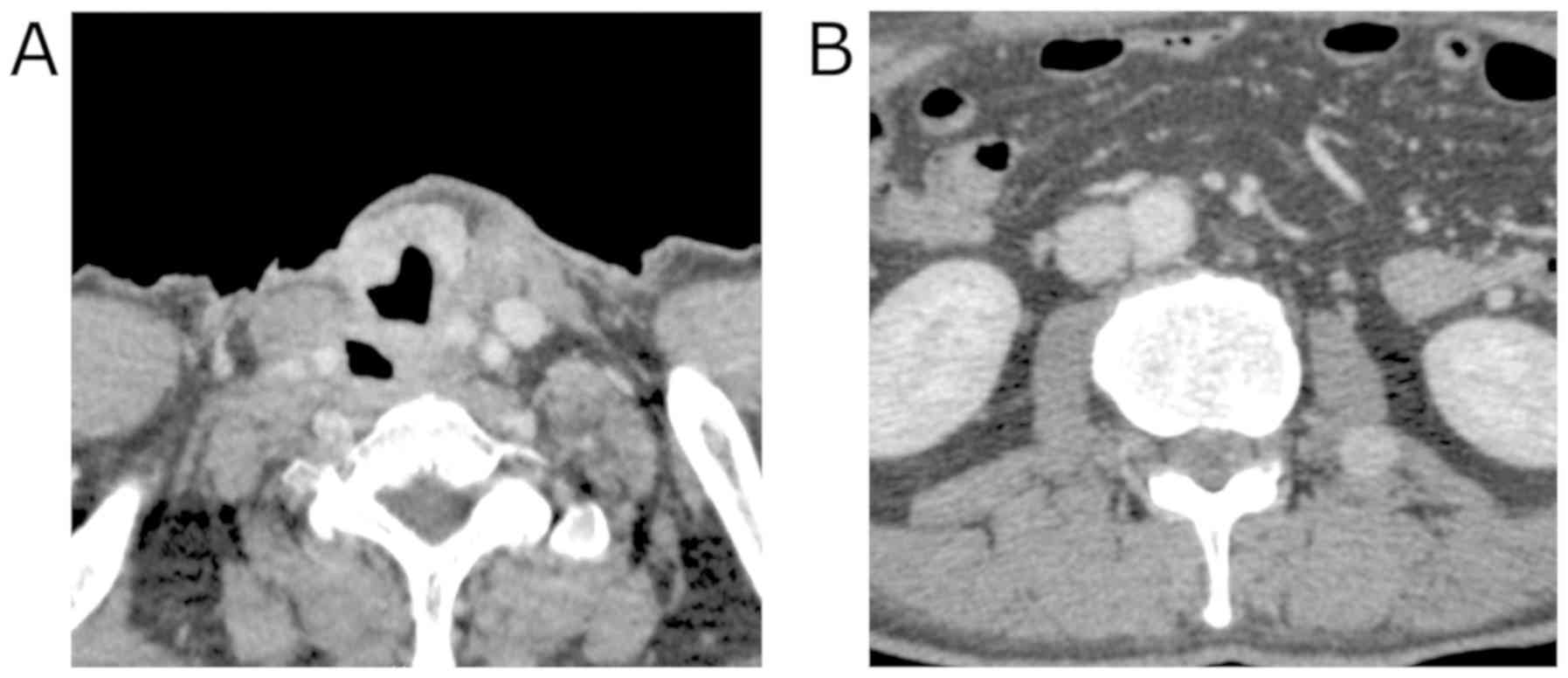
Contrast-enhanced computed tomography (CT) imaging
revealed the 40 mm mass with heterogeneous enhancement occupying
the entire right lobe of the thyroid gland. The margin of the tumor
was not clear and the tumor appeared to invade the trachea and the
esophagus (Fig. 1). Enlarged right
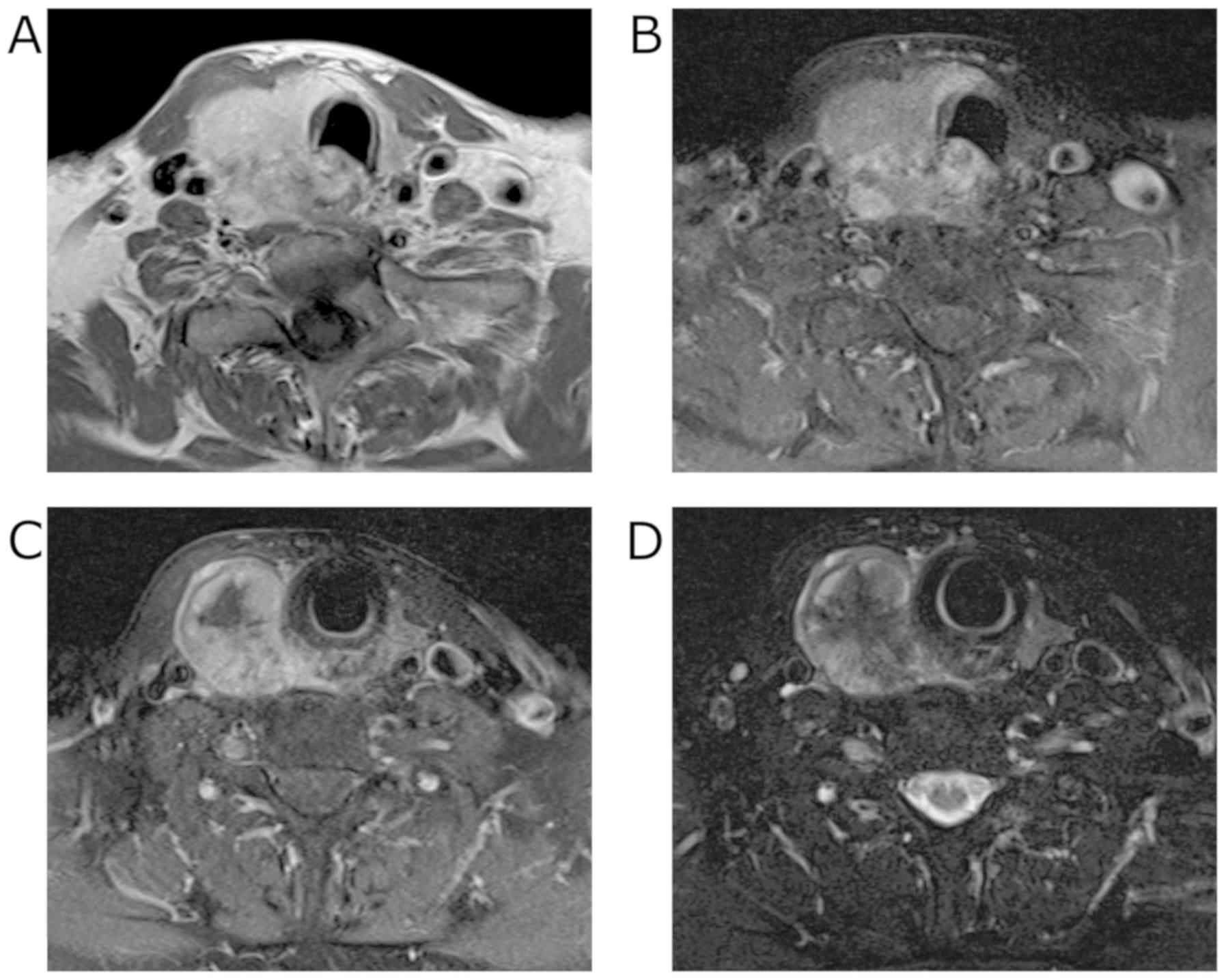
internal jugular lymph nodes were also detected. Magnetic resonance
imaging (MRI) revealed an enhancing mass with irregular margins in
the right lobe of the thyroid gland. Centrally, the tumor
demonstrated low signal intensity on T2 weighted images (T2WI) and
showed almost no enhancement on contrast-enhanced T1 weighted
images (T1WI). The tumor appeared fibrotic, and no lipomatous
portion was detected (Fig. 2).
