Introduction
The incidence and mortality rates of colorectal
cancer (CRC) vary markedly worldwide. Globally, CRC is the third
most commonly diagnosed cancer in men and the second in women
(1), with 1.8 million new cases and
almost 861,000 deaths in 2018 according to the World Health
Organization GLOBOCAN database (2,3).
Approximately 25% of patients with CRC have
metastatic disease, with a clinically significant detrimental
effect on prognosis (4,5). With the administration of chemotherapy
and molecular targeted therapy, the median overall survival (OS)
time of metastatic CRC (mCRC) has improved from 12 to 34.9 months
(6,7). However, mCRC carries a poor prognosis
and cannot be cured with the currently available therapy options.
Chemotherapy designed to prolong survival and improve the quality
of life of patients is the mainstay of treatment (8).
TAS-102 (trifluridine and tipiracil hydrochloride, a
novel combination oral nucleoside antitumor agent) was first
approved in Japan in March 2014 and received US Food and Drug
Administration (FDA) approval in September 2015, after an
international phase III clinical trial in patients with refractory
metastatic colon cancer demonstrated an OS benefit with TAS-102
compared with placebo (9).
Bevacizumab is a selective monoclonal antibody
inhibitor of vascular endothelial growth factor (VEGF)-A; it was
FDA-approved for mCRC in 2005, after showing efficacy in
combination with 5-fluorouracil (5-FU)-based chemotherapy (10,11). The
combination regimen of a fluoropyrimidine with bevacizumab in mCRC
in the first-line setting was established on the basis of high
clinical effectiveness and no overlapping toxicity between agents.
Similarly, combining TAS-102 with bevacizumab may be beneficial.
Evidence of the activity of TAS-102 plus bevacizumab in mCRC was
reported in a phase I/II trial of the C-TASK FORCE study (12). In addition, in a phase II/III trial
of the TRUSTY study was initiated in 2017 and the study is
anticipated to be completed in 2022 (13). The aim of the present cohort study
was to evaluate the benefits of using the modified Glasgow
Prognostic Score (mGPS) as an inflammatory index and the
combination of TAS-102 with bevacizumab as salvage-line
treatment.
Patients and methods
Patients
This study included 17 patients with unresectable
mCRC who were confirmed to have the wild-type or mutant RAS gene.
The patients received salvage-line treatment with TAS-102 plus
bevacizumab at the Surgical Oncology Department of Gifu University
School of Medicine between March 2016 and August 2018. The level 1
dose was TAS-102 (Taiho Pharmaceutical Co., Ltd.) at 35
mg/m2 body surface area, administered orally twice per
day on days 1–5 and 8–12 of a 28-day cycle, plus bevacizumab at 5
mg/kg body weight, administered by intravenous infusion for 30 min
every 2 weeks. Tumor shrinkage in the 17 patients was evaluated
according to the Response Evaluation Criteria in Solid Tumors
(RECIST) version 1.1, based on the dose-limiting toxicity observed
in each cycle. The demographic and disease characteristics of the
patients were also recorded. On the basis of the results of
previous clinical studies (9),
bevacizumab plus standard regimens, such as FOLFOX, CAPOX and
FOLFIRI, is currently recommended as first-line treatment for mCRC
(Table I).
 | Table I.Baseline characteristics (before
salvage-line treatment). |
Table I.
Baseline characteristics (before
salvage-line treatment).
| Previous chemotherapy
and reason for discontinuation | All patients (n=17),
no. (%) |
|---|
| Fluoropyrimidine |
|
|
Refractory | 17 (100) |
|
Intolerant | 0 |
| Oxaliplatin |
|
|
Refractory | 16 (94) |
|
Intolerant | 1 (6) |
| Irinotecan |
|
|
Refractory | 15 (88) |
|
Intolerant | 2 (12) |
| Angiogenesis
inhibitor |
|
|
Refractory | 17 (100) |
|
Intolerant | 0 |
| Anti-EGFR
antibody |
|
|
Refractory | 8 (47) |
|
Intolerant | 1 (6) |
| RAS mutational
status |
|
|
Wild-type | 9 (53) |
|
Mutant | 8 (47) |
Written informed consent was obtained from all
patients enrolled in the present study. The study protocol
conformed to the ethical guidelines of the 1975 Declaration of
Helsinki and the guidelines of the regional ethical committees of
Zurich and Basel, Switzerland, and was approved by the
Institutional Review Board of the Gifu University Graduate School
of Medicine (no. 28-196).
RECIST 1.1 guideline
Computed tomography (CT) is currently considered the
best and most reproducible method for measuring lesions when
assessing response. The RECIST guideline defines measurability of
lesions on CT scan based on a slice thickness of ≤5 mm. When the CT
slice thickness is >5 mm, the minimum size of a measurable
lesion must be at least twice the slice thickness.
Evaluation of target lesions
Complete response (CR) is defined as disappearance
of all target lesions. Any pathological lymph nodes (whether target
or non-target) must display a reduction in the short axis to <10
mm. Partial response (PR) is defined as at least a 30% decrease in
the sum of the diameters of the target lesions as compared with the
baseline sum diameters. Progressive disease (PD) is defined as at
least a 20% increase in the sum of the diameters of the target
lesions compared with the smallest sum in the study (this includes
the baseline sum, if that is the smallest). In addition to the
relative increase of 20%, the sum must also exhibit an absolute
increase of at least 5 mm (of note, the appearance of one or more
new lesions is also considered as progression). Finally, stable
disease (SD) is defined as neither sufficient shrinkage to qualify
for PR, nor sufficient increase in size to qualify for PD as
compared with the smallest sum diameters in the study.
mGPS
The GPS has been reported to be a useful
inflammatory index for assessing the status of cachexia. This score
is composed of C-reactive protein (CRP) levels to reflect the
systemic inflammation status, and serum albumin levels to reflect
the nutritional status (14). At
present, mGPS is widely used to classify patients into three
groups, namely 0, 1 and 2. mGPS was calculated as follows: 0, CRP
≤1.0 mg/dl; 1, CRP >1.0 mg/dl; and 2, CRP >1.0 mg/dl and
albumin <3.5 mg/dl (15).
