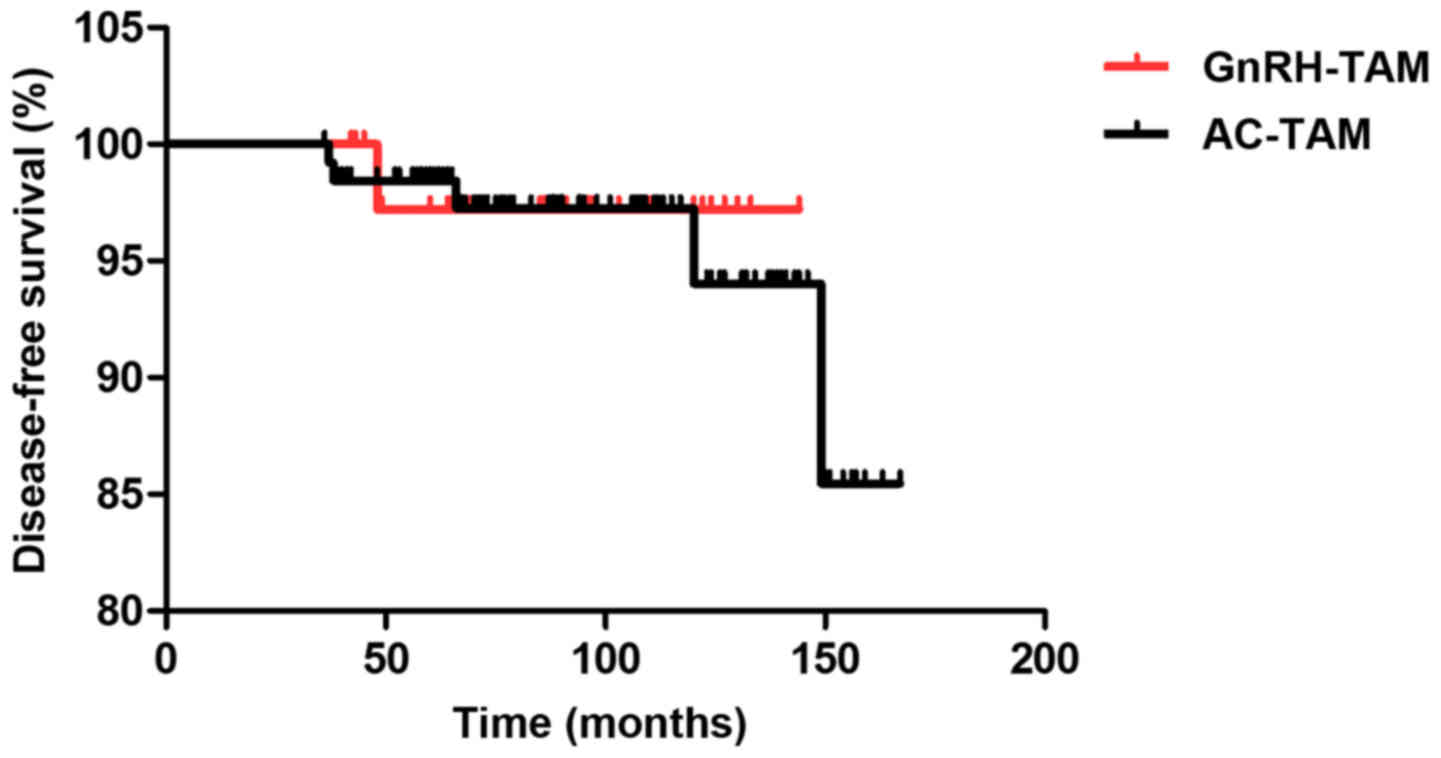
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tan BK, Lim GH, Czene K, Hall P and Chia
KS: Do Asian breast cancer patients have poorer survival than their
western counterparts? A comparison between Singapore and Stockholm.
Breast Cancer Res. 11:R42009. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sa-Nguanraksa D, Chuangsuwanich T,
Pongpruttipan T, Kummalue T, Rojananin S, Ratanawichhitrasin A,
Prasarttong-Osoth P, Chuthatisith S, Pisarnturakit P,
Aeumrithaicharoenchok W, et al: Vascular endothelial growth factor
634G/C polymorphism is associated with increased breast cancer risk
and aggressiveness. Mol Med Rep. 8:1242–1250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
National Cancer Institute, .
