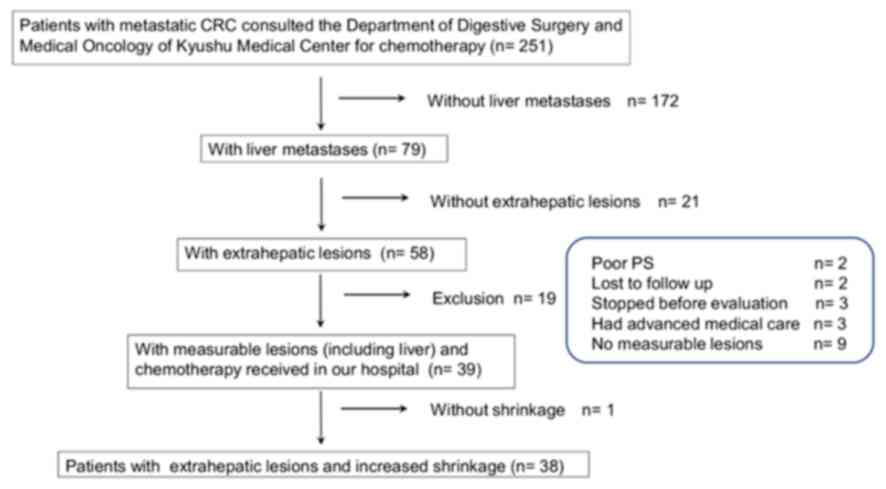
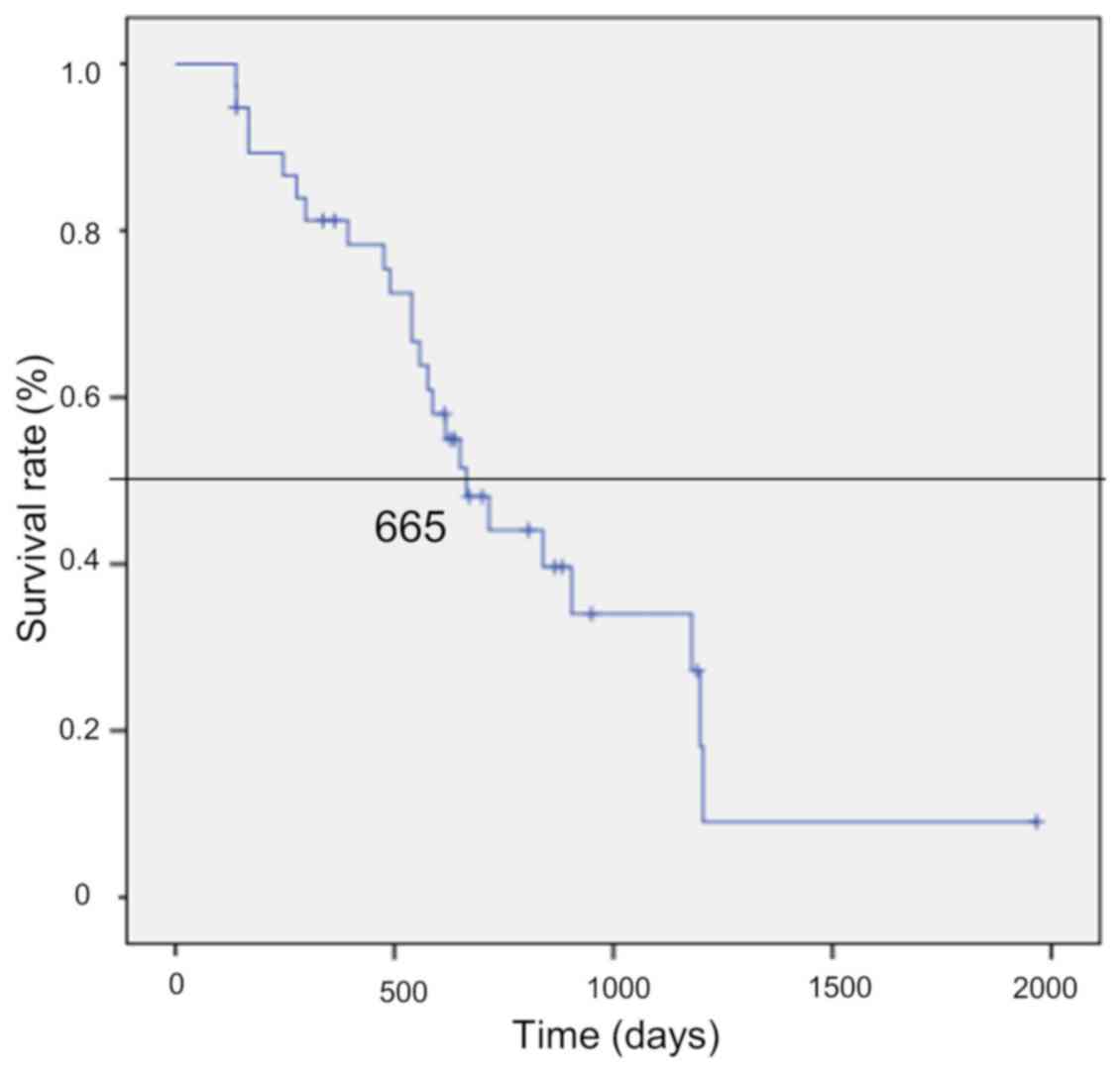
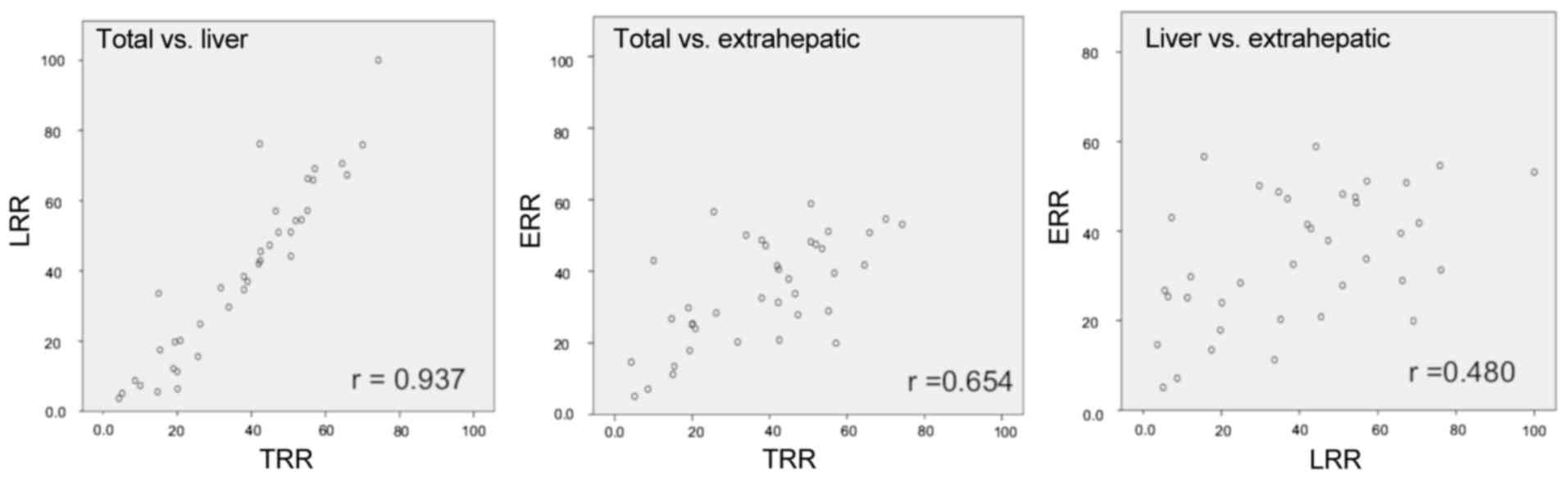