Statistical analysis
All data are presented as the mean ± standard
deviation. The Student's t-test, Wilcoxon's signed-rank test,
Kaplan-Meier method, log-rank test, and Pearson's product-moment
correlation coefficient were used to evaluate the data and
determine statistical significance. P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses were performed with SPSS 11.5J software (SPSS Japan,
Inc.).
Results
Study population
The present study included 17 patients with mCRC (12
men and 5 women; mean age, 60.4±13.4 years; range, 41–81 years),
all of whom had an Eastern Cooperative Oncology Group performance
status of 0–2. The study population was heavily pretreated: The
majority (71%) had received ≥4 prior regimens and, in addition to
fluoropyrimidine, irinotecan and oxaliplatin, all had received
bevacizumab (100%) and either cetuximab or panitumumab (47%). The
RAS gene mutation status was determined for all 17 patients: 9
(53%) had wild-type and 8 (47%) had mutant RAS (Table II). All 17 patients received TAS-102
plus bevacizumab at the dosages described in Patients and methods.
All patient had undergone primary tumor resection prior to
salvage-line treatment.
 | Table II.Patient characteristics. |
Table II.
Patient characteristics.
|
Characteristics | All patients
(n=17), no. (%) |
|---|
| Age (years), mean ±
SD | 60.4±13.4 |
| Sex |
|
|
Male | 12 (71) |
|
Female | 5 (29) |
| ECOG performance
status |
|
| 0 | 4 (23) |
| 1 | 10 (59) |
| 2 | 3 (18) |
| Primary site |
|
|
Right-sided colon | 5 (29) |
|
Left-sided colorectum | 12 (71) |
