Introduction
Granulocyte colony-stimulating factor (G-CSF) is a
naturally occurring glycoprotein that stimulates the proliferation
of precursor cells in the bone marrow and their maturation into
fully differentiated neutrophils (1). The lung is the most common site of
G-CSF-producing cancers. In the digestive system, particularly the
pancreas, G-CSF-producing cancers are very rare, and there is no
standardized treatment for these cancers. The prognosis of patients
with these cancers has been reported to be very poor (2). Herein, we report a rare case of a
G-CSF-producing pancreatic carcinoma associated with severe anemia
due to bleeding in the duodenum, which was successfully treated
with surgery.
Case presentation
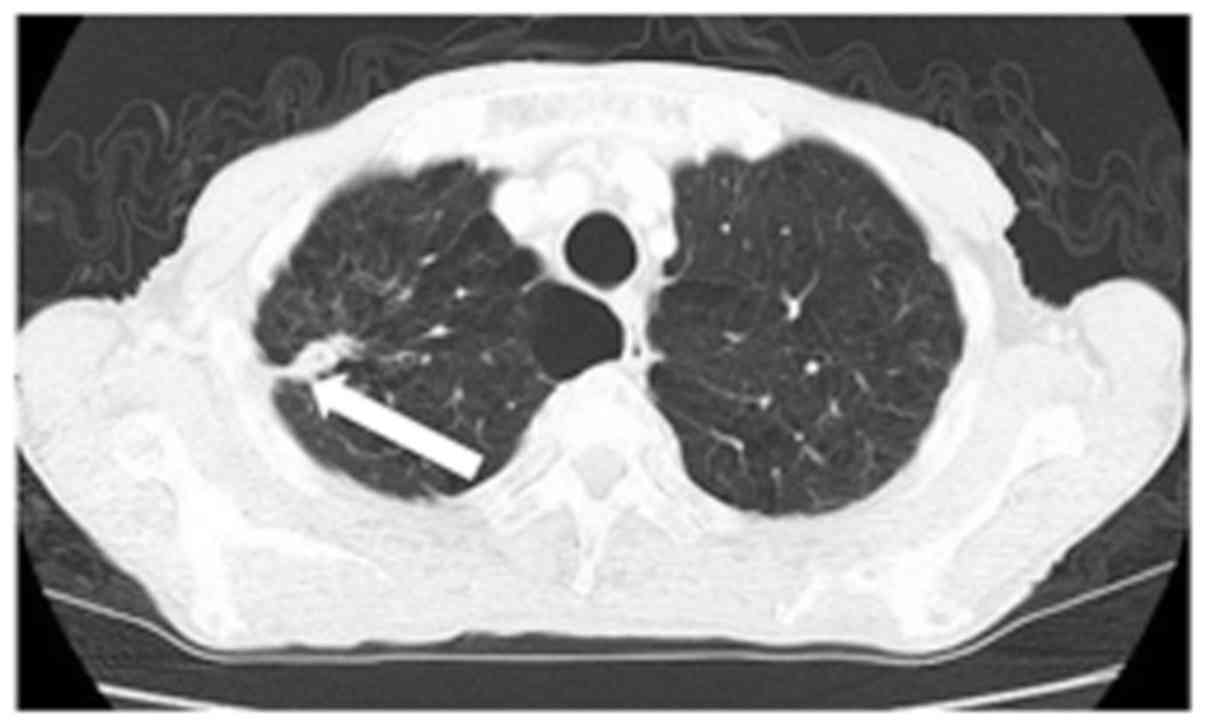
A 79-year-old man presented to another hospital with
slight fever. Contrast-enhanced computed tomography (CT) revealed a
40-mm tumor in the right lung field. Lung biopsy was performed, and
he was diagnosed with lung cancer. After a discussion regarding the
treatment options, he didn't want invasive treatment. He decided to
undergo radiotherapy (70 Gy) for 3 months, and the tumor size
decreased to 15 mm (Fig. 1). After 1
month, he underwent radiotherapy and experienced epigastralgia.