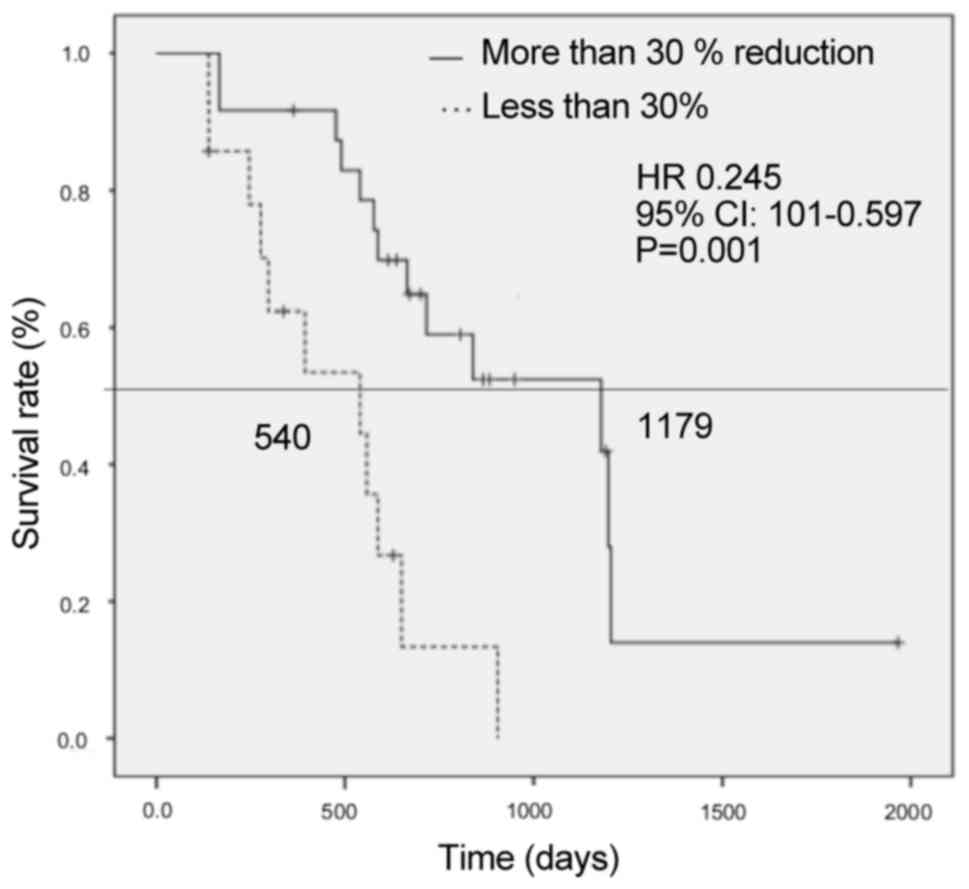
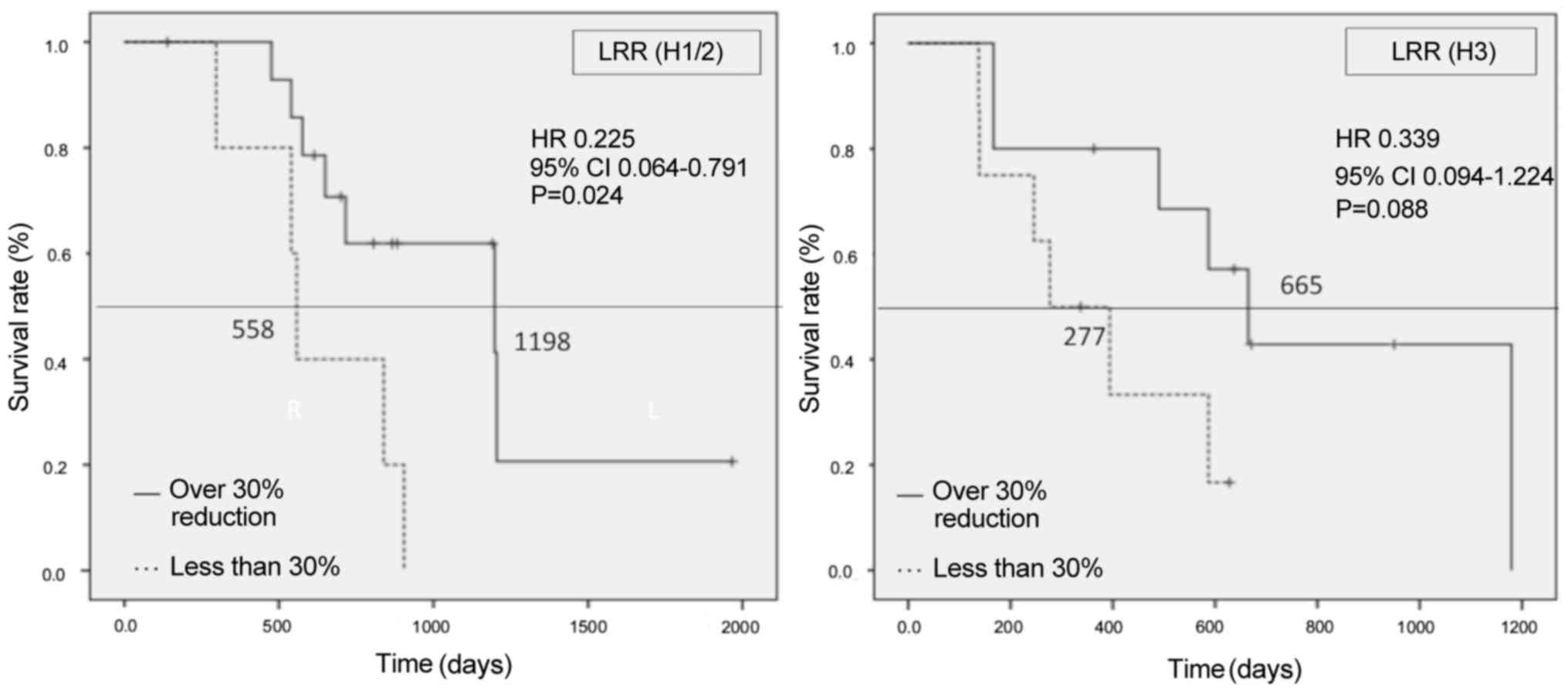
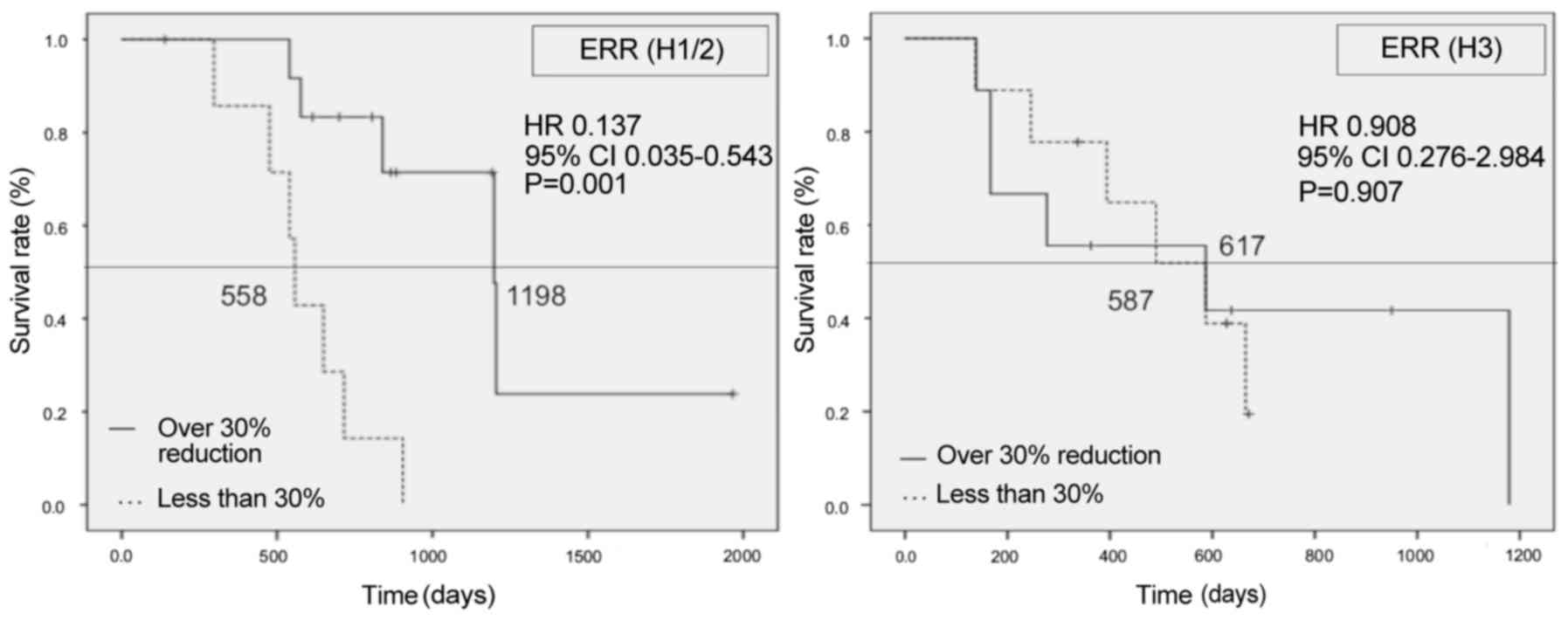