| Number of
metastatic organs/sites |
|
| 1 | 3 (18) |
| ≥2 | 14 (82) |
| Metastatic
organ |
|
|
Liver | 15 (88) |
|
Lung | 13 (76) |
| Lymph
nodes | 7 (41) |
|
Peritoneum | 5 (29) |
| Pathology |
|
|
Tub1 | 3 (18) |
|
Tub2 | 14 (82) |
Adverse events (classified according to the National
Cancer Institute Common Terminology Criteria for Adverse Events,
version 5.0) were observed in all 17 patients (16). Grade 3/4 toxicity was predominantly
hematological, consisting mostly of neutropenia (41%), leukopenia
(11%) and anemia (5%). Febrile neutropenia was not observed in any
of the patients. Non-hematological toxicity consisted mainly of
fatigue, nausea, proteinuria and anorexia, but it was rarely grade
≥3. There were no reported treatment-related deaths (Table III).
 | Table III.Frequency of adverse events and
laboratory abnormalities. |
Table III.
Frequency of adverse events and
laboratory abnormalities.
|
| TFTH + BV, n=17
(%) |
|---|
|
|
|
|---|
| Adverse events | All grade, n
(%) | Grade 3/4, n
(%) |
|---|
| Hematological |
|
|
|
Neutropenia | 11 (64) | 7 (41) |
|
Leukopenia | 11 (64) | 1 (11) |
|
Anemia | 8 (47) | 1 (5) |
|
Non-hematological |
|
|
|
Increased blood bilirubin | 7 (41) | 1 (5) |
|
Increased ALT | 7 (41) | 1 (5) |
|
Increased AST | 6 (35) | 1 (5) |
|
Increased ALP | 6 (35) | 1 (5) |
| Febrile
neutropenia | 0 (0) | 0 (0) |
|
Hypertension | 5 (29) | 1 (5) |
|
Anorexia | 7 (41) | 0 (0) |
| Oral
mucositis | 2 (11) | 0 (0) |
|
Proteinuria | 9 (52) | 0 (0) |
|
Nausea | 8 (47) | 0 (0) |
|
