Introduction
Breast cancer is the most frequent cancer among
women worldwide (1). The proportion
of patients with breast cancer, who were younger than 50 years
appears to be higher in Asian women compared with Western women
(2). In Thailand, more than one-half
of patients with breast cancer were diagnosed at an age younger
than 50 years (3,4). In total, ~54% of breast cancer is
hormone receptor-positive and the proportion is decreased in
younger patients (3,5).
Endocrine therapy, either alone or after
chemotherapy, is the most important treatment in hormone
receptor-positive breast cancer. Blockage of the oestrogen receptor
(ER) and its pathways is a key approach for the treatment of
hormone receptor-positive breast cancer (6). Tamoxifen (TAM) is a standard endocrine
therapy, especially in premenopausal women (7). In post-menopausal women, administration
of aromatase inhibitors results in better survival compared with
TAM (8,9). Ovarian ablation plus chemotherapy
resulted in improved long-term survival in ER-positive early breast
cancer (10). When comparing the
cyclophosphamide, methotrexate and fluorouracil regimen (CMF) of
chemotherapy with ovarian ablation/suppression, there was no
difference in terms of survival in hormone receptor-positive early
breast cancer (11–16). However, to the best of our knowledge,
at present, there is no randomised controlled trial that has
compared ovarian function suppression and TAM with the adriamycin
and cyclophosphamide (AC) regimen of chemotherapy. The addition of
ovarian function suppression to TAM in women who were premenopausal
after chemotherapy resulted in a better disease outcome (17).
The present study aimed to compare disease-free
survival (DFS) and overall survival (OS) in patients with early
breast cancer who received gonadotropin-releasing hormone (GnRH)
agonist-TAM (GnRH-TAM) alone compared with those who received
chemotherapy followed by TAM (AC-TAM).
Patients and methods
Patients
Female patients with breast cancer who were treated
at The Division of Head Neck and Breast Surgery, Department of
Surgery, Faculty of Medicine, Siriraj Hospital, Mahidol University
(Bangkok, Thailand) between January 2005 and December 2015 were
recruited. The inclusion criteria were premenopausal status, tumour
size ≤3 cm, node-negative, no distant metastasis, hormone
receptor-positive and did not receive neoadjuvant treatment. The
patients who rejected adjuvant chemotherapy received GnRH-TAM were
recruited in the GnRH-TAM group. The patients who received AC-TAM
were recruited in the AC-TAM group. The patients with incomplete
clinicopathological data were excluded. Medical records of the
eligible patients were reviewed. Premenopausal status was defined
as those who had menstruated within 1 year before surgery or had a
follicle-stimulating hormone level <30 IU/ml. All of the
patients who received GnRH-TAM without adjuvant chemotherapy were
included. The patients who received standard AC-TAM with comparable
demographic and clinicopathological parameters were recruited. The
tumor grade was classified according to Elston/Nottingham
modification of the Bloom-Richardson System (18,19). The
follow-up time was 36–167 months with a median follow-up of 77
months. The sample size was calculated using the two proportions
formula for a non-inferiority test (20). The parameters included in the formula
were as follows: i) The expected survival was 0.98 and 0.96 for
AC-TAM and GnRH-TAM, respectively; ii) one-sided significance
level, 0.05; iii) the power of test, 0.8; iv) non-inferiority
margin, 0.06; and v) the ratio of sample size, 3. This resulted in
a calculated sample size of 120 patients who received AC-TAM and 40
patients who received GnRH-TAM. During the recruitment period, 130
patients who received AC-TAM and 40 patients who received GnRH-TAM
met the inclusion criteria. All of the patients had complete
follow-up data. The present study was approved by The Siriraj
Institutional Review Board (certificate of approval no. Si
674/2016). No informed consent was obtained from the patients as
the present study was retrospective in nature.
Treatment
In the GnRH-TAM group, the patients received
subcutaneous injection of 10.8 mg goserelin every 3 months for 2–3
years and TAM (20 mg/day) for 5 years. In the AC-TAM group, AC was
administered every 3 weeks for 4 cycles (60 mg/m2
adriamycin intravenously plus 60 mg/m2 cyclophosphamide
intravenously every 21 days) followed by TAM (20 mg/day) for 5
years.