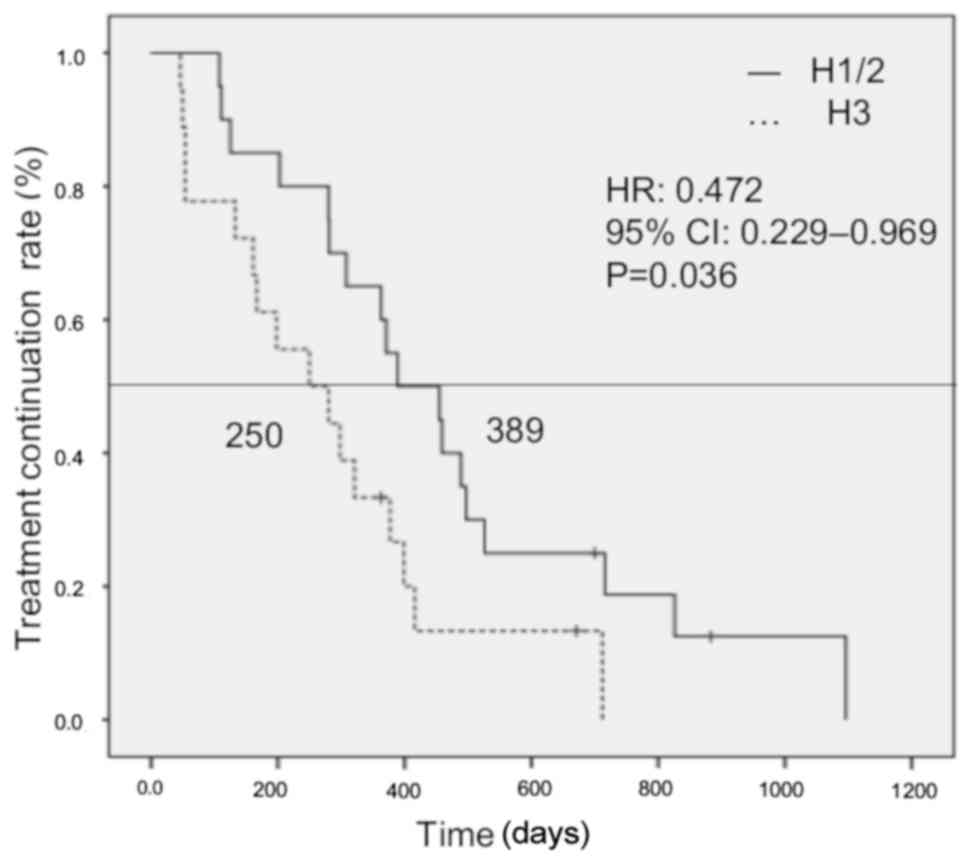
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of Worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Manfredi S, Lepage C, Hatem C, Coatmeur O,
Faivre J and Bouvier AM: Epidemiology and management of liver
metastases from colorectal cancer. Ann Surge. 244:254–259. 2006.