Diarrhea | 8 (47) | 0 (0) |
|
Vomiting | 2 (11) | 1 (5) |
|
Fatigue | 10 (58) | 0 (0) |
|
Fever | 2 (11) | 1 (5) |
| Skin
rash | 6 (35) | 0 (0) |
|
Epistaxis | 5 (29) | 0 (0) |
The first staging imaging scans (~after 2 cycles of
therapy) were available for review in all 17 patients. At the first
evaluation, 5 (29%) patients had PD, 12 (71%) patients had SD,
whereas none had PR to TAS-102 plus bevacizumab (Table IV). The respective median OS and
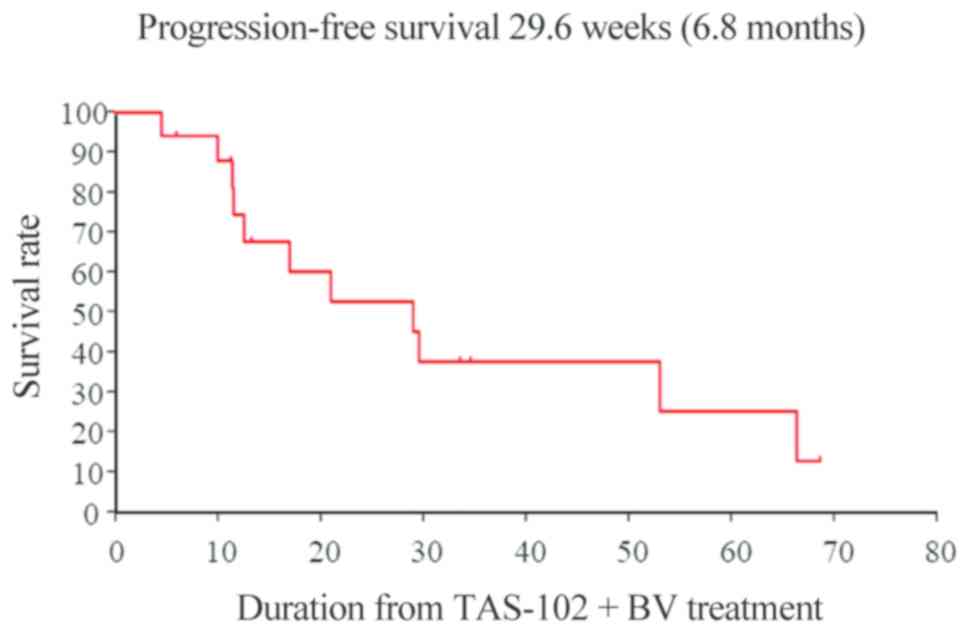
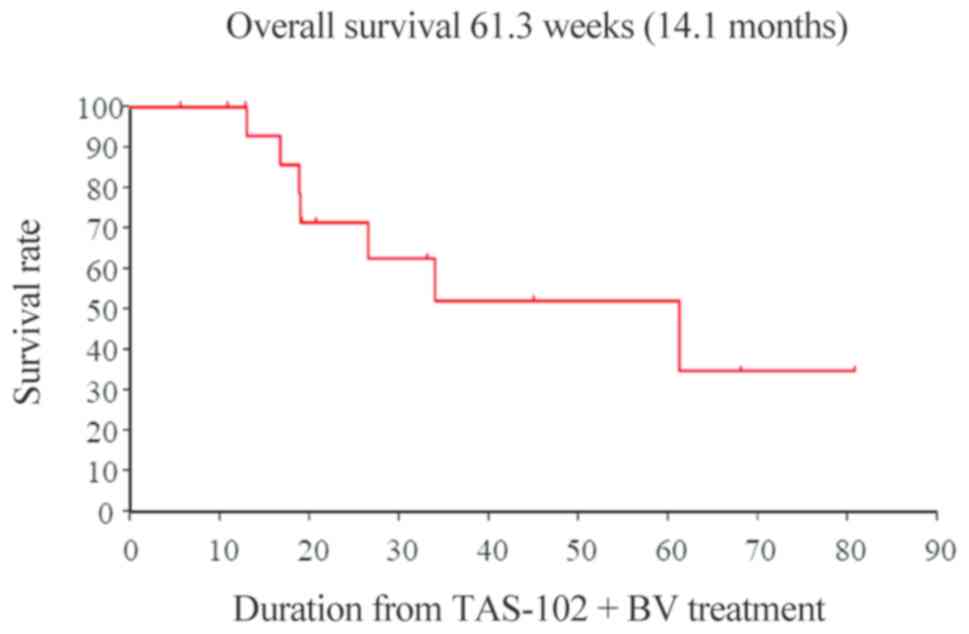
progression-free survival (PFS) times were 14.1 and 6.8 months,
respectively, which were somewhat better compared with the
respective 11.2 and 5.6 months reported in the C-TASK FORCE study
(12) (Figs. 1 and 2).
 | Table IV.Best response to treatment. |
Table IV.
Best response to treatment.
| Response to
treatment | TFTH + BV, n=17
(%) |
|---|
| Complete response
(CR) | 0 (0) |
| Partial response
(PR) | 0 (0) |
| Stable disease
(SD) | 12 (71) |
| Progressive disease
(PD) | 5 (29) |
| Not evaluable
(NE) | 0 (0) |
| Overall response
(CR + PR) | 0 (0) |
| Disease control (CR
+ PR + SD) | 12 (71) |
Furthermore, patients with mCRC receiving
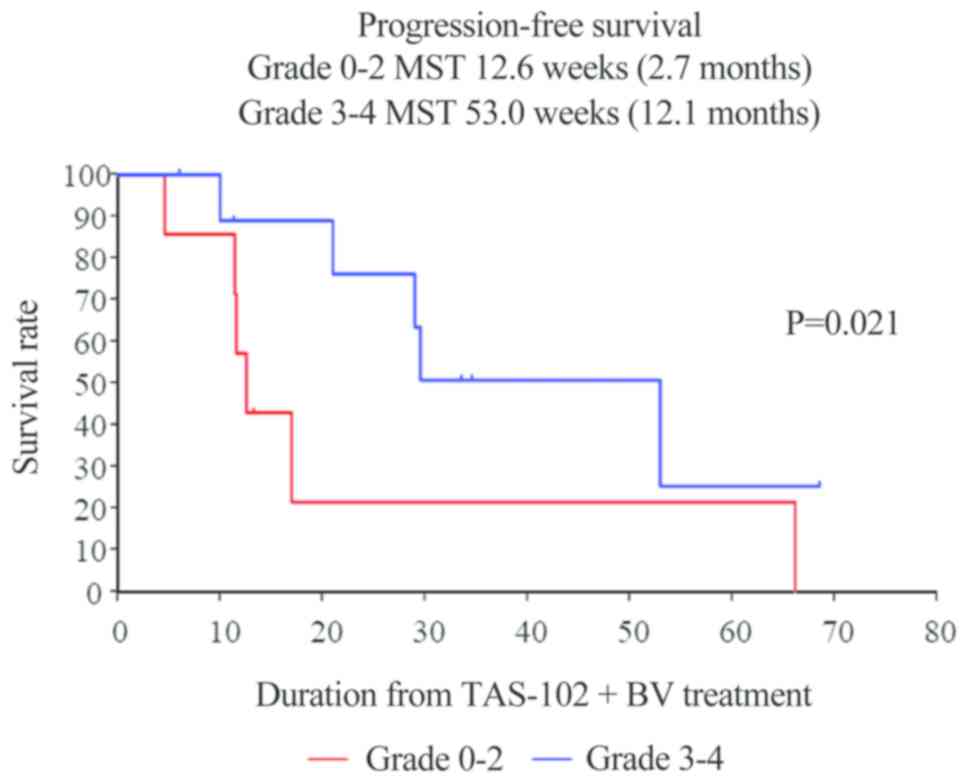
salvage-line therapy who developed chemotherapy-induced neutropenia
at 1 month (CIN-1-month) had significantly improved PFS (grade 0–2
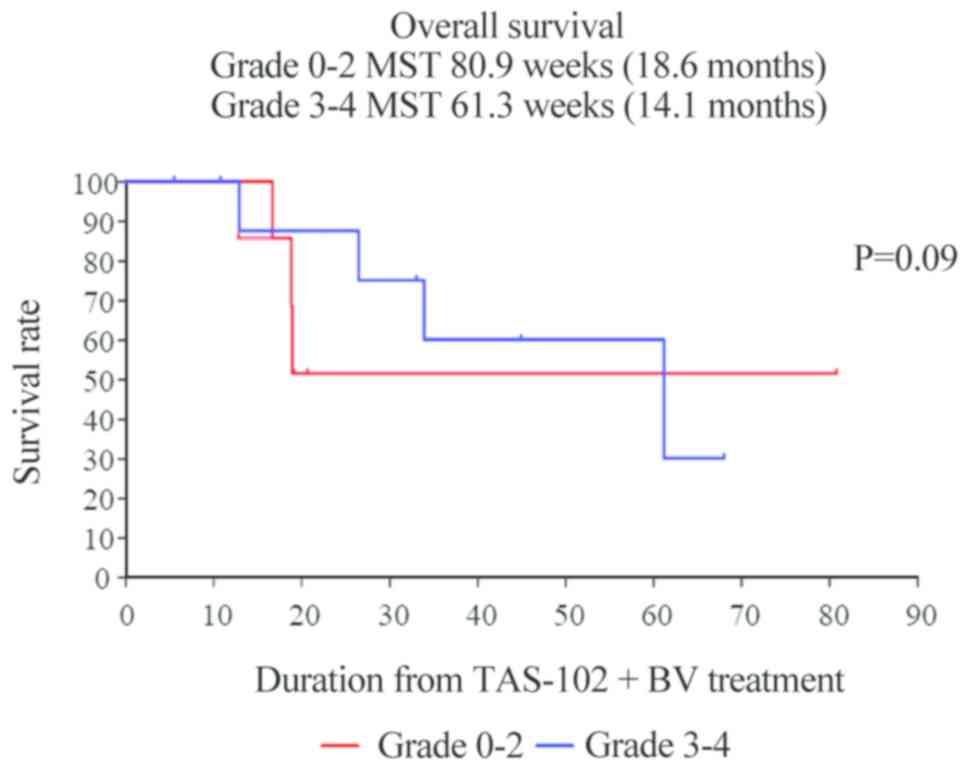
vs. 3–4: 2.7 vs. 12.1 months, respectively; P=0.021) (Fig. 3). Unfortunately, individuals who
developed CIN-1-month exhibited no significant improvement in OS
(Fig. 4).