Statistical analysis
Continuous parameters are presented as the mean ±
SD. Categorical parameters are presented as the frequency and
percentage. The DFS period was defined as the time from the
operation to disease recurrence or death (whichever occurred first)
and the OS period was defined as the time from the operation to
death from any cause. The categorical characteristics of the
patients were compared between the two treatment groups using
Pearson's χ2 test. The continuous parameters of the two
treatment groups were compared using independent t-test. DFS and OS
were assessed using Kaplan-Meier curves and compared using a
log-rank test. The Cox proportional hazards model was used to
calculate the adjusted hazard ratios. P<0.05 was considered to
indicate a statistically significant difference. The statistical
analysis was performed using SPSS software version 21 (IBM
Corp.).
Results
Patients and tumour
characteristics
The total number of the patients recruited was 170.
The mean age at diagnosis was 44.4±6.3 years. In total, 40 patients
received GnRH-TAM and 130 patients received AC-TAM. The baseline
characteristics of the patients, tumours and treatments are
presented in Table I. There was no
significant difference in all pathological parameters. All patients
with HER2 overexpression had a tumour size <2 cm and did not
receive HER2-targeted therapy. In the GnRH-TAM group, a higher
proportion of the patients underwent breast-conserving surgery
followed by postoperative radiotherapy. All of the patients in the
GnRH-TAM group experienced hot flashes but could tolerate and
received complete treatment. Most of the patients in the AC-TAM
group had common side effects of chemotherapy, including alopecia
and nausea. No severe adverse effects, such as cardiotoxicity were
observed in the AC-TAM group.
 | Table I.Characteristics of the patients. |
Table I.
Characteristics of the patients.
| Characteristics | GnRH-TAM, n=40 n
(%) | AC-TAM, n=130 n
(%) | P-value |
|---|
| Age, mean ± SD
(years) | 45.1±3.5 | 44.2±6.9 | 0.30a |
| Tumour size |
| ≤20
mm | 30 (75.0) | 91 (70.0) | 0.54 |
| >20
mm | 10 (25.0) | 39 (30.0) |
|
| Histology |
|
Invasive ductal carcinoma | 40 (100.0) | 123 (94.6) | 0.13 |
|
Invasive lobular
carcinoma | 0 | 7 (5.4) |
|
| Tumour grade |
| Grade
1 | 13 (33.3) | 23 (17.7) | 0.11 |
| Grade
2 | 23 (59.0) | 97 (74.6) |
|
| Grade
3 | 3 (7.7) | 10 (7.7) |
|
| Lymphovascular
invasion |
| No | 36 (90.0) | 122 (93.8) | 0.41 |
|
Yes | 4 (10.0) | 8 (6.2) |
|
| HER2 |
|
Negative | 37 (92.5) | 109 (83.8) | 0.17 |
|
Positive | 3 (7.5) | 21 (16.2) |
|
| Type of
surgery |
| Breast
conserving surgery | 27 (67.5) | 56 (43.1) | 0.01 |
| Total
mastectomy | 13 (32.5) | 74 (56.9) |
|
Survival analysis
The median follow-up time was 77 (36–167) months.