View Article : Google Scholar
|
|
4
|
Heinemann V, Stintzing S, Modest DP,
Giessen-Jung C, Michl M and Mansmann UR: Early tumor shrinkage
(ETS) and depth of response (DpR) in the treatment of patients with
metastatic colorectal cancer (mCRC). Eur J Cancer. 51:1927–1936.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schmoll HJ, Van Cutsem E, Stein A,
Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde
CJ, Balmana J, Regula J, et al: ESMO consensus guidelines for
management of patients with colon and rectal cancer. A personalized
approach to clinical decision making. Ann Oncol. 23:2479–2516.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Cutsem E, Dicato M, Arber N, Berlin J,
Cervantes A, Ciardiello F, De Gramont A, Diaz-Rubio E, Ducreux M,
Geva R, et al: Molecular markers and biological targeted in
metastasis colorectal cancer: Expert opinion and recommendations
derived from the 11th ESNO/World Congress on Gastrointestinal
Cancer, Barcelona, 2009. Ann Oncol. 21 (Suppl 6):vi1–10. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ye LC, Liu TS, Ren L, Wei Y, Zhu DX, Zai
SY, Ye QH, Yu Y, Xu B, Qin XY and Xu J: Randomized controlled trial
of cetuximab plus chemotherapy for patients with LRAS wild-type
unresectable colorectal liver-limited metastases. J Clin Oncol.
31:1931–1938. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Folprecht G, Gruenberger T, Bechstein WO,
Raab HR, Lordick F, Hartmann JT, Lang H, Frilling A, Stoehlmacher
J, Weitz J, et al: Tumor response and secondary resectability of
colorectal liver metastases following neoadjuvant chemotherapy with
cetuximab: The CELIM randomized phase II trial. Lancet Oncol.
11:38–47. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Van Cutsem E, Köhne CH, Hitre E, Zaluski
J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G,
et al: Cetuximab and chemotherapy as initial treatment for
metastatic colorectal cancer. N Engl J Med. 360:1408–1417. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bokemeyer C, Bondarenko I, Makhson A,
Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G,
Stroh C, et al: Fluororacil, leucovorin, and oxaliplatin with and
without cetuximab in the first line treatment of metastatic
colorectal cancer. J Clin Oncol. 27:663–671. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saltz LB, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Bevacizumab in combination with oxaliplatin-based
chemotherapy as first-line therapy in metastatic colorectal cancer:
A randomized phase III study. J Clin Oncol. 26:2013–2019. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Douillard JY, Oliner KS, Siena S,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Panitumumab-FOLFOX4 treatment and RAS mutations
in colorectal cancer. N Engl J Med. 369:1023–1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluoriuracil, and
leucovolin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fakih MG: Metastatic colorectal cancer:
Current state and future directions. J Clin Oncol. 33:1809–1824.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller
C, Kahl C, Seipelt G, et al: FOLFIRI plus cetuximab versus FOLFIRI
plus bevacizumab as first-line treatment for patients with
metastatic colorectal cancer (FIRE-3): A randomized, open-label,
phase III trial. Lancet Oncol. 15:1065–1075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Osumi H, Matsusaka S, Suenaga M, Wakatsuki
T, Ogura M, Ozaka M, Shinozaki E, Chin K and Mizunuma N:
Quantitative analysis of the impact of deepness of response on
survival time following patients with metastatic colorectal cancer
treated by chemotherapy and anti-EGFR monoclonal antibodies. J Clin
Oncol 32 (3 Suppl). S4932014. View Article : Google Scholar
|
|
17
|
Peeters M, Price TJ, Cervantes A, Sobrero
A, Ducreux MP, André T, Lordick F, Punt CJA, Koukakis R, Terwey J
and van Custem E: Tumour shrinkage and response outcomes during