Hospital-Based Cancer Registry 2017. http://www.nci.go.th/th/File_download/Nci%20Cancer%20Registry/HOSPITAL-BASED%202016%20Revise%204%20Final.pdfDecember
4–2018
|
|
5
|
Lertsanguansinchai P, Chottetanaprasith T,
Chatamra K, Sampatanukul P, Wannakrairot P, Rojpornpradit P,
Shotelersuk K, Lertbutsayanukul C, Boonjunwetwat D and Vajragupta
L: Estrogen and progesterone receptors status in Thai female breast
cancer patients: An analysis of 399 cases at King Chulalongkorn
Memorial Hospital. J Med Assoc Thai. 85 (Suppl 1):S193–S202.
2002.PubMed/NCBI
|
|
6
|
Johnston SJ and Cheung KL: Endocrine
therapy for breast cancer: A model of hormonal manipulation. Oncol
Ther. 6:141–156. 2018. View Article : Google Scholar
|
|
7
|
National Comprehensive Cancer Network, .
NCCN Clinical Practice Guidelines in Oncology (NCCN GuidelinesR).
Breast Cancer. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdfDecember
4–2018
|
|
8
|
Coates AS, Keshaviah A, Thürlimann B,
Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch
M, Gelber RD, Colleoni M, et al: Five years of letrozole compared
with tamoxifen as initial adjuvant therapy for postmenopausal women
with endocrine-responsive early breast cancer: Update of study BIG
1–98. J Clin Oncol. 25:486–492. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arimidex, Tamoxifen, Alone or in
Combination (ATAC) Trialists' Group, ; Forbes JF, Cuzick J, Buzdar
A, Howell A, Tobias JS and Baum M: Effect of anastrozole and
tamoxifen as adjuvant treatment for early-stage breast cancer:
100-month analysis of the ATAC trial. Lancet Oncol. 9:45–53. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ovarian ablation in early breast cancer, .
Overview of the randomised trials. Early Breast Cancer Trialists'
Collaborative Group. Lancet. 348:1189–1196. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Adjuvant ovarian ablation versus CMF
chemotherapy in premenopausal women with pathological stage II
breast carcinoma, . The Scottish trial. Scottish Cancer Trials
Breast Group and ICRF Breast Unit, Guy's Hospital, London. Lancet.
341:1293–1298. 1993.PubMed/NCBI
|
|
12
|
Ejlertsen B, Dombernowsky P, Mouridsen HT
and Kamby C; American Society of Clinical Oncology, : Comparable
effect of ovarian (OA) and CMF chemotherapy in premenopausal
hormonal receptor positive breast cancer patients (PRP). Proc Am
Soc Clin Oncol. 18:pp. 66a(abstract). 1999, https://www.tib.eu/en/search/id/BLCP%3ACN030034114/Comparable-Effect-of-Ovarian-Ablation-OA-and-CMF
|
|
13
|
Boccardo F, Rubagotti A, Amoroso D, Mesiti
M, Romeo D, Sismondi P, Giai M, Genta F, Pacini P, Distante V, et
al: Cyclophosphamide, methotrexate, and fluorouracil versus
tamoxifen plus ovarian suppression as adjuvant treatment of
estrogen receptor-positive pre-/perimenopausal breast cancer
patients: Results of the Italian Breast Cancer Adjuvant Study Group
02 randomized trial. simpleboccardo@hp380.ist.unige.it.
J Clin Oncol. 18:2718–2727. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jakesz R, Hausmaninger H, Samonigg H,
Kubista E, Depisch D, Fridrik M, Stierer M, Gnant M, Steger G, Kolb
R, et al: Comparison of adjuvant therapy with tamoxifen and
goserelin vs. CMF in premenopausal stage I and II
hormone-responsive breast cancer patients: Four-year results of
Austrian Breast Cancer Study Group (ABCSG) trial 5. Eur J Cancer.
35 (Suppl 4):S831999. View Article : Google Scholar
|
|
15
|
Jakesz R, Hausmaninger H, Kubista E, Gnant
M, Menzel C, Bauernhofer T, Seifert M, Haider K, Mlineritsch B,
Steindorfer P, et al: Randomized adjuvant trial of tamoxifen and
goserelin versus cyclophosphamide, methotrexate, and fluorouracil:
Evidence for the superiority of treatment with endocrine blockade
in premenopausal patients with hormone-responsive breast
cancer-Austrian Breast and Colorectal Cancer Study Group Trial 5. J
Clin Oncol. 20:4621–4627. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roché H, Kerbrat P, Bonneterre J, Fargeot
P, Fumoleau P, Monnier A, Clavère P, Goudier MJ, Chollet P,
Guastalla JP and Serin D: Complete hormonal blockade versus
epirubicin-based chemotherapy in premenopausal, one to three
node-positive, and hormone-receptor positive, early breast cancer
patients: 7-year follow-up results of French Adjuvant Study Group
06 randomised trial. Ann Oncol. 17:1221–1227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Francis PA, Regan MM, Fleming GF, Láng I,
Ciruelos E, Bellet M, Bonnefoi HR, Climent MA, Da Prada GA,
Burstein HJ, et al: Adjuvant ovarian suppression in premenopausal
breast cancer. N Engl J Med. 372:436–446. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bloom HJ and Richardson WW: Histological
grading and prognosis in breast cancer; a study of 1,409 cases of
which 359 have been followed for 15 years. Br J Cancer. 11:359–377.