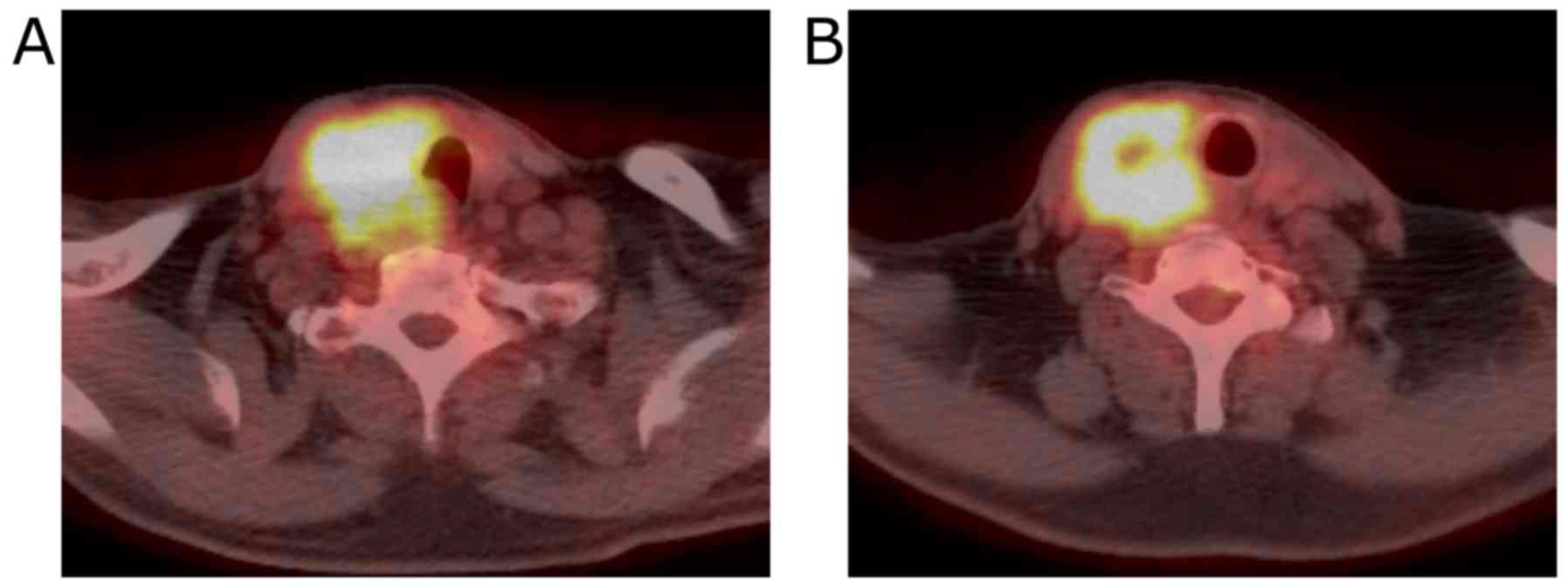
Fluorodeoxyglucose-positron emission tomography (FDG-PET) showed a
hypermetabolic mass in the right lobe of the thyroid gland. No
evidence of distant metastases or recurrent thymus tumor was
detected (Fig. 3).
Although FNA showed no evidence of cellular atypia,
malignancy was suspected because of the local extension of the
tumor, and total thyroidectomy with right modified radical neck
dissection as well as tracheal and esophageal resections were
performed.
A 7 cm tumor was identified in the right lobe
infiltrating the second tracheal ring, the esophageal muscle, and
the right recurrent laryngeal nerve. Furthermore, the tumor was
surrounded by fibrous tissue extending from the mediastinum into
the larynx/pharynx and the common carotid artery, and frozen
section of the fibrous tissue revealed spindle cells. The tumor was
resected with the right side of the 1st to 3rd tracheal ring, the
esophageal and cricopharyngeus muscles, and the right recurrent
laryngeal nerve and upper mediastinal dissection was performed.
Even though the extensive local invasion of the tumor over the neck
and mediastinum, grossly complete resection of the tumor was
achieved.