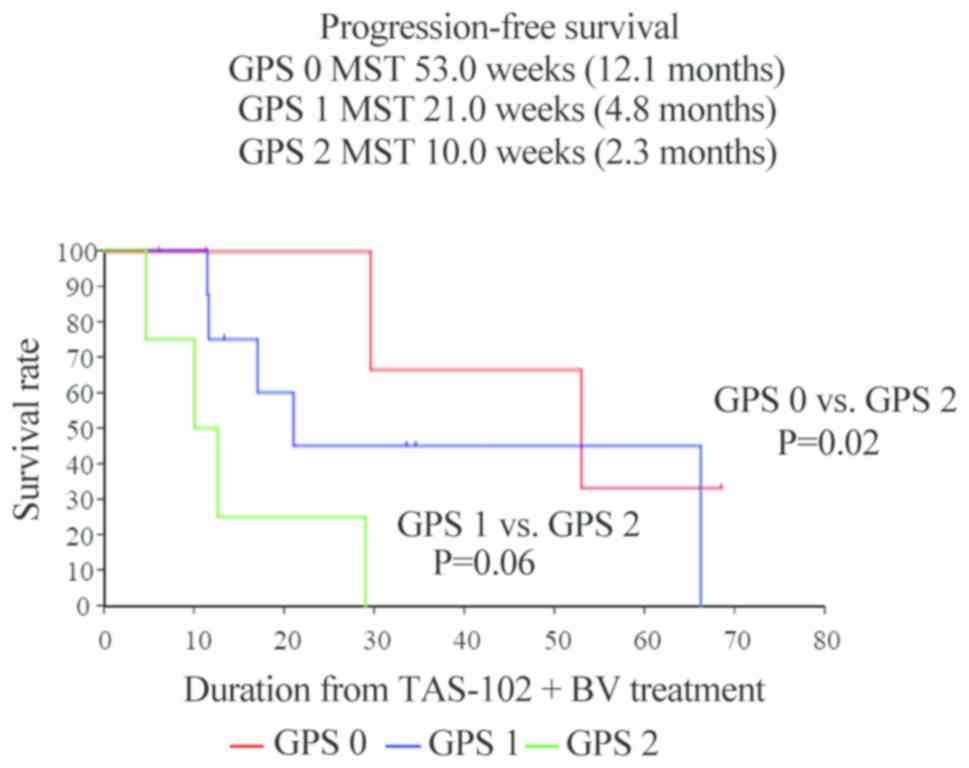
The median PFS was significantly different among the
three mGPS groups (mGPS 0 vs. mGPS 2, P=0.02; mGPS 1 vs. mGPS 2,
P=0.06). The median PFS in the mGPS 0, 1 and 2 groups was 12.1, 4.8
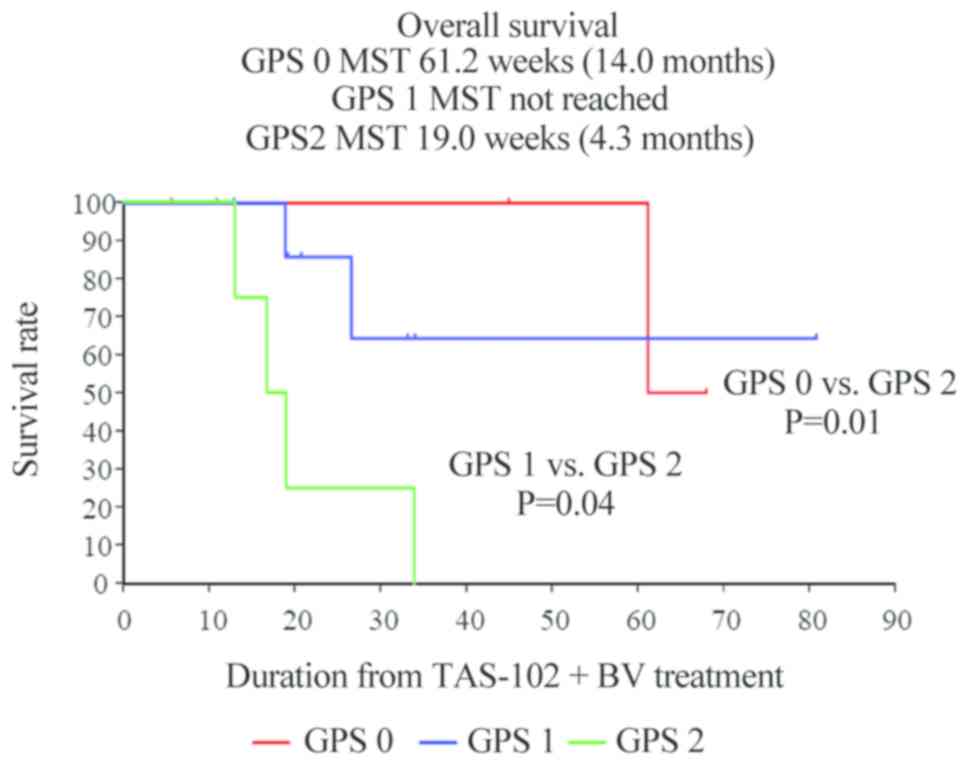
and 2.3 months, respectively. The median OS was also significantly
among the three groups (mGPS 0 vs. mGPS 2, P=0.01; mGPS 1 vs. mGPS
2, P=0.04). The median OS in the mGPS 0, 1 and 2 groups was 14.0,
not reached, and 4.3 months, respectively (Figs. 5 and 6).
Discussion
Recently, chemotherapy for CRC has markedly
progressed. In particular, the treatment for advanced or metastatic
CRC has significantly improved due to the development of the FOLFOX
and FOLFIRI regimens. Furthermore, the introduction of targeted
therapy has further increased the effectiveness of CRC treatment
(17–20).
The development of molecular targeted agents has
contributed to the prolongation of mCRC patient survival. One
anti-VEGF agent, bevacizumab, and two anti-epidermal growth factor
receptor (EGFR) agents, cetuximab and panitumumab, have shown
clinical benefits in the first-, second- and salvage-line setting
in combination with cytotoxic chemotherapy. Moreover, RAS mutations
have been proven to be a negative biomarker for anti-EGFR therapy
in recent retrospective analyses (6,21).
As salvage-line chemotherapy, regorafenib was the
first small-molecule multikinase inhibitor to offer a survival
benefit in mCRC that has progressed after all standard therapies
have been exhausted. In the CORRECT study, no patients achieved a
CR; however, 5 patients receiving regorafenib and 1 patient
assigned to placebo exhibited a PR, with objective response rates
of 1.0 and 0.4%, respectively (P=0.19). As CR and PR were obtained
in only a few patients, regorafenib is unlikely to fully achieve a
complete or partial disease response. However, disease control was
achieved in 41% of the patients assigned regorafenib and in 15% of
the patients assigned placebo (P<0.0001). The median duration of
SD was 2.0 months in the regorafenib group and 1.7 months in the
placebo group (22).