Laboratory tests showed abnormal values for white blood cell count
(12,700/µl; reference range, 3,500–9,800/µl), neutrophil percentage
(83.1%; reference range, 44–74%), hemoglobin level (6.3 g/dl;
reference range, 13.5–17.5 g/dl), and C-reactive protein (CRP)
level (14.2 mg/dl; reference range, 0–0.6 g/dl). He did not have
any signs of infection. Tumor maker levels were as follows:
Carcinoembryonic antigen, 5.9 ng/dl (upper reference limit, 5.0
ng/dl); s-pancreas-1 cancer antigen, 41.0 U/ml (upper reference
limit, 30.0 U/ml); squamous cell carcinoma antigen, 5.4 ng/ml
(upper reference limit, 1.5 ng/ml); and carbohydrate antigen 19–9,
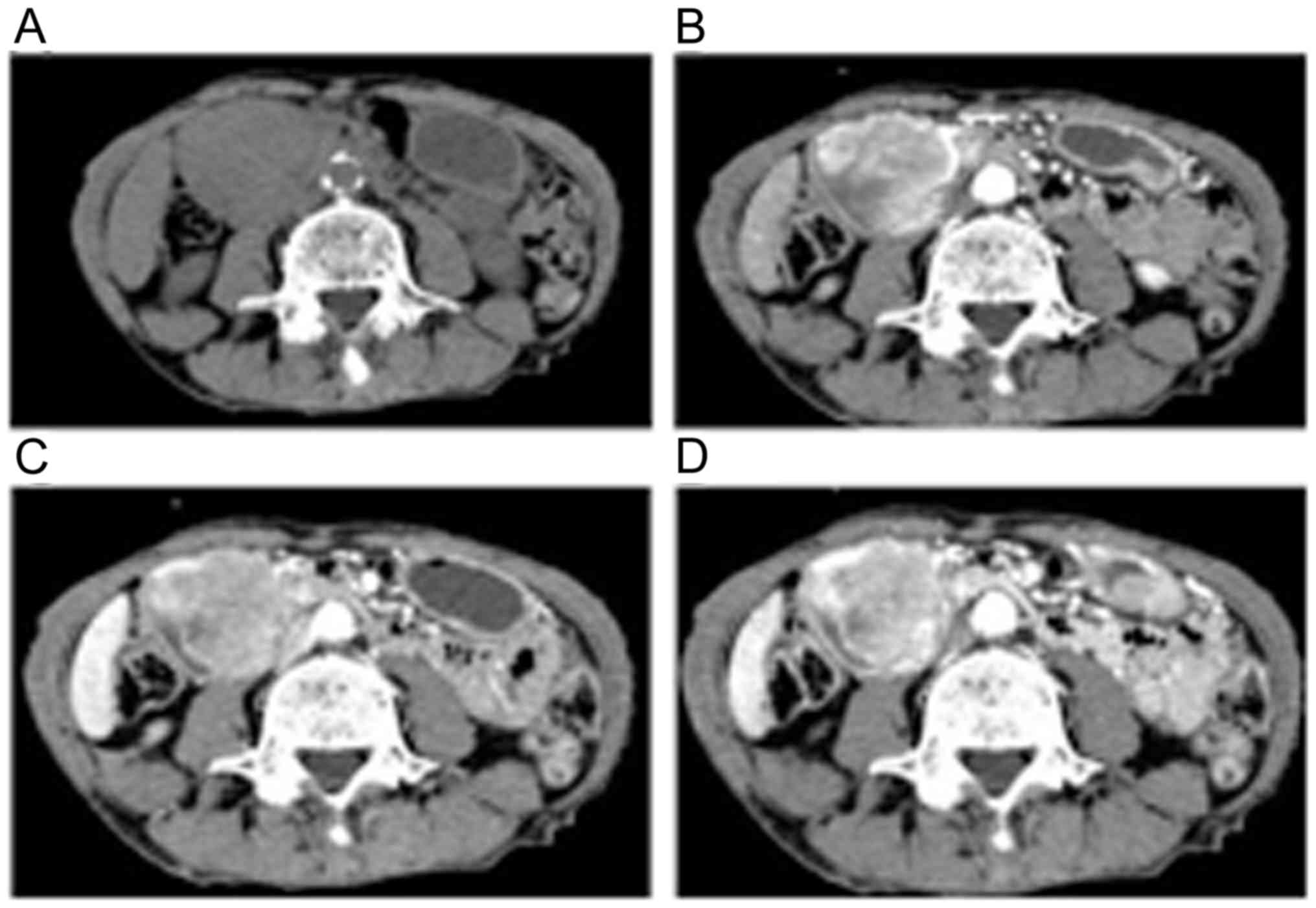