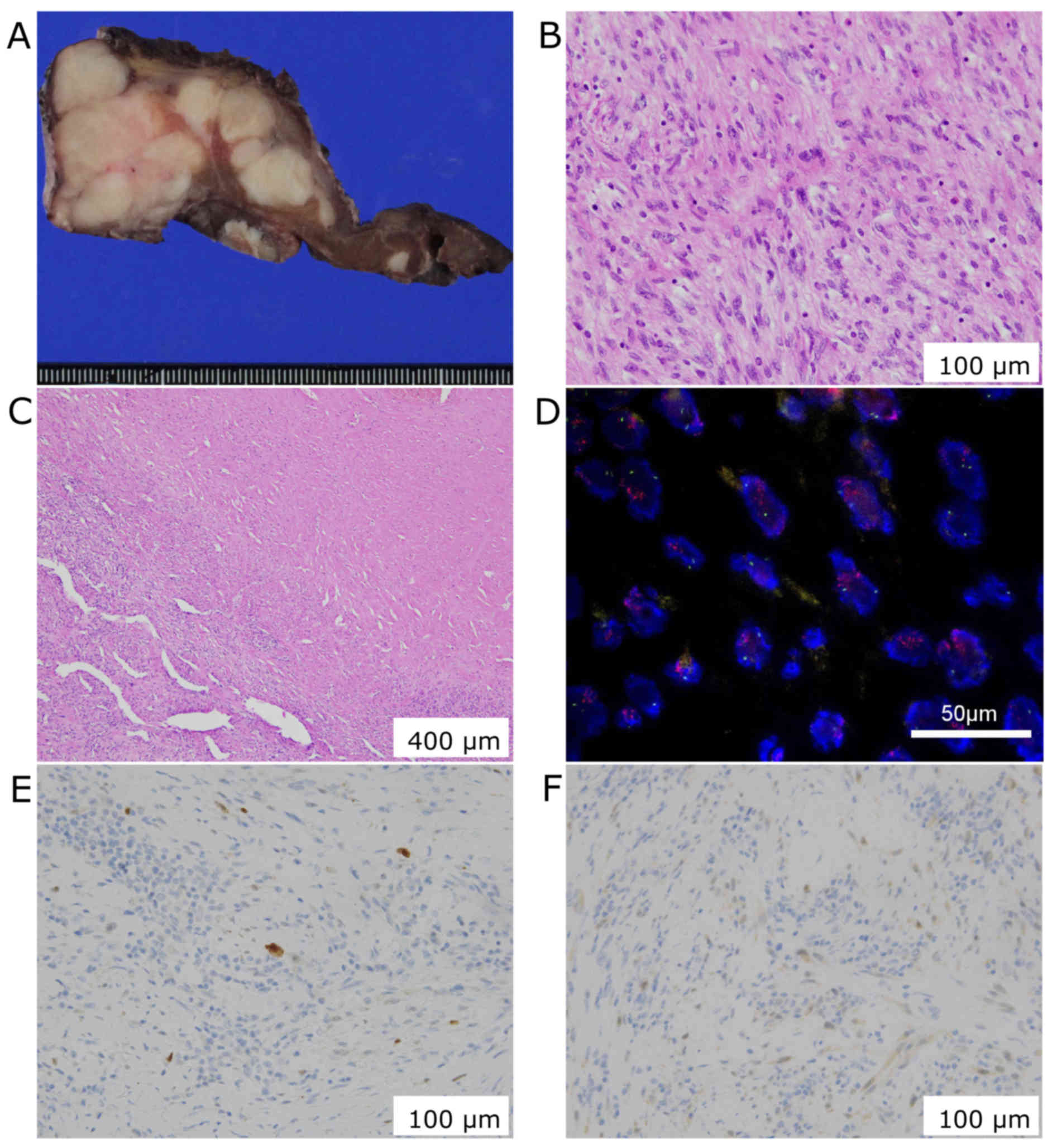
Histological examination showed hypercellularity and
infiltrative overgrowth of spindle cells with nuclear atypia. The
right lobe was completely replaced by the tumor without any
lipid-laden cells, and the central part of the tumor was replaced
by fibrotic tissue. The tumor lacks epithelial component and cells
were rarely stained with p53. These findings didn't support a
diagnosis of anaplastic carcinoma of the thyroid. Further, the
tumor didn't express S100, desmin, α-smooth muscle actin, myogenin,
myoglobin, and CD34 suggesting a dedifferentiated tumor.
Overexpression of human murine double minute 2 (MDM2) and cyclin D
kinase 4 (CKD4) were detected, and the tumor was diagnosed as DDLPS
with cervical lymph node metastases (Fig. 4).
Postoperatively, the patient received adjuvant
radiation (22 Gy in 11 fractions +35 Gy in 14 fractions delivered
to the operative field) and chemotherapy (4 courses of 1,200
mg/m2 gemcitabine and 90 mg/m2 docetaxel).