In salvage-line treatment of mCRC, TAS-102 was
reported to significantly improve OS compared with placebo in the
RECOURSE trial. In the tumor-response population (502 patients in
the TAS-102 group and 258 in the placebo group), 8 patients in the
TAS-102 group had a PR, and 1 patient in the placebo group had a
CR, resulting in objective response rates of 1.6% with TAS-102 and
0.4% with placebo (P=0.29). Disease control (CR, PR or SD, assessed
at least 6 weeks after randomization) was achieved in 221 patients
(44%) in the TAS-102 group and 42 patients (16%) in the placebo
group (P<0.001). Patients in the TAS-102 group received the
study drug for a mean ± standard deviation of 12.7±12.0 weeks
(median, 6.7; range, 0.1–78.0 weeks), and patients in the placebo
group received the study drug for a mean of 6.8±6.1 weeks (median,
5.7; range, 0.1–63.7 weeks). The assessment of tumor status with
regard to KRAS demonstrated that 49% of the patients had wild-type
tumors and 51% had mutant tumors. A benefit from treatment with
TAS-102 was observed in both patient subgroups (20). In the CORRECT trial,
treatment-related adverse events occurred in 93% patients receiving
regorafenib and in 61% of those receiving placebo. The most common
adverse events of grade ≥3 associated with regorafenib were
hand-foot skin reaction (17%), fatigue (10%), diarrhea (n=36, 7%),
hypertension (n=36, 7%), and rash or desquamation (n=29, 6%)
(22).
Although regorafenib and TAS-102 are effective as
salvage-line therapies, OS and PFS remain unsatisfactory with these
treatments. Recently, the phase I/II C-TASK FORCE trial was
conducted to investigate the efficacy and safety of TAS-102 plus
bevacizumab in the salvage-line setting. The median OS was 11.2
months and the PFS was 5.6 months. In addition, although the
relative risk was only 4.0%, the disease control rate was 72%, with
tolerable toxicity. However, the sample size was quite small
(n=25). The assessment of tumor status with regard to KRAS revealed
that 49% of the patients had wild-type and 51% had mutant tumors. A
benefit from treatment with TAS-102 was also observed in both
patient subgroups (12).
At present, there are almost no reports on the
antitumor effect of combined TAS-102 and bevacizumab. Tsukihara
et al evaluated the mechanism underlying the enhanced
antitumor effect of combined TAS-102 and bevacizumab (23). They measured trifluridine (FTD) and
its phosphorylated forms in tumors, as these are the active
components and metabolites of TAS-102. Phosphorylated FTD levels
were increased by combining TAS-102 and bevacizumab in both SW48
and HCT116 tumors (23). In
addition, Jain et al reported that tumor blood vessels are
generally poorly organized and hyperpermeable, with an impaired
gradient between the vascular and interstitial pressure and,
consequently, offer a diminished blood supply (24). They further reported that these
vessels may also limit the accumulation of FTD in tumors.
Bevacizumab inhibits angiogenesis by antagonizing VEGF, and may
therefore normalize tumor vasculature, thus improving tumor blood
supply and increasing FTD accumulation and its subsequent
phosphorylation in the tumor.
The combination regimen of a fluoropyrimidine with
bevacizumab in mCRC in the first-line setting was established on
the basis of high clinical effectiveness and no overlapping
toxicity between agents. It is considered that sustained VEGF
inhibition achieves and maintains tumor regression. The addition of
bevacizumab, aflibercept, or ramucirumab to chemotherapy has shown
a survival benefit in mCRC in the second-line setting (25,26).
However, little is known on the efficacy and safety of continued
administration of angiogenesis inhibitors with cytotoxic
chemotherapy beyond progression after second-line therapy.
Bevacizumab combined with a chemotherapy regimen has a fully
manageable toxicity profile. From these viewpoints, combining
TAS-102 with bevacizumab may prove to be beneficial.
Kasi et al reported that CIN-1-month after
starting TAS-102 appears to be a prognostic and/or predictive
biomarker of both PFS and OS in patients with mCRC undergoing
salvage-line therapy (26). In the
present study, patients who developed CIN-1-month had significantly
improved PFS (grade 0–2 vs. 3–4: 2.7 vs. 12.1 months, respectively;
P=0.021), although OS was not significantly improved (data not
shown).
Parallel to the increase in the serum CRP level,
hypoalbuminemia has been observed in various types of tumors and
verified as a negative prognostic factor (27–29). The
association between hypoalbuminemia and reduced survival in
patients with cervical cancer is affected by several factors.
Hypoalbuminemia reflects a progressive nutritional decline in
cancer patients, and is associated with cancer-related cachexia
(30).
The GPS was shown to be a powerful prognostic
factor. This cumulative prognostic score is based on pre-treatment
values of CRP and albumin, is objective, and has shown superior
prognostic value in various cancers. A correlation between
preoperative GPS and CRC survival has been reported (31). In the same manner, GPS was shown to
be a powerful prognostic factor in cancer patients, irrespective of
tumor site (32). Whether the
prognostic value of the GPS is modified by the addition of
non-cytotoxic agents with high activity and tolerable toxicity,
such as salvage-line TAS-102 plus bevacizumab, remains unknown. In
our experience, mGPS allowed prediction of PFS and OS in patients
with mCRC receiving salvage-line therapy, such as TAS-102 plus
bevacizumab.