There was no death that occurred during the follow-up period. Only
one local recurrence in the conserved breast occurred in the
GnRH-TAM group at 48 months after surgery. In the AC-TAM group,
there were 3 patients with loco-regional recurrence of the
conserved breast, chest wall and axillary lymph nodes at 37, 47 and
120 months after surgery, respectively. Additionally, in the AC-TAM
group, 1 patient had lung metastasis and another patient had lung
and liver metastasis at 66 and 149 months, respectively. Survival
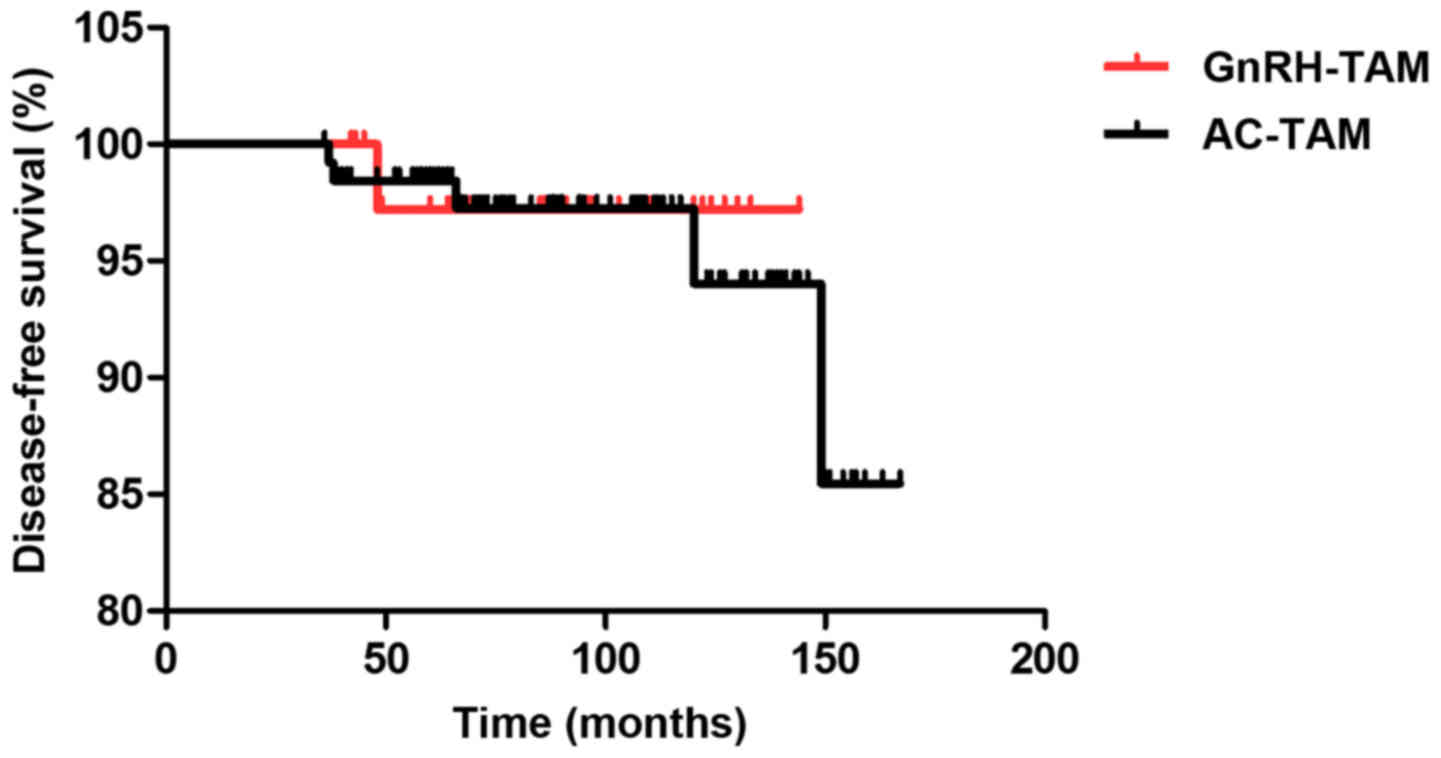
analysis by Kaplan-Meier method revealed no difference in DFS for
each parameter (Table II). OS
analysis was not conducted in the present study as all patients
survived and there was no death in the follow-up period.
Multivariate analysis by Cox regression showed that lymphovascular
invasion tended to be an independent prognostic factor for lower
DFS; however, this was not significant. The survival curves of DFS
by each treatment group showed no significant difference between
the GnRH-TAM group and AC-TAM group (P=0.858; Fig. 1).
 | Table II.Disease-free survival among different
parameters and treatment groups. |
Table II.
Disease-free survival among different
parameters and treatment groups.
| Parameters | Case | Event | 5-year
survival | 10-year
survival |
P-valuea | Hazard ratio (95%
CI) |
P-valueb |
|---|
| Age at
diagnosis |
| ≤45
years | 91 | 3 | 1.00 | 0.93 | 0.85 | 1.54
(0.27–8.82) | 0.63 |
| >45
years | 79 | 3 | 0.96 | 0.96 |
|
|
|
| Tumour size |
| ≤20
mm | 121 | 4 | 0.98 | 0.94 | 0.93 | 1.01
(0.17–5.88) | 0.99 |
| >20
mm | 49 | 2 | 0.98 | 0.95 |
|
|
|
| Tumour grade |
| Grade
1 | 36 | 0 | 1.00 | 1.00 | 0.19 | N/A | 0.98 |
| Grade
2/3 | 133 | 6 | 0.98 | 0.93 |
|
|
|
| Lymphovascular
invasion |
|
Absence | 158 | 4 | 0.99 | 0.98 | 0.06 | 4.78
(0.84–27.29) | 0.08 |
|
Presence | 12 | 2 | 0.92 | 0.76 |
|
|
|
| HER2 status |
|
Negative | 146 | 6 | 0.98 | 0.94 | 0.44 | N/A | 0.99 |
|
Positive | 24 | 0 | 1.00 | 1.00 |
|
|
|
| Surgery |
| Breast
conserving | 83 | 4 | 0.97 | 0.90 | 0.22 | 0.29
(0.04–1.89) | 0.20 |
| Total
mastectomy | 87 | 2 | 0.99 | 0.99 |
|
|
|
| Systemic
treatment |
|
GnRH-TAM | 40 | 1 | 0.97 | 0.97 | 0.86 | 1.90
(0.19–18.80) | 0.58 |
|
AC-TAM | 130 | 5 | 0.98 | 0.94 |
|
|
|
Discussion
Hormonal therapy after chemotherapy or hormonal
therapy alone is the main treatment option in hormone
receptor-positive, HER2-negative early breast cancer (7). The decision of treatment depends on the
aggressiveness of the disease and the condition of the patients. In
the present study, patients with breast cancer were recruited, who
completely rejected chemotherapy due to concerns for quality of
life. GnRH-TAM did not demonstrate inferior survival when compared
with AC-TAM in hormone receptor-positive, HER2-negative early
breast cancer.