297.3 IU/ml (upper reference limit, 37.0 IU/ml). Abdominal CT
revealed a 55-mm hypervascular tumor at the second part of the
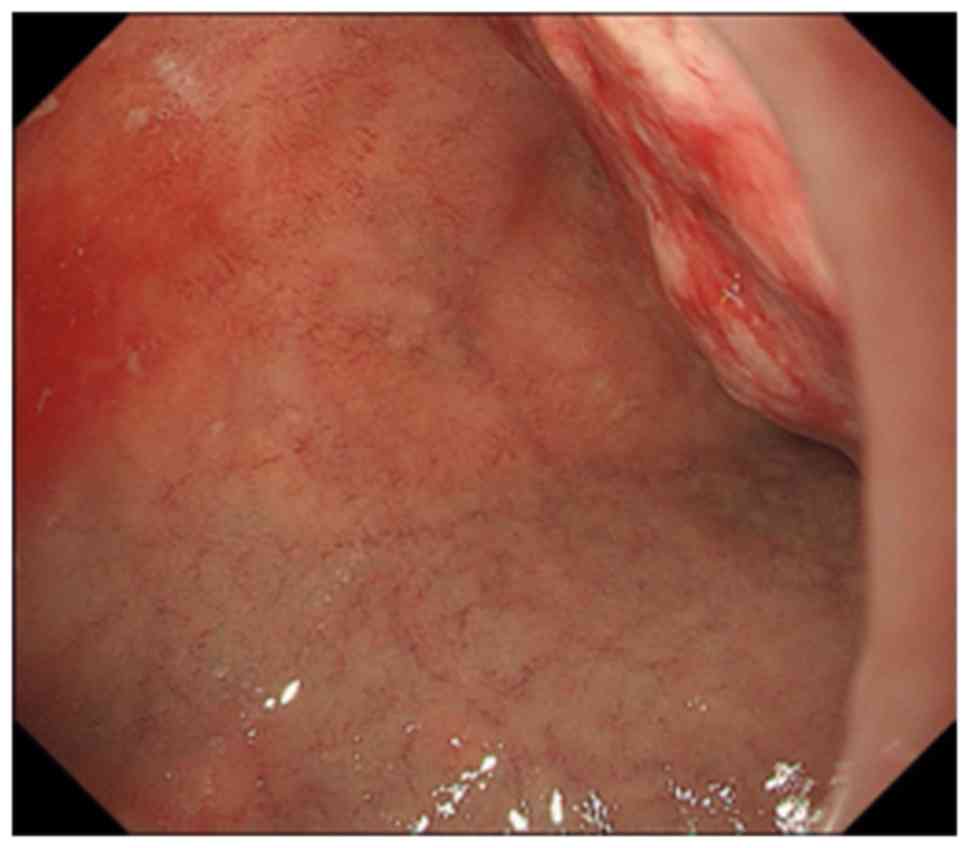
duodenum (Fig. 2). Upper endoscopy
revealed that the tumor was the lumens of the first and second
parts of the duodenum, mainly at the posterior wall. A clot was
noted on the tumor, but no ulcer was present (Fig. 3). Pathological examination identified
a poorly differentiated adenocarcinoma. Based on these results, we
made differential diagnoses of primary duodenum carcinoma and
metastatic duodenal cancer associated with the right lung cancer.
We performed subtotal stomach-preserving pancreaticoduodenectomy to
control bleeding.