In the present study, the median PFS was
significantly different for each factor of CIN-1 and each mGPS
group, whereas the median OS was not significantly different.
However, in a study of this sample size, it may be concluded that
this result is important as real world data in future prospective
studies. mGPS as an inflammatory index is also a useful predictor
marker in the salvage-line setting.
The results of the present study have shown that in
salvage-line therapy for patients with mCRC, bevacizumab enhances
the antitumor effect of TAS-102, with superior median OS and PFS
times compared with those reported in the C-TASK FORCE study.
In conclusion, a clinical study of combined TAS-102
and bevacizumab therapy is currently in phase II and phase III, and
we expect its outcome to be highly informative. Although the sample
size of the present study was small, the findings confirm the
prognostic accuracy of the mGPS score in salvage-line therapy for
patients with mCRC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of materials and data
All the datasets generated and analyzed in the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
NM, HF and TT conceived and designed the study. NM,
TT, HF, TS, MF, YI, YT, RM, ToT, SM, HI, YT, KYa, MF and KYo
acquired the data. NM analyzed and interpreted the data and drafted
the manuscript. NM, TT and KYo performed critical revision of the
manuscript. KYo supervised the study. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1975
Helsinki declaration and its later amendments or comparable ethical
standards. Informed consent was obtained from all individual
participants included in the study.
Patient consent for publication
Not applicable.
Competing interests
K. Yoshida has received honoraria for lectures from
Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd.,
Takeda Pharmaceutical Co., Ltd., Eli Lilly and Company, Daiichi
Sankyo Co., Ltd., Ono Pharmaceutical Co., Ltd., Merck Serono Co.,
Ltd., Novartis Pharma K.K., and Sanofi K.K.; and research funding
from Ajinomoto Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co.,
Ltd., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd.,
Taiho Pharmaceutical Co., Ono Pharmaceutical Co., and Yakult Honsha
Co., Ltd. outside the submitted work.
T. Takahashi has received honoraria for lectures
from Takeda Pharmaceutical Co., Ltd. All remaining authors declare
that they have no conflicts of interest.
References
|
1
|
Center MM, Jemal A and Ward E:
International trends in colorectal cancer incidence rates. Cancer
Epidemiol Biomarkers Prev. 18:1688–1694. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheng AL, Li J, Vaid AK, Ma BB, The C, Ahn
JB, Bello M, Charoentum C, Chen LT, de Lima Lopes G Jr, et al:
Adaptation of international guidelines for metastatic colorectal
cancer: An Asian consensus. Clin Colorectal Cancer. 13:145–155.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancersin 185
countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lemmens V, van Steenbergen L,
Janssen-Heijnen M, Martijn H, Rutten H and Coebergh JW: Trends in
colorectal cancer in the south of the Netherlands 1975–2007: rectal
cancer survival levels with colon cancer survival. Acta Oncol.
49:784–796. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Cutsem E, Borràs JM, Castells A,
Ciardiello F, Ducreux M, Haq A, Schmoll HJ and Tabernero J:
Improving outcomes in colorectal cancer: where do we go from here?
Eur J Cancer. 49:2476–2485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Cutsem E, Köhne CH, Hitre E, Zaluski
J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G,
et al: Cetuximab and chemotherapy as initial treatment for
metastatic colorectal cancer. N Engl J Med. 360:1408–1417. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamada Y, Denda T, Gamoh M, Iwanaga I,
Yuki S, Shimodaira H, Nakamura M, Yamaguchi T, Ohori H, Kobayashi
K, et al: S-1 and irinotecan plus bevacizumab vs. mFOLFOX6 or
CapeOX plus bevacizumab as first-line treatment in patients with
metastatic colorectal cancer (TRICOLORE): A randomized, open-label,
phase III, noninferiority trial. Ann Oncol. 29:624–631. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Van Cutsem E, Cervantes A, Adam R, Sobrero
A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson
A, Bodoky G, et al: ESMO consensus guidelines for the management of
patients with metastatic colorectal cancer. Ann Oncol.
27:1386–1422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mayer RJ, Van Cutsem E, Falcone A, Yoshino
T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero
J, Komatsu Y, et al: RECOURSE Study Group. Randomized trial of
TAS-102 for refractory metastatic colorectal cancer. N Engl J.
372:1909–1919. 2015. View Article : Google Scholar
|
|
10
|
Melnyk O, Zimmerman M, Kim KJ and Shuman
M: Neutralizing anti-vascular endothelial growth factor antibody
inhibits further growth of established prostate cancer and
metastases in a pre-clinical model. J Urol. 161:960–963. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Klement G, Baruchel S, Rak J, Man S, Clark
K, Hicklin DJ, Bohlen P and Kerbel RS: Continuous low-dose therapy
with vinblastine and VEGF receptor-2 antibody induces sustained
tumor regression without overt toxicity. J Clin Invest.
105:R15–R24. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuboki Y, Nishina T, Shinozaki E, Yamazaki
K, Shitara K, Okamoto W, Kajiwara T, Matsumoto T, Tsushima T,
Mochizuki N, et al: TAS-102 plus bevacizumab for patients with
metastatic colorectal cancer refractory to standard therapies
(C-TASK FORCE): An investigator-initiated, open-label, single-arm,
multicentre, phase 1/2 study. Lancet Oncol. 18:1172–1181. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshino T, Oki E, Nozawa H,
Eguchi-Nakajima T, Taniguchi H, Morita S, Takenaka N, Ozawa D and
Shirao K: Rationale and design of the TRUSTY study: A randomised,
multicentre, open-label phase II/III study of
trifluridine/tipiracil plus bevacizumab versus irinotecan,
fluoropyrimidine plus bevacizumab as second-line treatment in
patients with metastatic colorectal cancer progressive during or
following first-line oxaliplatin-based chemotherapy. ESMO Open.