1957. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: Experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chow SC, Shao J and Wang H: Sample size
calculations in clinical researchMarcel Dekker; New York: 2003
|
|
21
|
Jonat W, Kaufmann M, Sauerbrei W, Blamey
R, Cuzick J, Namer M, Fogelman I, de Haes JC, de Matteis A, Stewart
A, et al: Goserelin versus cyclophosphamide, methotrexate, and
fluorouracil as adjuvant therapy in premenopausal patients with
node-positive breast cancer: The Zoladex Early Breast Cancer
Research Association Study. J Clin Oncol. 20:4628–4635. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kaufmann M, Graf E, Jonat W, Eiermann W,
Vescia S, Geberth M, Conrad B, Gademann G, Albert US, Loibl S, et
al: A randomised trial of goserelin versus control after adjuvant,
risk-adapted chemotherapy in premenopausal patients with primary
breast cancer-GABG-IV B-93. Eur J Cancer. 43:2351–2358. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), : Effects of chemotherapy and
hormonal therapy for early breast cancer on recurrence and 15-year
survival: An overview of the randomised trials. Lancet.
365:1687–1717. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Francis PA, Pagani O, Fleming GF, Walley
BA, Colleoni M, Láng I, Gómez HL, Tondini C, Ciruelos E, Burstein
HJ, et al: Tailoring adjuvant endocrine therapy for premenopausal
breast cancer. N Engl J Med. 379:122–137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Davidson NE, O'Neill AM, Vukov AM, Osborne
CK, Martino S, White DR and Abeloff MD: Chemoendocrine therapy for
premenopausal women with axillary lymph node-positive, steroid
hormone receptor-positive breast cancer: Results from INT 0101
(E5188). J Clin Oncol. 23:5973–5982. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ejlertsen B, Mouridsen HT, Jensen MB,
Bengtsson NO, Bergh J, Cold S, Edlund P, Ewertz M, de Graaf PW,
Kamby C and Nielsen DL: Similar efficacy for ovarian ablation
compared with cyclophosphamide, methotrexate, and fluorouracil:
From a randomized comparison of premenopausal patients with
node-positive, hormone receptor-positive breast cancer. J Clin
Oncol. 24:4956–4962. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
International Breast Cancer Study Group
(IBCSG), ; Castiglione-Gertsch M, O'Neill A, Price KN, Goldhirsch
A, Coates AS, Colleoni M, Nasi ML, Bonetti M and Gelber RD:
Adjuvant chemotherapy followed by goserelin versus either modality
alone for premenopausal lymph node-negative breast cancer: A
randomized trial. J Natl Cancer Inst. 95:1833–1846. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kaufmann M, Jonat W, Blamey R, Cuzick J,
Namer M, Fogelman I, de Haes JC, Schumacher M and Sauerbrei W;
Zoladex Early Breast Cancer Research Association (ZEBRA) Trialists'
Group, : Survival analyses from the ZEBRA study. goserelin
(Zoladex) versus CMF in premenopausal women with node-positive
breast cancer. Eur J Cancer. 39:1711–1717. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schmid P, Untch M, Kossé V, Bondar G,
Vassiljev L, Tarutinov V, Lehmann U, Maubach L, Meurer J,
Wallwiener D and Possinger K: Leuprorelin acetate every-3-months
depot versus cyclophosphamide, methotrexate, and fluorouracil as
adjuvant treatment in premenopausal patients with node-positive
breast cancer: The TABLE study. J Clin Oncol. 25:2509–2515. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thomson CS, Twelves CJ, Mallon EA and
Leake RE; Scottish Cancer Trials Breast Group; Scottish Cancer
Therapy Network, : Adjuvant ovarian ablation vs. CMF chemotherapy
in premenopausal breast cancer patients: Trial update and impact of
immunohistochemical assessment of ER status. Breast. 11:419–429.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
von Minckwitz G, Graf E, Geberth M,
Eiermann W, Jonat W, Conrad B, Brunnert K, Gerber B, Vescia S,
Wollert J and Kaufmann M: CMF versus goserelin as adjuvant therapy
for node-negative, hormone-receptor-positive breast cancer in
premenopausal patients: A randomised trial (GABG trial IV-A-93).