Ovarian function suppression has been shown to
improve the outcome of high-risk premenopausal patients when
combined with TAM, but this benefit was not established in a
low-risk group (17). Only a few
previous studies, to the best of our knowledge, have illustrated
that GnRH agonists are suitable substitutes for chemotherapy in
hormone-responsive patients with breast cancer (21,22).
Meta-analysis by Early Breast Cancer Trialist's Collaborative Group
showed that patients with breast cancer under the age of 50 years
who received ablation of ovarian function had better long-term
survival regardless of hormone receptor status (10,23). In
premenopausal women with hormone receptor-positive breast cancer,
ovarian function suppression plus TAM resulted in increased 8-year
DFS and OS rates (24). Subgroup
analysis showed that in the patients who did not receive prior
chemotherapy, the favourable outcome was more emphasized (24). The effect of ovarian ablation in the
improvement of survival was more influential in the patients who
did not receive chemotherapy; chemotherapy may itself suppress
ovarian function in late premenopausal women.
Several previous randomised control trials compared
ovarian function suppression/ovarian ablation to CMF alone in
hormone receptor-positive premenopausal patients with early breast
cancer there was no difference between the two treatment regimens
in DFS and OS (22,25–31). The
efficacy of ovarian function suppression or ovarian ablation is
similar to that of chemotherapy and may replace chemotherapy as an
alternative choice of treatment. Previous randomised control trials
compared medical ovarian function ablation using a GnRH agonist
with CMF in hormone receptor-positive premenopausal women, and
found no difference in both DFS and OS (26–28,31).
This finding was observed in both node-negative patients (27,31) and
node-positive patients (26,28). However, patients who received GnRH
agonist alone demonstrated better quality of life compared with
those who received adjuvant chemotherapy (32–34).
Several previous studies compared a combination of ovarian function
suppression plus TAM to chemotherapy alone in hormone receptor
positive, premenopausal early breast cancer. The results suggested
that combined endocrine therapy is a reasonable alternative to
chemotherapy (15,16,35,36).
Furthermore, in hormone receptor-positive patients, administration
of 3.6 mg goserelin every 4 weeks for 3 years plus TAM for 5 years
was more effective than CMF (15).
In terms of quality of life, the patients receiving GnRH analog had
less deterioration in quality of life over the first 6 months when
compared with those receiving the CMF regimen (37). In the present study, the patients who
received GnRH-TAM had no adverse effects and were able to complete
the course of treatment. In addition, the patients in the GnRH-TAM
group could avoid the side-effects of chemotherapy, including
alopecia, permanent infertility, chemotherapy-induced memory
problems and febrile neutropenia (38).
To the best of our knowledge, at present, there is
no randomised controlled trial that compared combined ovarian
function suppression and TAM with sequential AC-TAM. The present
retrospective study found comparable survival outcome between these
two treatment regimens similar to other previous retrospective
studies of a Korean population (39,40).
There was only one local recurrence in the GnRH-TAM group while in
the AC-TAM group, 2 patients with distant metastasis were
identified. No mortality occurred in the enrolled patients. Further
long-term follow-up might be required for luminal subtype breast
cancer.