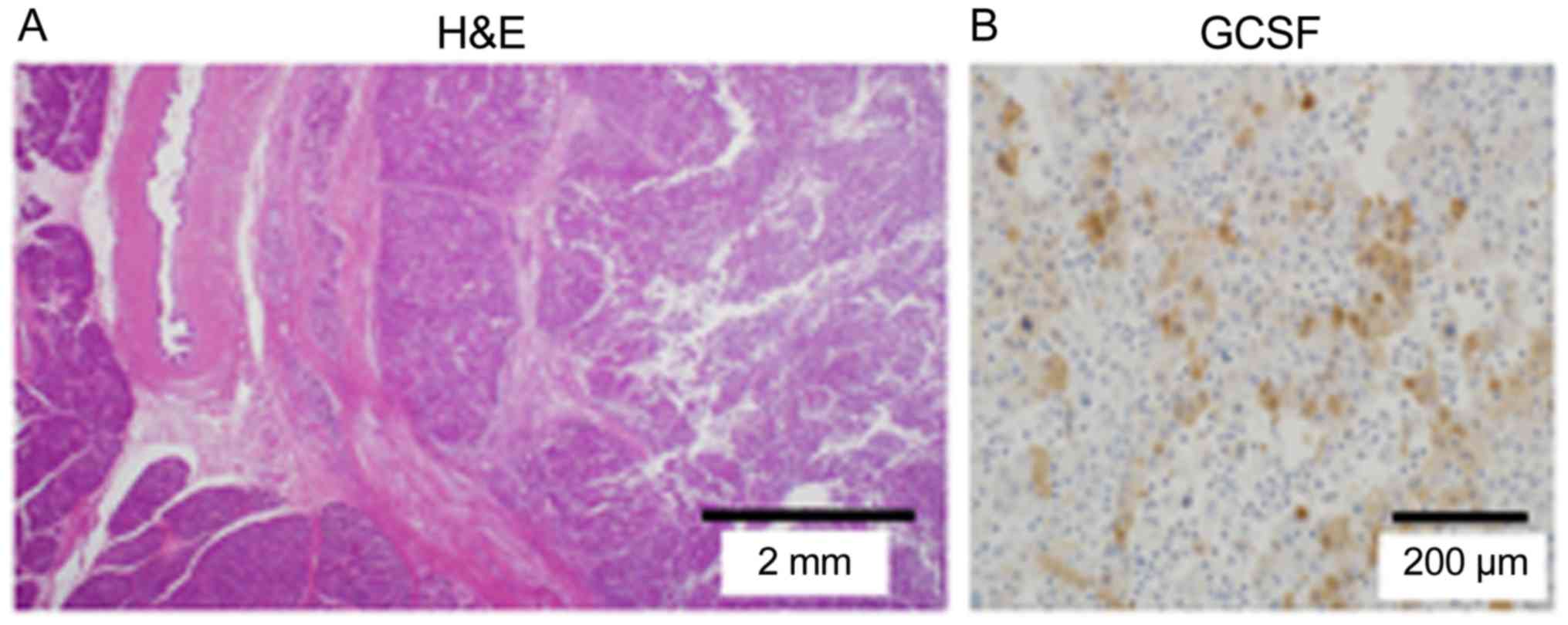
The excised tumor measured 86×55×54 mm. The tumor
mainly occupied the pancreatic head and compressed the pancreatic
parenchyma and common bile duct. It comprised a poorly
differentiated adenocarcinoma, and prominent neutrophil
infiltration was noted around the tumor. In addition, we identified
metastasis in 1 of 13 lymph nodes and lymphatic and venous
invasion. The final pathological diagnosis was T3N1aM0, Stage IIB.
On immunohistochemical examination, which involves the process of
selectively identifying antigens in cells of a tissue section by
exploiting the principle of antibodies binding specifically to
antigens in biological tissues, the tumor was positive for G-CSF
and negative for thyroid transcription factor 1 (TTF-1).
Evaluations of a lung biopsy specimen indicated an adenocarcinoma
with clear cells and TTF-1 positivity (Fig. 4). Based on these results, we made a
final diagnosis of G-CSF-producing primary pancreatic cancer.