Two months postoperatively, however, the patient presented with
growth of the residual disease in the neck. Four months
postoperatively, metastases to the psoas muscle, as well as to the
axillary and intra-thoracic lymph nodes were identified (Fig. 5). Nine months postoperatively, the
patient died from severe bleeding due to local tumor progression in
the neck.
Discussion
In the head and neck region, LPS is very rare and
represents only 1% of head and neck sarcomas (15). Correct diagnosis is difficult before
surgical resection, and Davis reported that one third of patients
with head and neck LPS had an initial pathologic misdiagnosis
(4). Incorrect diagnosis may lead to
delayed or inadequate treatment. In our case, the patient was
initially suspected to have anaplastic thyroid carcinoma or
lymphoma because of the rapid extension of the tumor. Recurrence of
WDLPS of the thymus was not considered because of the long interval
from the initial resection of that tumor. However, according to
previous reports summarized in the Table
I, LPS can recur long after the initial resection. In a patient
with a history of LPS, the possibility of a recurrence or
metastasis should be considered, even if the suspect lesion is in
the thyroid.
 | Table I.Summary of published cases of
liposarcoma of the thyroid gland. |
Table I.
Summary of published cases of
liposarcoma of the thyroid gland.
| Case no. | Age (years old) | Sex |
Primary/metastatic | Interval from the
initial treatment | Primary site | Initial
treatment | Subtype | Adjuvant
treatment | Observation period
(months) |
Recurrence/metastasis | Prognosis | (Refs.) |
|---|
| 1 | 80 | M | Primary |
|
| Partial
thyroidectomy | WDLPS | None | 24 | – | DOA | (4) |
| 2 | 23 | M | Primary |
|
| Partial
thyroidectomy | MLPS | None | 22 | – | NED | (5) |
| 3 | 56 | F | Primary |
|
| Undescribed | MLPS | None | 2 | Undescribed | DOD | (6) |
| 4 | 35 | F | Primary |
|
| Partial
thyroidectomy | MLPS | None | 189 | Skin, bone, Lung,
liver | DOD | (9) |
| 5 | 71 | M | Primary |
|
| Partial
thyroidectomy | PLS | RTx | 6 | – | NED | (9) |
| 6 | 49 | F | Primary |
|
| Partial
thyroidectomy | MLPS | RTx | 10 | Lung, liver | DOD | (7) |
| 7 | 71 | M | Primary |
|
| Partial
thyroidectomy | PLS | RTx | 24 | Lung, bone | AWD | (7) |
| 8 | 40 | M | Primary |
|
| Total
thyroidectomy | WDLPS | RTx | 24 | – | NED | (8) |
| 9 | 59 | F | Primary |
|
| Partial
thyroidectomy | MLPS | None | 15 | Lung, bone | DOD | (2) |
| 10 | 72 | F | Primary |
|
| Undescribed | Undescribed | RTx | More than 24 | – | NED | (10) |
| 11 | 65 | F | Primary |
|
| Total
thyroidectomy | Undescribed | RTx | More than 24 | – | NED | (10) |
| 12 | 51 | M | Metastatic | 28 months | Thigh | Partial
thyroidectomy | MLPS | CTx | 6 | Thigh, lung | AWD | (14) |
| 13 | 30 | F | Metastatic | 30 months | Thigh | None | PLS | Undescribed | Undescribed | Pelvis | AWD | (11) |
| 14 | 55 | F | Metastatic | 36 months | Buttock | Undescribed | PLS | Undescribed | Undescribed | Bone | AWD | (12) |
| 15 | 86 | F | Metastatic | Two decades | Thigh | Partial
thyroidectomy | MLPS | RTx | 21 | Lung | DOD | (13) |
LPSs are mostly idiopathic and etiology of LPS still
remains unclear. Similar to other sarcomas, complex karyotypic
defects leading to genetic instability and disturbances in cell
cycle genes are reported to cause LPSs (16). The complex karyotypic defects can be
induced by radiation, however, LPSs are less frequent in radiation
induced sarcomas. Further, LPSs are less frequently seen in
patients with genetic syndromes than in patients with sporadic soft
tissue sarcomas (17).