Cost-utility analysis of GnRH agonist and adjuvant
chemotherapy showed that GnRH agonist is more cost-effective than
the docetaxel cyclophosphamide (TC) regimen (41). However, in Thailand, TC is reserved
for the patients who have a higher risk for cardiotoxicity when
they receive the AC regimen due to cumulative dose-related and
permanent cardiotoxicity of anthracycline (42), and the higher cost of a TC regimen.
Administration of 10.8 mg goserelin every 3 months as in the
present study is convenient, reduces costs, with no adverse
effects. The efficacy of ovarian function suppression was
comparable to 3.6 mg monthly doses (43). The present study indicated that 10.8
mg 3-monthly administration of goserelin does not result in a
different outcome, and is safe and practical to use.
There are limitations to the present study; it is a
retrospective study conducted in a single institute with a limited
number of patients in GnRH-TAM group. This may cause selection bias
in the analysis. According to The National Comprehensive Cancer
Network Clinical Practice Guideline (version 3.2018) (7), this group of patients should be
evaluated by a 21-gene reverse transcription-PCR assay to classify
the risk of recurrence. Further studies including this assay might
be beneficial and a multicentre-randomised controlled study is
required for the prospective comparison of the efficacies of
GnRH-TAM and AC-TAM regimens in premenopausal patients with early
stage breast cancer.
In conclusion, it was demonstrated that in
premenopausal women with hormone receptor-positive, node-negative
early breast cancer, adjuvant treatment with GnRH-TAM had similar
survival outcomes and improved the quality of life compared with
patients who were treated with AC-TAM. This adjuvant treatment
regimen represents a valid option in this group of patients.
Acknowledgements
The abstract was presented at The Primary Therapy of
Early Breast Cancer 15th St. Gallen International Breast Cancer
Conference 15–18 March 2017 in Vienna, Austria and published as
abstract no. P007 in The Breast 32 (Suppl 1): 2017. The authors
would like to thank Miss Surat Phumphuang (Division of Head Neck
and Breast Surgery, Department of Surgery, Faculty of Medicine,
Siriraj Hospital, Mahidol University), for coordination of the
research work.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or used during this study
are available from the corresponding author on reasonable
request.
Authors' contributions
DS performed the statistical analysis, manuscript
preparation, revised and completed the manuscript for important
content. TK collected the patient data, and performed the
statistical analysis and manuscript preparation. WP helped with the
study design, data acquisition and manuscript preparation, and
revised the manuscript. PO was involved in the treatment of the
patients, provided ideas, and contributed to the conception and
design of the study, the critical point of discussion and the
completion of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Siriraj
Institutional Review Board (certificate of approval no. Si
674/2016). No informed consent was obtained from the patients as
the present study was retrospective in nature.
Patient consent for publication
Not applicable as the present study was
retrospective in nature.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AC
|
adriamycin and cyclophosphamide
|
|
TAM
|
tamoxifen
|
|
GnRH-TAM
|
gonadotropin-releasing hormone agonist
plus tamoxifen
|
|
AC-TAM
|
adriamycin and cyclophosphamide plus
tamoxifen
|
|
TC
|
docetaxel and cyclophosphamide
|
|
CMF
|
cyclophosphamide, methotrexate and
fluorouracil
|
|
OS
|
overall survival
|
|
DFS
|
disease-free survival
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tan BK, Lim GH, Czene K, Hall P and Chia
KS: Do Asian breast cancer patients have poorer survival than their
western counterparts? A comparison between Singapore and Stockholm.
Breast Cancer Res. 11:R42009. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sa-Nguanraksa D, Chuangsuwanich T,
Pongpruttipan T, Kummalue T, Rojananin S, Ratanawichhitrasin A,
Prasarttong-Osoth P, Chuthatisith S, Pisarnturakit P,
Aeumrithaicharoenchok W, et al: Vascular endothelial growth factor
634G/C polymorphism is associated with increased breast cancer risk
and aggressiveness. Mol Med Rep. 8:1242–1250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
National Cancer Institute, .
Hospital-Based Cancer Registry 2017. http://www.nci.go.th/th/File_download/Nci%20Cancer%20Registry/HOSPITAL-BASED%202016%20Revise%204%20Final.pdfDecember
4–2018
|
|
5
|
Lertsanguansinchai P, Chottetanaprasith T,
Chatamra K, Sampatanukul P, Wannakrairot P, Rojpornpradit P,
Shotelersuk K, Lertbutsayanukul C, Boonjunwetwat D and Vajragupta
L: Estrogen and progesterone receptors status in Thai female breast
cancer patients: An analysis of 399 cases at King Chulalongkorn
Memorial Hospital. J Med Assoc Thai. 85 (Suppl 1):S193–S202.