second-line panitumumab (pmab) + FOLFIRI vs FOLFIRI treatment. Ann
Oncol. 25 (Suppl 4):iv186–iv187. 2014. View Article : Google Scholar
|
|
18
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nordlinger B, Van Cutsem E, Gruenberger T,
Glimelius B, Poston G, Rougier P, Sobrero A and Ychou M; European
Colorectal Metastases Treatment Group; Sixth International
Colorectal Liver Metastases Workshop, : Combination of surgery and
chemotherapy and the role of targeted agents in the treatment of
patients with colorectal liver metastases: Recommendations from an
expert panel. Ann Oncol. 20:985–992. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adam R, Lucidi V and Bismuth H: Hepatic
colorectal metastases: Methods of improving resectability. Surge
Clin North Am. 84:659–671. 2004. View Article : Google Scholar
|
|
21
|
Pawlik TM and Choti MA: Surgical therapy
for colorectal metastases to the liver. J Gastrointest Surg.
11:1057–1077. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sharma S, Camci C and Jobbour N:
Management of hepatic metastasis from colorectal cancers: An
update. J Hepatobiliary Pancreat Surg. 15:570–580. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Himuro N, Minakata T, Oshima Y, Kataoka D,
Yamamoto S and Kadokura M: Prognostic indicators after resection of
pulmonary metastases from colon and rectal cancer. J Jpn Assoc
Chest Surg. 30:136–142. 2016. View Article : Google Scholar
|
|
24
|
Elias D, Sideris L, Pocard M, Ouellet JF,
Boige V, Lasser P, Pignon JP and Ducreux M: Results of R0 resection
for colorectal liver metastases associated with extrahepatic
disease. Ann Surg Oncol. 11:274–280. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adam R, de Gramont A, Figueras J, Kokudo
N, Kunstlinger F, Loyer E, Poston G, Rougier P, Rubbia-Brandt L,
Sobrero A, et al: Managing synchronous liver metastases from
colorectal cancer: A multidisciplinary international consensus.
Cancer Treat Rev. 41:729–741. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoshidome H, kimura F, Shimizu H, Ohysuka
M and Miyazaki M: Advances in surgical treatment for colorectal
liver metastases. Nippon Shokakibyo Gakkai Zasshi. 106:1438–1446.
2009.(In Japanese). PubMed/NCBI
|
|
27
|
Yang YY, Fleshman JW and Strasberg SM:
Detection and management of extrahepatic colorectal cancer in
patients with resectable liver metastases. J Gastrointest Surg.
11:929–944. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Adam R: Developing strategies for liver
metastases from colorectal cancer. Semin Oncol Apr 34 (2 Suppl 1).
S7–S11. 2007.
|
|
29
|
Lam VW, Spiro C, Laurence JM, Johnston E,
Hollands MJ, Pleass HC and Richardson AJ: A systematic review of
clinical response and survival outcomes of downsizing systemic
chemotherapy and rescue liver surgery in patients with initially
unresectable colorectal liver metastases. Ann Surg Oncol.
19:1292–1301. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Watanabe T, Itabashi M, Shimada Y, Tanaka
S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, et
al: Japanese society for cancer of the colon and rectum (JSCCR)
guidelines 2014 for treatment of colorectal cancer. Int J Clin
Oncol. 20:207–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Borner MM: Neoadjuvant chemotherapy for
unresectable liver metastases of colorectal cancer-too good to be
true? Ann Oncol. 10:623–626. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cummings LC, Payes JD and Cooper GS:
Survival after hepatic resection in metastatic colorectal cancer: A
population-based study. Cancer. 109:718–726. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bogaerts J, Ford R, Sargent D, Schwartz
LH, Rubinstein L, Lacombe D, Eisenhauer E, Verweij J and Therasse
P; RECIST Working Party, : Individual patient data analysis to
assess modifications to the RECIST criteria. Eur J Cancer.