Eur J Cancer. 42:1780–1788. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
de Haes H, Olschewski M, Kaufmann M,
Schumacher M, Jonat W and Sauerbrei W; Zoladex Early Breast Cancer
Research Association Trialists Group, : Quality of life in
goserelin-treated versus cyclophosphamide + methotrexate +
fluorouracil-treated premenopausal and perimenopausal patients with
node-positive, early breast cancer: The Zoladex Early Breast Cancer
Research Association Trialists Group. J Clin Oncol. 21:4510–4516.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hürny C, Bernhard J, Coates AS,
Castiglione-Gertsch M, Peterson HF, Gelber RD, Forbes JF, Rudenstam
CM, Simoncini E, Crivellari D, et al: Impact of adjuvant therapy on
quality of life in women with node-positive operable breast cancer.
International Breast Cancer Study Group. Lancet. 347:1279–1284.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nystedt M, Berglund G, Bolund C, Fornander
T and Rutqvist LE: Side effects of adjuvant endocrine treatment in
premenopausal breast cancer patients: A prospective randomized
study. J Clin Oncol. 21:1836–1844. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Boccardo F, Rubagotti A, Amoroso D,
Sismondi P, Genta F, Nenci I, Piffanelli A, Farris A, Castagnetta
L, Traina A, et al: Chemotherapy versus tamoxifen versus
chemotherapy plus tamoxifen in node-positive, oestrogen-receptor
positive breast cancer patients. An update at 7 years of the 1st
GROCTA (Breast Cancer Adjuvant Chemo-Hormone Therapy Cooperative
Group) trial. Eur J Cancer. 28:673–680. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Roché H, Mihura J, de Lafontan B,
Reme-Saumon M, Martel P, Dubois JB and Naja A: PP-5-6 castration
and tamoxifen versus chemotherapy (FAC) for premenopausal, node and
receptors positive breast cancer patients: A randomized trial with
a 7 years median follow up. Eur J Cancer. 32 (Suppl 2):S351996.
View Article : Google Scholar
|
|
37
|
Bernhard J, Zahrieh D, Castiglione-Gertsch
M, Hürny C, Gelber RD, Forbes JF, Murray E, Collins J, Aebi S,
Thürlimann B, et al: Adjuvant chemotherapy followed by goserelin
compared with either modality alone: The impact on amenorrhea, hot
flashes, and quality of life in premenopausal patients-the
International Breast Cancer Study Group Trial VIII. J Clin Oncol.
25:263–270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Partridge AH, Burstein HJ and Winer EP:
Side effects of chemotherapy and combined chemohormonal therapy in
women with early-stage breast cancer. J Natl Cancer Inst Monogr.
135–142. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim HJ, Lee JS, Park EH, Lim WS, Sei JY,
Koh BS, Son BH, Ahn JH, Jeong KH, Kim SB and Ahn SH: Short term
results from GHRH analogue use in pre-menopausal breast cancer in
Korea. Eur J Surg Oncol. 35:936–941. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sohn G, Ahn SH, Kim HJ, Son BH, Lee JW, Ko
BS, Lee Y, Lee SB and Baek S: Survival outcome of combined GnRH
agonist and tamoxifen is comparable to that of sequential
adriamycin and cyclophosphamide chemotherapy plus tamoxifen in
premenopausal patients with lymph-node-negative,
hormone-responsive, HER2-negative, T1-T2 breast cancer. Cancer Res
Treat. 48:1351–1362. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cheng TF, Wang JD and Uen WC: Cost-utility
analysis of adjuvant goserelin (Zoladex) and adjuvant chemotherapy
in premenopausal women with breast cancer. BMC Cancer. 12:332012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Florescu M, Cinteza M and Vinereanu D:
Chemotherapy-induced cardiotoxicity. Maedica (Buchar). 8:59–67.
2013.PubMed/NCBI
|
|
43
|
Masuda N, Iwata H, Rai Y, Anan K, Takeuchi
T, Kohno N, Takei H, Yanagita Y and Noguchi S: Monthly versus
3-monthly goserelin acetate treatment in pre-menopausal patients
with estrogen receptor-positive early breast cancer. Breast Cancer
Res Treat. 126:443–451. 2011. View Article : Google Scholar : PubMed/NCBI
|