3:e0004112018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Douglas E and McMillan DC: Towards a
simple objective framework for the investigation and treatment of
cancer cachexia: The glasgow prognostic score. Cancer Treat Rev.
40:685–691. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takeno S, Hashimoto T, Shibata R, Maki K,
Shiwaku H, Yamana I, Yamashita R and Yamashita Y: The
high-sensitivity modified Glasgow prognostic score is superior to
the modified Glasgow prognostic score as a prognostic predictor in
patients with resectable gastric cancer. Oncology. 87:205–214.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Common Terminology Criteria for Adverse
Events (CTCAE) Version5.0 Published Nov 27, 2017.
|
|
17
|
Douillard JY, Cunningham D, Roth AD,
Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J,
Alakl M, et al: Irinotecan combined with fluorouracil compared with
fluorouracil alone as first-line treatment for metastatic
colorectal cancer: A multicentre randomised trial. Lancet.
355:1041–1047. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tournigand C, André T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et
al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: A randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Falcone A, Ricci S, Brunetti I, Pfanner E,
Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W,
Fanchini L, et al: Gruppo Oncologico Nord Ovest.Phase III trial of
infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan
(FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and
irinotecan (FOLFIRI) as first-line treatment for metastatic
colorectal cancer: The Gruppo Oncologico Nord Ovest. J Clin Oncol.
25:1670–1676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grothey A, Sargent D, Goldberg RM and
Schmoll HJ: Survival of patients with advanced colorectal cancer
improves with the availability of fluorouracil-leucovorin,
irinotecan, and oxaliplatin in the course of treatment. J Clin
Oncol. 22:1209–1214. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grothey A, Van Cutsem E, Sobrero A, Siena
S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et
al: Regorafenib monotherapy for previously treated metastatic
colorectal cancer (CORRECT): An international, multicenter,
randomised, placebo-controlled phase 3 trial. Lancet. 381:303–312.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsukihara H, Nakagawa F, Sakamoto K,
Ishida K, Tanaka N, Okabe H, Uchida J, Matsuo K and Takechi T:
Efficacy of combination chemotherapy using a novel oral
chemotherapeutic agent, TAS-102, together with bevacizumab,
cetuximab, or panitumumab on human colorectal cancer xenografts.
Oncol Rep. 33:2135–2142. 2015.PubMed/NCBI
|
|
24
|
Jain RK: Normalizing tumor vasculature
with anti-angiogenic therapy: A new paradigm for combination
therapy. Nat Med. 7:987–989. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Van Cutsem E, Tabernero J, Lakomy R,
Prenen H, Prausová J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko
V, Ferry D, et al: Addition of aflibercept to fluorouracil,
leucovorin, and irinotecan improves survival in a phase III
randomized trial in patients with metastatic colorectal cancer
previously treated with an oxaliplatin-based regimen. J Clin Oncol.
30:3499–3506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tabernero J, Yoshino T, Cohn AL,
Obermannova R, Bodoky G, Garcia-Carbonero R, Ciuleanu TE, Portnoy
DC, Van Cutsem E, Grothey A, et al: Ramucirumab versus placebo in
combination with second-line FOLFIRI in patients with metastatic
colorectal carcinoma that progressed during or after first-line
therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine
(RAISE): A randomised, double-blind, multicentre, phase 3 study.
Lancet Oncol. 16:499–508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kasi PM, Kotani D, Cecchini M, Shitara K,
Ohtsu A, Ramanathan RK, Hochster HS, Grothey A and Yoshino T:
Chemotherapy induced neutropenia at 1-month mark is a predictor of
overall survival in patients receiving TAS-102 for refractory
metastatic colorectal cancer: A cohort study. BMC Cancer.
16:4672016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McMillan DC: An inflammation-based
prognostic score and its role in the nutrition-based management of
patients with cancer. Proc Nutr Soc. 67:257–262. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Deans DA, Tan BH, Wigmore SJ, Ross JA, de
Beaux AC, Paterson-Brown S and Fearon KC: The influence of systemic
inflammation, dietary intake and stage of disease on rate of weight
loss in patients with gastro-oesophageal cancer. Br J Cancer.
100:63–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morley JE, Thomas DR and Wilson MM:
Cachexia: Pathophysiology and clinical relevance. Am J Clin Nutr.
83:735–743. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ishizuka M, Nagata H, Takagi K, Horie T
and Kubota K: Inflammation-based prognostic score is a novel
predictor of postoperative outcome in patients with colorectal
cancer. Ann Surg. 246:1047–1051. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Proctor MJ, Morrison DS, Talwar D, Balmer
SM, O'Reilly DS, Foulis AK, Horgan PG and McMillan DC: An
inflammation- based prognostic score (mGPS) predicts cancer
survival independent of tumour site: A glasgow inflammation outcome
study. Br J Cancer. 104:726–732. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Prager GW, Braemswig KH, Martel A, Unseld
M, Heinze G, Brodowicz T, Scheithauer W, Kornek G and Zielinski CC:
Baseline carcinoembryonic antigen (CEA) serum levels predict
bevacizumab-based treatment response in metastatic colorectal
cancer. Cancer Sci. 105:996–1001. 2014. View Article : Google Scholar : PubMed/NCBI
|