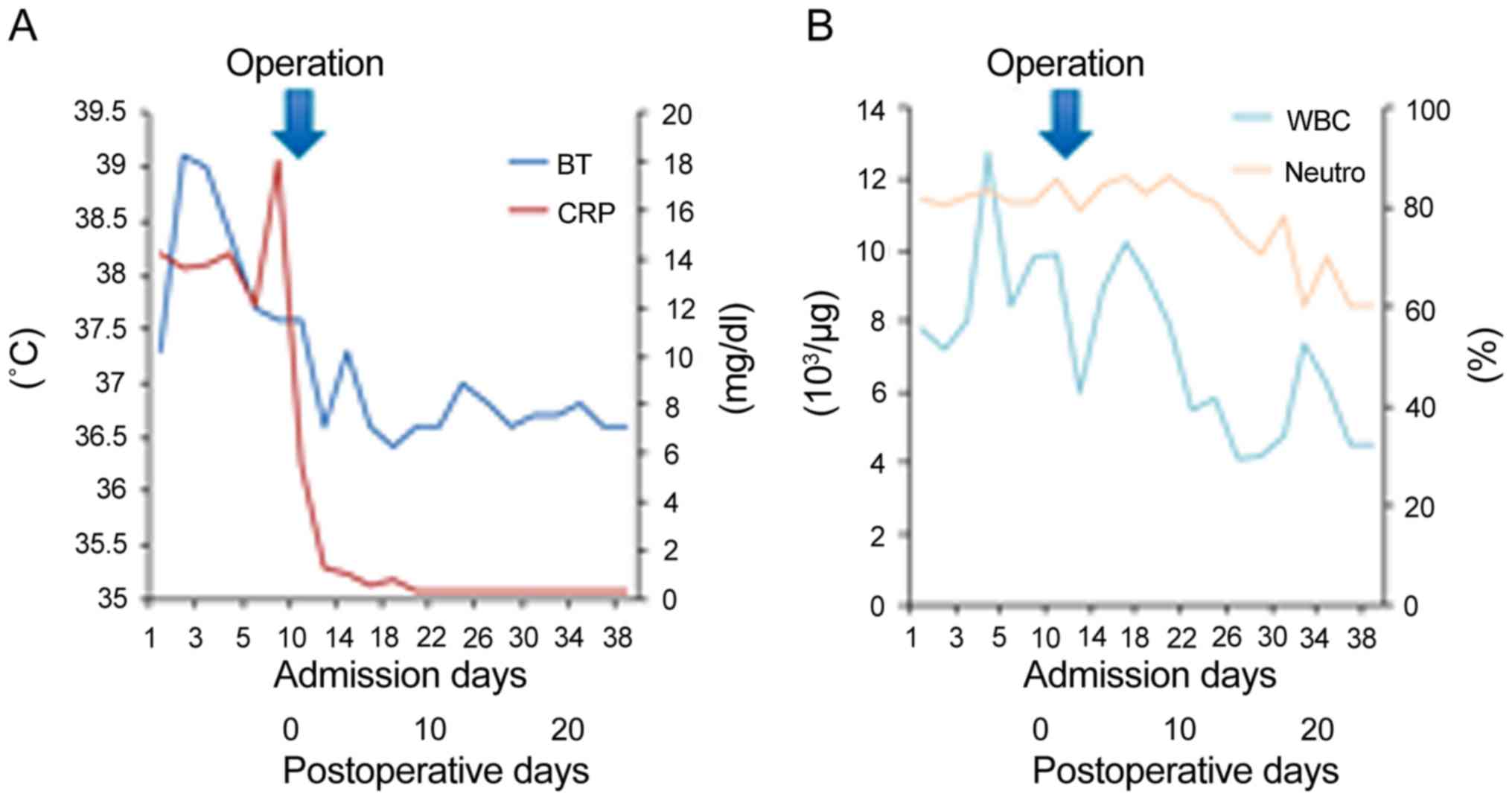
The CRP level decreased from 14.2 mg/dl on the day
before the surgery to 0.3 mg/dl on postoperative day (POD) 13.
Although he experienced delayed gastric emptying after the surgery,
he was discharged on POD 38 (Fig.
5). He was administered S-1 twice a day as adjuvant
chemotherapy for 2 weeks but suffered from adverse drug reaction.
We stopped administrating. At 18 months after surgery, he is
currently alive without recurrence including lung cancer.
Discussion
A G-CSF-producing tumor in the pancreas is very
rare. The first case of G-CSF-producing lung cancer was reported by
Asano in 1977, and many reports on such tumors have been
subsequently published (3). Usually,
patients with G-CSF-producing tumors have fever and leukocyte
elevation without having an infection because of G-CSF elevation in
the blood. The diagnostic criteria for G-CSF-producing tumors are
as follows: i) Extreme leukocytosis; ii) elevated G-CSF activity;
iii) decreased white blood cell count after tumor resection, and
iv) detection of G-CSF production in the tumor (3). In the present study, the patient met
three of aforementioned diagnostic criteria.
We searched PubMed using the terms ‘granulocyte
colony-stimulating factor’ and ‘pancreatic cancer’ and identified
nine reported cases of G-CSF-producing pancreatic cancers during
the period of 1989–2018 (Table I).