2002.PubMed/NCBI
|
|
6
|
Johnston SJ and Cheung KL: Endocrine
therapy for breast cancer: A model of hormonal manipulation. Oncol
Ther. 6:141–156. 2018. View Article : Google Scholar
|
|
7
|
National Comprehensive Cancer Network, .
NCCN Clinical Practice Guidelines in Oncology (NCCN GuidelinesR).
Breast Cancer. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdfDecember
4–2018
|
|
8
|
Coates AS, Keshaviah A, Thürlimann B,
Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch
M, Gelber RD, Colleoni M, et al: Five years of letrozole compared
with tamoxifen as initial adjuvant therapy for postmenopausal women
with endocrine-responsive early breast cancer: Update of study BIG
1–98. J Clin Oncol. 25:486–492. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arimidex, Tamoxifen, Alone or in
Combination (ATAC) Trialists' Group, ; Forbes JF, Cuzick J, Buzdar
A, Howell A, Tobias JS and Baum M: Effect of anastrozole and
tamoxifen as adjuvant treatment for early-stage breast cancer:
100-month analysis of the ATAC trial. Lancet Oncol. 9:45–53. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ovarian ablation in early breast cancer, .
Overview of the randomised trials. Early Breast Cancer Trialists'
Collaborative Group. Lancet. 348:1189–1196. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Adjuvant ovarian ablation versus CMF
chemotherapy in premenopausal women with pathological stage II
breast carcinoma, . The Scottish trial. Scottish Cancer Trials
Breast Group and ICRF Breast Unit, Guy's Hospital, London. Lancet.
341:1293–1298. 1993.PubMed/NCBI
|
|
12
|
Ejlertsen B, Dombernowsky P, Mouridsen HT
and Kamby C; American Society of Clinical Oncology, : Comparable
effect of ovarian (OA) and CMF chemotherapy in premenopausal
hormonal receptor positive breast cancer patients (PRP). Proc Am
Soc Clin Oncol. 18:pp. 66a(abstract). 1999, https://www.tib.eu/en/search/id/BLCP%3ACN030034114/Comparable-Effect-of-Ovarian-Ablation-OA-and-CMF
|
|
13
|
Boccardo F, Rubagotti A, Amoroso D, Mesiti
M, Romeo D, Sismondi P, Giai M, Genta F, Pacini P, Distante V, et
al: Cyclophosphamide, methotrexate, and fluorouracil versus
tamoxifen plus ovarian suppression as adjuvant treatment of
estrogen receptor-positive pre-/perimenopausal breast cancer
patients: Results of the Italian Breast Cancer Adjuvant Study Group
02 randomized trial. simpleboccardo@hp380.ist.unige.it.
J Clin Oncol. 18:2718–2727. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jakesz R, Hausmaninger H, Samonigg H,
Kubista E, Depisch D, Fridrik M, Stierer M, Gnant M, Steger G, Kolb
R, et al: Comparison of adjuvant therapy with tamoxifen and
goserelin vs. CMF in premenopausal stage I and II
hormone-responsive breast cancer patients: Four-year results of
Austrian Breast Cancer Study Group (ABCSG) trial 5. Eur J Cancer.
35 (Suppl 4):S831999. View Article : Google Scholar
|
|
15
|
Jakesz R, Hausmaninger H, Kubista E, Gnant
M, Menzel C, Bauernhofer T, Seifert M, Haider K, Mlineritsch B,
Steindorfer P, et al: Randomized adjuvant trial of tamoxifen and
goserelin versus cyclophosphamide, methotrexate, and fluorouracil:
Evidence for the superiority of treatment with endocrine blockade
in premenopausal patients with hormone-responsive breast
cancer-Austrian Breast and Colorectal Cancer Study Group Trial 5. J
Clin Oncol. 20:4621–4627. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roché H, Kerbrat P, Bonneterre J, Fargeot
P, Fumoleau P, Monnier A, Clavère P, Goudier MJ, Chollet P,
Guastalla JP and Serin D: Complete hormonal blockade versus
epirubicin-based chemotherapy in premenopausal, one to three
node-positive, and hormone-receptor positive, early breast cancer
patients: 7-year follow-up results of French Adjuvant Study Group
06 randomised trial. Ann Oncol. 17:1221–1227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Francis PA, Regan MM, Fleming GF, Láng I,
Ciruelos E, Bellet M, Bonnefoi HR, Climent MA, Da Prada GA,
Burstein HJ, et al: Adjuvant ovarian suppression in premenopausal
breast cancer. N Engl J Med. 372:436–446. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bloom HJ and Richardson WW: Histological
grading and prognosis in breast cancer; a study of 1,409 cases of
which 359 have been followed for 15 years. Br J Cancer. 11:359–377.