45:248–260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yamaguchi T, Mori T, Takahashi K,
Matsumoto H, Miyamoto H and Kato T: A new classification system for
liver metastases from colorectal cancer in Japanese multicenter
analysis. Hepatogastroenterology. 55:173–178. 2008.PubMed/NCBI
|
|
35
|
Cassidy J, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Randomized phase III study of capecitabine plus
oxaliplatin compared with fluorouracil/folinic acid plus
oxaliplatin as first-line therapy for metastatic colorectal cancer.
J Clin Oncol. 26:2006–2012. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Klinger M, Tamandl D, Eipeldauer S, Hacker
S, Herberger B, Kaczirek K, Dorfmeister M, Gruenberger B and
Gruenberger T: Bevacizumab improves pathological response of
colorectal cancer liver metastases treated with XELOX/FOLFOX. Ann
Surge Oncol. 17:2059–2065. 2010. View Article : Google Scholar
|
|
37
|
Zorzi D, Chun YS, Madoff DC, Abdalla EK
and Vauthey JN: Chemotherapy with bevacizumab does not affect liver
regeneration after portal vein embolization in the treatment of
colorectal liver metastases. Ann Surg Oncol. 15:2765–2772. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Veen T and Søreide K: Can molecular
biomarkers replace a clinical risk score for resectable colorectal
liver metastasis? World J Gastrointest Oncol. 9:98–104. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brudvik KW, Kopetz SE, Li L, Conrad C,
Aloia TA and Vauthey JN: Meta-analysis of KRAS mutations and
survival after resection of colorectal liver metastases. Br J Surg.
102:1175–1183. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Passot G, Denbo JW, Yamashita S, Kopetz
SE, Chun YS, Maru D, Overman MJ, Brudvik KW, Conrad C, Aloia TA and
Vauthey JN: Is Hepatectomy justified for Patients with RAS mutant
colorectal liver metastases? An analysis of 524 patients undergoing
curative liver resection. Surgery. 161:332–340. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bokemeyer C, Kohne C, Rougier P, Stroh C,
Schlichting M and Van Cutsem E: Cetuximab with chemotherapy (CT) as
first-line treatment for metastatic colorectal cancer (mCRC):
Analysis of the CRYSTAL and OPUS studies according to KRAS and BRAF
mutation status. (ASCO Annual Meeting, abstract no. 3506). J Clin
Oncol. 28:15s2010. View Article : Google Scholar
|
|
42
|
Maughan TS, Adams RA, Smith CG, Meade AM,
Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL,
et al: Addition of cetuximab to oxaliplatin-based first-line
combination chemotherapy for treatment of advanced colorectal
cancer: Results of the randamised phase 3 MRC COIN trial. Lancet.
377:2103–2114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Odisio BC, Yamashita S, Huang SY, Harmoush
S, Kopetz SE, Ahrar K, Shin Chun Y, Conrad C, Aloia TA, Gupta S, et
al: Local tumour progression after percutaneous ablation of
colorectal liver metastases according to RAS mutation status. Br J
Surge. 104:760–768. 2017. View Article : Google Scholar
|
|
44
|
Vauthey JN, Zimmitti G, Kopetz SE, Shindoh
J, Chen SS, Andreou A, Curley SA, Aloia TA and Maru DM: RAS
mutation status predicts survival and patterns of recurrence in
patients undergoing hepatectomy for colorectal liver metastases.
Ann Surg. 258:619–626; discussion 626–627. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Margonis GA, Kim Y, Spolverato G, Ejaz A,
Gupta R, Cosgrove D, Anders R, Karagkounis G, Choti MA, Pawlik TM,
et al: Association between specific mutations in KRAS Codon
12 and colorectal liver metastasis. JAMA Surg. 150:722–729. 2015.
View Article : Google Scholar : PubMed/NCBI
|