The mean age of the 10 patients, including our patient, was 66
years (range, 50–83 years), and there was a predominance in male
patients (males, 9; female, 1). Clinically, most patients presented
with fever, weight loss, and back pain. Histologically, seven
patients had anaplastic carcinomas, two had poorly differentiated
adenocarcinomas, and one had a glandular squamous cell carcinoma.
Five patients underwent surgery, and eight patients received
chemotherapy. Patient survival was very poor, with a median
survival period of 132 days after diagnosis. The treatment options
reported for this type of cancer include surgical resection,
chemotherapy, palliative care, or a combination of these; however,
there is no standard treatment. Pathological analyses have
identified severe hematogenous metastases in most autopsy reports.
Even after curative resection in patients with G-CSF-producing
pancreatic cancer, the prognosis is very poor (4). The poor prognosis of these patients
might be associated with G-CSF elevation. To evaluate the serum
G-CSF we used that the Quantikine Human G-CSF Immunoassay is a 3.5
or 4.5 h solid phase ELISA designed to measure G-CSF in cell
culture supernates, serum, and plasma. It contains E.
coli-expressed recombinant human G-CSF and antibodies raised
against the protein. It has been shown to accurately quantitate
recombinant human G-CSF. A previous study reported a correlation
between cancer cell-derived G-CSF and the production of atypical
T-cell-suppressive neutrophils in breast cancer and mentioned that
G-CSF overexpression increased tumor growth (12).
 | Table I.Reported cases of granulocyte
colony-stimulating factor-producing pancreatic cancer. |
Table I.
Reported cases of granulocyte
colony-stimulating factor-producing pancreatic cancer.
| Author, year | Age | Sex | Symptoms | Location | Pathological
diagnosis | Operation | Chemo |
Prognosisa | (Refs.) |
|---|
| Ohwada et al,
1989 | 83 | M | Back pain | Body | scc | No | 5FU, THP, MMC | Dead (4 months) | (4) |
| Kawakami et
al, 2007 | 56 | M | Back pain | Body and tail | por | No | GEM, S-1 | Dead (4 months) | (5) |
| Nakajima et
al, 2008 | 63 | M | Weight loss | – | ana | No | – | Dead (11 days) | (2) |
| Murata et al,
2009 | 59 | M | Epigastralgia | Body and tail | ana | Yes | GEM+Radiation | Dead (8 months) | (6) |
| Ikeda et al,
2013 | 70 | F | Weight loss | Body and tail | ana | No | S-1 | Dead (88 days) | (7) |
| Kitade et al,
2015 | 68 | M | Weight loss,
Fever | Tail | ana | Yes | S-1 | Dead (83 days) | (8) |
| Hayashi et al,
2016 | 50 | M | Fever | Body | ana | No | S-1, GEM | Dead (123 days) | (9) |
| Vinzens et al,
2017 | 67 | M | Abdominal pain | Tail | ana | Yes 5-FU | Oxa, CPT-11, (34
days) | Dead | (10) |
| Seki et al,
2018 | 65 | M | Not described | Head | ana | Yes | – | Dead (58 days) | (11) |
| Present study | 79 | M | Fever | Head | por | Yes | S-1 | Alive without
recurrence |
|
Our patient had anemia due to bleeding from an
erosive lesion in the duodenum associated with pancreatic head
cancer invasion. Pancreatic head cancer has a greater tendency to
cause symptoms, such as bleeding and obstructive jaundice, than
pancreatic body and tail cancers. The tumor location is possibly
associated with the detection of this G-CSF-producing cancer
without metastasis. Also, the level of white blood cell count was
relatively lower than previously reported cases in our case. Early
detection may be associated with low white blood cell count. Our
patient is alive without an evidence of recurrence 18 months after
the surgery. To our knowledge, this is the first report of a
patient with G-CSF-producing pancreatic cancer to survive for more
than 1 year after treatment. Early detection and complete tumor
resection might have improved our patient's survival. Currently,
there is no standard treatment for G-CSF-producing pancreatic
cancer; however, surgery may be the appropriate first choice for
cancers without evidence of remote metastasis.
G-CSF-producing pancreatic cancer is rare, and the
prognosis is considered to be very poor. We reported the case of a
patient with G-CSF-producing pancreatic cancer who has survived
without recurrence for 18 months after curative surgery. Therefore,
curative surgery should be considered in patients with localized
G-CSF-producing pancreatic cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
The supervision of the current study was by YJ; KT,
HN, KY, MN, MH, AK, TN, SO, SA, YK, ES, YY, SH, HT, KH, HU
interpreted the clinical data and TA and TE wrote, review and/or
revised the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Competing interests
No authors report any competing interest.