1957. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: Experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chow SC, Shao J and Wang H: Sample size
calculations in clinical researchMarcel Dekker; New York: 2003
|
|
21
|
Jonat W, Kaufmann M, Sauerbrei W, Blamey
R, Cuzick J, Namer M, Fogelman I, de Haes JC, de Matteis A, Stewart
A, et al: Goserelin versus cyclophosphamide, methotrexate, and
fluorouracil as adjuvant therapy in premenopausal patients with
node-positive breast cancer: The Zoladex Early Breast Cancer
Research Association Study. J Clin Oncol. 20:4628–4635. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kaufmann M, Graf E, Jonat W, Eiermann W,
Vescia S, Geberth M, Conrad B, Gademann G, Albert US, Loibl S, et
al: A randomised trial of goserelin versus control after adjuvant,
risk-adapted chemotherapy in premenopausal patients with primary
breast cancer-GABG-IV B-93. Eur J Cancer. 43:2351–2358. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), : Effects of chemotherapy and
hormonal therapy for early breast cancer on recurrence and 15-year
survival: An overview of the randomised trials. Lancet.
365:1687–1717. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Francis PA, Pagani O, Fleming GF, Walley
BA, Colleoni M, Láng I, Gómez HL, Tondini C, Ciruelos E, Burstein
HJ, et al: Tailoring adjuvant endocrine therapy for premenopausal
breast cancer. N Engl J Med. 379:122–137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Davidson NE, O'Neill AM, Vukov AM, Osborne
CK, Martino S, White DR and Abeloff MD: Chemoendocrine therapy for
premenopausal women with axillary lymph node-positive, steroid
hormone receptor-positive breast cancer: Results from INT 0101
(E5188). J Clin Oncol. 23:5973–5982. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ejlertsen B, Mouridsen HT, Jensen MB,
Bengtsson NO, Bergh J, Cold S, Edlund P, Ewertz M, de Graaf PW,
Kamby C and Nielsen DL: Similar efficacy for ovarian ablation
compared with cyclophosphamide, methotrexate, and fluorouracil:
From a randomized comparison of premenopausal patients with
node-positive, hormone receptor-positive breast cancer. J Clin
Oncol. 24:4956–4962. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
International Breast Cancer Study Group
(IBCSG), ; Castiglione-Gertsch M, O'Neill A, Price KN, Goldhirsch
A, Coates AS, Colleoni M, Nasi ML, Bonetti M and Gelber RD:
Adjuvant chemotherapy followed by goserelin versus either modality
alone for premenopausal lymph node-negative breast cancer: A
randomized trial. J Natl Cancer Inst. 95:1833–1846. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kaufmann M, Jonat W, Blamey R, Cuzick J,
Namer M, Fogelman I, de Haes JC, Schumacher M and Sauerbrei W;
Zoladex Early Breast Cancer Research Association (ZEBRA) Trialists'
Group, : Survival analyses from the ZEBRA study. goserelin
(Zoladex) versus CMF in premenopausal women with node-positive
breast cancer. Eur J Cancer. 39:1711–1717. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schmid P, Untch M, Kossé V, Bondar G,
Vassiljev L, Tarutinov V, Lehmann U, Maubach L, Meurer J,
Wallwiener D and Possinger K: Leuprorelin acetate every-3-months
depot versus cyclophosphamide, methotrexate, and fluorouracil as
adjuvant treatment in premenopausal patients with node-positive
breast cancer: The TABLE study. J Clin Oncol. 25:2509–2515. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thomson CS, Twelves CJ, Mallon EA and
Leake RE; Scottish Cancer Trials Breast Group; Scottish Cancer
Therapy Network, : Adjuvant ovarian ablation vs. CMF chemotherapy
in premenopausal breast cancer patients: Trial update and impact of
immunohistochemical assessment of ER status. Breast. 11:419–429.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
von Minckwitz G, Graf E, Geberth M,
Eiermann W, Jonat W, Conrad B, Brunnert K, Gerber B, Vescia S,
Wollert J and Kaufmann M: CMF versus goserelin as adjuvant therapy
for node-negative, hormone-receptor-positive breast cancer in
premenopausal patients: A randomised trial (GABG trial IV-A-93).