According to the classification of the World Health
Organization, LPSs are a heterogeneous group classified into four
subtypes based on morphology and genetic findings, namely: Atypical
lipomatous tumors (ALT)/WDLPS, DDLPS, myxoid LPS (MLPS), and
pleomorphic LPS (PLS) (18). Each
subtype exhibits different clinical behaviors. DDLPS, first
described by Evans in 1979 (19), is
characterized by the transition from an adipocyte-rich,
well-differentiated region within a tumor to a non-lipogenic,
spindle cell-rich region. It develops de novo in most cases, and
25–40% of patients show progression from ALT/WDLPS to DDLPS.
Although ALT/WDLPS has a low metastatic potential, DDLPS shows a
strong propensity for distant lung metastasis and local recurrence
(18,20). In the present case, the tumor
extended rapidly and adjuvant radiation and chemotherapy were not
effective in halting its progression, consistent with features of
DDLPS. It is difficult to determine whether the tumor was a local
recurrence or metastasis of the original LPS of the thymus,
particularly because there appeared to be infiltration of the tumor
into the mediastinum. We thought of this case as metastasis as main
lesion was located in the thyroid gland and not in mediastinum.
DDLPS shares radiologic features with WDLPS, and
dedifferentiation is usually suggested by the presence of a focal,
nodular, nonlipomatous region greater than 10 mm in size (21). These non-adipose foci are easily
detected on MRI. Although WDLPS shows high signal intensities on
both T1WI and T2WI, dedifferentiated regions appear to show low
intensity area on both sequences (22). In our case, because of its aggressive
characteristics, anaplastic carcinoma or lymphoma was initially
suspected. Usually, anaplastic carcinoma of the thyroid gland is
heterogeneous with areas of necrosis and mixed signal on T1 and
T2WI and moderate-to-marked enhancement, and lymphoma has
homogenous mild enhancement and mild T2 hyperintense signal
compared with the surrounding normal thyroid tissues (23). As anaplastic carcinoma and DDLPS
share some features on MRI, it is difficult to distinguish between
them preoperatively.
Histologically, ALT/WDLPS and DDLPS are
characterized by the amplification of the 12q13-15 chromosome
region encoding for potential oncogenes including mouse double
minute 2 (MDM2) and cyclin dependent kinase-4 (CDK4). DDLPS is
characterized by the transition of regions of the tumor from
adipocyte-rich, well-differentiated cells to non-lipogenic, spindle
cell-rich cells (18). In this case,
any lipid-laden cells were not observed in the tumor and it was
partly replaced by fibrotic tissues as expected from the
pre-operative images.
Standard treatment for DDLPS is wide surgical
excision, which is frequently followed by radiation. Adjuvant
chemotherapy is often performed as well. Even with
multidisciplinary treatment, however, DDLPS often recurs locally
and rapidly, and can metastasize to lung, bone, or liver, and
disease progression is difficult to control (21). Recently, molecular therapies
including tyrosine kinase inhibitors, mouse double minute 2 (MDM2)
antagonists, cyclin dependent kinase-4 (CDK4) antagonists,
peroxisome proliferator-activated receptor gamma (PPAR-γ) agonists,
and Nelfinavir have been shown to have some therapeutic effects on
DDLPS (18), and further clinical
studies are warranted to establish novel therapeutic strategies for
DDLPS.
We have presented, to our knowledge, the first case
of dedifferentiated LPS of the thyroid gland. The case presented
difficulties in regards to initial diagnosis, and demonstrated the
very aggressive features of DDLPS despite aggressive surgery and
chemoradiation. To ensure timely and accurate initial diagnosis,
the differential of any thyroid mass must be a possible metastatic
lesion, particularly in a patient with a history of previous
malignancy. Novel therapeutic strategies are needed for better
outcomes in patients with DDLPS.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
YKis acquired the data, performed the literature
review and wrote the manuscript. MK, YKit, MK, IT, MS, KO acquired
the data and contributed clinical advice. AKO evaluated the images
and YY evaluated the specimens. All authors read and approved the
final manuscript.
Ethics and consent to participate
Not applicable.