References
|
1
|
Joshita S, Nakazawa K, Koike S, Kamijo A,
Matsubayashi K, Miyabayashi H, Furuta K, Kitano K, Yoshizawa K and
Tanaka E: A case of granulocyte-colony stimulating factor-producing
hepatocellular carcinoma confirmed by immunohistochemistry. J
Korean Med Sci. 25:476–480. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakajima A, Takahashi H, Inamori M, Abe Y,
Kobayashi N, Kubota K and Yamanaka S: Anaplastic carcinoma of the
pancreas producing granulocyte-colony stimulating factor: A case
report. J Med Case Rep. 2:3912008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Asano S, Urabe A, Okabe T, Sato N and
Kondo Y: Demonstration of granulopoietic factor(s) in the plasma of
nude mice transplanted with a human lung cancer and in the tumor
tissue. Blood. 49:845–852. 1977.PubMed/NCBI
|
|
4
|
Ohwada S, Miyamoto Y, Fujii T, Kuribara T,
Teshigawara O, Oyama T, Ishii H, Joshita T and Izuo M: Colony
stimulating factor producing carcinoma of the pancreas-a case
report. Gan No Rinsho. 35:523–527. 1989.(In Japanese). PubMed/NCBI
|
|
5
|
Kawakami H, Kuwatani M, Fujiya Y,
Uebayashi M, Konishi K, Makiyama H, Hashino S, Kubota K, Itoh T and
Asaka M: A case of granulocyte-colony stimulating factor producing
ductal adenocarcinoma of the pancreas. Nihon Shokakibyo Gakkai
ZasshiZasshi. 104:233–238. 2007.(In Japanese).
|
|
6
|
Murata T, Terasaki M, Sakaguchi K, Okubo
M, Fukami Y, Nishimae K, Kitayama Y and Hoshi S: A case of
anaplastic carcinoma of the pancreas producing granulocyte-colony
stimulating factor. Clin J Gastroenterol. 2:109–114. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ikeda S, Okubo K, Shibahara H, Narita M,
Morita K, Takeuchi A, Kanazawa H, Ito T, Nishimura D and Katada N:
An autopsy of G-CSF-producing anaplastic carcinoma of the pancreas
with impaired accumulation on FDG-PET after S-1 chemotherapy. Gan
To Kagaku Ryoho. 40:789–792. 2013.(In Japanese). PubMed/NCBI
|
|
8
|
Kitade H, Yanagida H, Yamada M, Satoi S,
Yoshioka K, Shikata N and Kon M: Granulocyte-colony stimulating
factor producing anaplastic carcinoma of the pancreas treated by
distal pancreatectomy and chemotherapy: Report of a case. Surg Case
Rep. 1:462015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hayashi H, Eguchi N, Sumimoto K, Matsumoto
K, Azakami T, Sumida T, Tamura T, Sumii M, Uraoka N and Shimamoto
F: Autopsy of anaplastic carcinoma of the pancreas producing
granulocyte colony-stimulating factor. Nihon Shokakibyo Gakkai
ZasshiZasshi. 113:1408–1415. 2016.(In Japanese).
|
|
10
|
Vinzens S, Zindel J, Zweifel M, Rau T
Gloor B and Wochner A: Granulocyte colony-stimulating factor
producing anaplastic carcinoma of the pancreas: Case report and
review of the literature. Anticancer Res. 37:223–228. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seki H, Yasui N, Shimada A, Matsumoto H
and Domoto H: Resection of a granulocyte colony-stimulating
factor-producing anaplastic carcinoma of the pancreas, associated
with humoral hypercalcemia of malignancy. Gan To Kagaku Ryoho.
45:859–862. 2018.(In Japanese). PubMed/NCBI
|
|
12
|
Cavalloni G, Sarotto I, Pignochino Y,
Gammaitoni L, Migliardi G, Sgro L, Piacibello W, Risio M, Aglietta
M and Leone F: Granulocyte-colony stimulating factor upregulates
ErbB2 expression on breast cancer cell lines and converts primary
resistance to trastuzumab. Anticancer Drugs. 19:689–696. 2008.
View Article : Google Scholar : PubMed/NCBI
|