Eur J Cancer. 42:1780–1788. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
de Haes H, Olschewski M, Kaufmann M,
Schumacher M, Jonat W and Sauerbrei W; Zoladex Early Breast Cancer
Research Association Trialists Group, : Quality of life in
goserelin-treated versus cyclophosphamide + methotrexate +
fluorouracil-treated premenopausal and perimenopausal patients with
node-positive, early breast cancer: The Zoladex Early Breast Cancer
Research Association Trialists Group. J Clin Oncol. 21:4510–4516.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hürny C, Bernhard J, Coates AS,
Castiglione-Gertsch M, Peterson HF, Gelber RD, Forbes JF, Rudenstam
CM, Simoncini E, Crivellari D, et al: Impact of adjuvant therapy on
quality of life in women with node-positive operable breast cancer.
International Breast Cancer Study Group. Lancet. 347:1279–1284.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nystedt M, Berglund G, Bolund C, Fornander
T and Rutqvist LE: Side effects of adjuvant endocrine treatment in
premenopausal breast cancer patients: A prospective randomized
study. J Clin Oncol. 21:1836–1844. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Boccardo F, Rubagotti A, Amoroso D,
Sismondi P, Genta F, Nenci I, Piffanelli A, Farris A, Castagnetta
L, Traina A, et al: Chemotherapy versus tamoxifen versus
chemotherapy plus tamoxifen in node-positive, oestrogen-receptor
positive breast cancer patients. An update at 7 years of the 1st
GROCTA (Breast Cancer Adjuvant Chemo-Hormone Therapy Cooperative
Group) trial. Eur J Cancer. 28:673–680. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Roché H, Mihura J, de Lafontan B,
Reme-Saumon M, Martel P, Dubois JB and Naja A: PP-5-6 castration
and tamoxifen versus chemotherapy (FAC) for premenopausal, node and
receptors positive breast cancer patients: A randomized trial with
a 7 years median follow up. Eur J Cancer. 32 (Suppl 2):S351996.
View Article : Google Scholar
|
|
37
|
Bernhard J, Zahrieh D, Castiglione-Gertsch
M, Hürny C, Gelber RD, Forbes JF, Murray E, Collins J, Aebi S,
Thürlimann B, et al: Adjuvant chemotherapy followed by goserelin
compared with either modality alone: The impact on amenorrhea, hot
flashes, and quality of life in premenopausal patients-the
International Breast Cancer Study Group Trial VIII. J Clin Oncol.
25:263–270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Partridge AH, Burstein HJ and Winer EP:
Side effects of chemotherapy and combined chemohormonal therapy in
women with early-stage breast cancer. J Natl Cancer Inst Monogr.
135–142. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim HJ, Lee JS, Park EH, Lim WS, Sei JY,
Koh BS, Son BH, Ahn JH, Jeong KH, Kim SB and Ahn SH: Short term
results from GHRH analogue use in pre-menopausal breast cancer in
Korea. Eur J Surg Oncol. 35:936–941. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sohn G, Ahn SH, Kim HJ, Son BH, Lee JW, Ko
BS, Lee Y, Lee SB and Baek S: Survival outcome of combined GnRH
agonist and tamoxifen is comparable to that of sequential
adriamycin and cyclophosphamide chemotherapy plus tamoxifen in
premenopausal patients with lymph-node-negative,
hormone-responsive, HER2-negative, T1-T2 breast cancer. Cancer Res
Treat. 48:1351–1362. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cheng TF, Wang JD and Uen WC: Cost-utility
analysis of adjuvant goserelin (Zoladex) and adjuvant chemotherapy
in premenopausal women with breast cancer. BMC Cancer. 12:332012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Florescu M, Cinteza M and Vinereanu D:
Chemotherapy-induced cardiotoxicity. Maedica (Buchar). 8:59–67.
2013.PubMed/NCBI
|
|
43
|
Masuda N, Iwata H, Rai Y, Anan K, Takeuchi
T, Kohno N, Takei H, Yanagita Y and Noguchi S: Monthly versus
3-monthly goserelin acetate treatment in pre-menopausal patients
with estrogen receptor-positive early breast cancer. Breast Cancer
Res Treat. 126:443–451. 2011. View Article : Google Scholar : PubMed/NCBI
|