Patient consent for publication
Written informed consent was obtained for the
publication of data and materials.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Blumberg JM, Jedrych J, Costa J and Judson
B: Cervical dedifferentiated liposarcoma with meningothelial-like
whorling. Head Neck Pathol. 6:476–480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang GW, Li YX and Hu ZL: Primary myxoid
liposarcoma of the thyroid gland. J Clin Pathol. 62:1037–1038.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Azar AR, Weynand B, Daumerie C and Coche
E: Metastatic liposarcoma of the thyroid gland. Br J Radiol.
76:750–752. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Davis EC, Ballo MT, Luna MA, Patel SR,
Roberts DB, Nong X and Sturgis EM: Liposarcoma of the head and
neck: The University of Texas M. D. Anderson Cancer Center
experience. Head Neck. 31:28–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nielsen VT, Knudsen N and Holm IE:
Liposarcoma of the thyroid gland. Tumori. 72:499–502. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Griem KL, Robb PK, Caldarelli DD and
Templeton AC: Radiation-induced sarcoma of the thyroid. Arch
Otolaryngol Head Neck Surg. 115:991–993. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Andrion A, Gaglio A, Dogliani N, Bosco E
and Mazzucco G: Liposarcoma of the thyroid gland. Fine-needle
aspiration cytology, immunohistology, and ultrastructure. Am J Clin
Pathol. 95:675–679. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mitra A, Fisher C, Rhys-Evans P and Harmer
C: Liposarcoma of the thyroid. Sarcoma. 8:91–96. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kilic M, Keskek M, Albayrak L, Ertan T,
Gocmen E and Koc M: Liposarcoma of the thyroid gland: A case
report. Acta Chir Belg. 107:73–74. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Awad WI, Evans PHR, Nicholson AG and
Goldstraw P: Liposarcoma of the thyroid gland mimicking
retrosternal goiter. Ann Thorac Surg. 75:566–568. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumar V, Raj A and Rathore PK: Liposarcoma
of thyroid gland: A review of the gained experience. Indian J
Cancer. 51:548–549. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bashir H, Nawaz MK, Shah MA and Ahmad E:
Pleomorphic liposarcoma metastatic to the thyroid gland. Clin Nucl
Med. 27:9–10. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brandwein-Gensler M, Urken M and Wang B:
Collision tumor of the thyroid: A case report of metastatic
liposarcoma plus papillary thyroid carcinoma. Head Neck.
26:637–641. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tysome JR, Sandison A and Clarke PM:
Myxoid liposarcoma metastatic to the thyroid gland: A case report
and literature review. J Laryngol Otol. 120:511–513. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Golledge J, Fisher C and Rhys-Evans PH:
Head and neck liposarcoma. Cancer. 76:1051–1058. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hui JY: Epidemiology and etiology of
sarcomas. Surg Clin North Am. 96:901–914. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Penel N, Grosjean J, Robin YM,
Vanseymortier L, Clisant S and Adenis A: Frequency of certain
established risk factors in soft tissue sarcomas in adults: A
prospective descriptive study of 658 cases. Sarcoma.
2008:4593862008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De Vita A, Mercatali L, Recine F, Pieri F,
Riva N, Bongiovanni A, Liverani C, Spadazzi C, Miserocchi G,
Amadori D, et al: Current classification, treatment options, and
new perspectives in the management of adipocytic sarcomas. Onco
Targets Ther. 9:6233–6246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Evans HL: Liposarcoma: A study of 55 cases
with a reassessment of its classification. Am J Surg Patho.
3:507–523. 1970. View Article : Google Scholar
|
|
20
|
Singer S, Antonescu CR, Riedel E and
Brennan MF: Histologic subtype and margin of resection predict
pattern of recurrence and survival for retroperitoneal liposarcoma.
Ann Surg. 238:358–370. 2003.PubMed/NCBI
|
|
21
|
Murphey MD, Arcara LK and Fanburg-Smith J:
Imaging of Musculoskeletal Liposarcoma with radiologic-pathologic
correlation. RadioGraphics. 25:1371–1395. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kito M, Yoshimura Y, Isobe K, Aoki K,
Suzuki S, Tanaka A, Okamoto M, Sano K and Kato H: Clinical outcome
of dedifferentiated liposarcoma in the extremities: A retrospective
case series of 7 patients. J Orthop Sci. 21:673–677. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aiken AH: Imaging of thyroid cancer. Semin
Ultrasound CT MR. 33:138–149. 2012. View Article : Google Scholar : PubMed/NCBI
|