Introduction
Interleukin 2 (IL-2), which has been identified in
activated T cells, stimulates an immune response on target cells
via a high affinity receptor composed of α, β and γ subunits. The
interaction between IL-2 and its receptors on target cells plays a
pivotal role in activating and maintaining immune responses. The
soluble form of the IL2R α subunit (CD25) appears to be released
into the serum from the membranes of activated lymphocytes after
shedding by proteolytic cleavage. Soluble IL-2 receptor α (sIL2R)
is detected in the serum of healthy individuals and increases in
association with various types of inflammation or neoplasms
(1–3). However, the mechanism and functional
significance of elevated sIL2R levels for each pathological
condition remain unclear.
Serum sIL2R levels have been found to be elevated in
most types of hematolymphoid neoplasms, including Hodgkin's
lymphomas, non-Hodgkin lymphomas, acute lymphoblastic leukemia
(ALL), chronic lymphocytic leukemia (CLL), multiple myeloma, and
others (3). Moreover, the highest
levels of sIL2R have been reported in adult T-cell
lymphoma/leukemia (ATLL) (4) and
hairy cell leukemia (5). In patients
with hematolymphoid neoplasms, most of the serum sIL2R is derived
from the neoplastic cells themselves, directly reflecting the tumor
burden and disease activity; therefore, it may be considered as a
true tumor marker. In certain B-cell lymphomas, proteinases derived
from tumor-associated macrophages in the tumor microenvironment
also appear to play an important role in producing sIL2R (6). In several non-lymphoid solid tumors,
serum sIL2R levels are significantly higher compared with those in
healthy individuals, although this increase is not as notable as
that observed in hematolymphoid neoplasms (1,7,8). In non-lymphoid solid tumors, increased
serum sIL2R levels may originate from tumor cells, as well as from
activated lymphoid cells, circulating mononuclear cells, or
tumor-infiltrating lymphocytes (3).
Furthermore, elevated sIL2R levels have been detected in a growing
number of pathological conditions, including infections, autoimmune
or other inflammatory diseases, allograft rejection, graft-vs.-host
disease after allogeneic hematopoietic stem cell transplantation,
and hemophagocytic lymphohistiocytosis (3).
Although the mechanisms and clinical significance of
elevated sIL2R levels are unclear, the clinical usefulness of sIL2R
has been evaluated for the diagnosis, staging, prognosis and
post-treatment monitoring in lymphomas or other diseases (3). However, the availability of data on the
significance of sIL2R levels in the differential diagnosis of
lymphoma from other diseases in the clinical setting is limited
(1,7,8). In the
present study, patients with suspected lymphoma were
retrospectively analyzed and the sIL2R levels were measured, the
final diagnoses were compared, and then the diagnostic
characteristics and clinical parameters that affect the diagnostic
value were evaluated.
Patients and methods
Ethics approval
The present study was approved by the Ethics
Committee of the University of Toyama. The use of an opt-out method
enabled refusal to participate in disclosure documents.
Patient population
A total of 248 consecutive adult patients (aged ≥18
years) who had suspected malignant lymphoma and had their serum
sIL2R levels measured by attending physicians in Toyama University
Hospital between January 2004 and December 2007 were
retrospectively analyzed.
Data collection
Data on clinical parameters, including age, sex,
white blood cell (WBC) count, C-reactive protein (CRP) levels
(normal range: 0.00–0.14 mg/dl), serum lactate dehydrogenase (LDH)
levels (normal range: 124–222 U/l) and serum sIL2R levels, were
extracted from the medical record database. Clinical symptoms at
presentation, initial differential diagnosis and final diagnosis
were reviewed in the medical records for each patient. Standard
histological diagnosis of lymphoma by hematopathologists was based
on the World Health Organization 2008 classification.
sIL2R measurement
The serum sIL2R levels were measured with a
sandwich-enzyme immunoassay (IL-2R test; BML; cat. no.
221ADAMX00007000). The normal range was defined as 122–496 U/ml
following the manufacturer's information.
Statistical analyses
The sensitivity (Sn) and specificity (Sp) of the
sIL2R levels for the diagnosis of lymphoma and other lymphoid
neoplasms were evaluated, and the positive and negative predictive
values were also determined. A receiver operating characteristic
(ROC) curve was used to determine the diagnostic accuracy and
cutoff value of sIL2R. Differences between the two groups were
evaluated using the Mann-Whitney U test. Comparisons among multiple
groups were made using the Kruskal-Wallis test, and post hoc group
comparisons were performed with the Steel-Dwass test. A
multivariate logistic regression model was used to determine risk
factors for lymphomas. All data were considered statistically
significant if the P-values were <0.05. The analyses were
performed using EZR version 2.4-0 (Saitama Medical Center, Jichi
Medical University), which is a graphical user interface for R (The
R Foundation for Statistical Computing) (9).
Results
Patient characteristics
A total of 248 patients with lymphoma suspected by
attending physicians based on clinical, laboratory or imaging
findings were included in the present study. Lymphoma was suspected
due to varying reasons. The common presentations and differential
diagnoses for lymphomas included solid tumors with uncommon
presentation, inflammatory symptoms, liver dysfunction,
neurological symptoms of unexplained etiology, or even unexplained
complaints. The final diagnosis of lymphoma subtype or differential
diagnoses were established. The patient characteristics are
summarized in Table I. Of the 248
patients, 97 were diagnosed with aggressive lymphomas, 23 with
indolent lymphomas, 8 with plasma cell disorders including plasma
cell myeloma, and 5 with CLL. The remaining 115 patients were
diagnosed with neoplasms other than lymphoid. The most common
diagnosis was non-lymphoid solid tumor (n=50), followed by
infection (n=32), non-infectious inflammatory disorders (autoimmune
diseases or allergy; n=18) and miscellaneous (n=15).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Diagnosis | n | M/F | Mean age (range),
years | Fever (+/-) |
|---|
| Lymphomas | 120 | 71/49 | 64.1 (21–90) | 16/104 |
|
Aggressive | 97 | 62/35 | 64.6 (21–90) | 15/82 |
|
Indolent | 23 | 9/14 | 61.8 (32–82) | 1/22 |
| Other lymphoid
neoplasms |
| Plasma
cell disorders | 8 | 4/4 | 62.3 (48–70) | 0/8 |
| CLL | 5 | 1/4 | 70.4 (54–83) | 0/5 |
| Non-hematolymphoid
tumors | 50 | 27/23 | 65.4 (19–87) | 5/45 |
|
Histologically proven | 43 | 22/21 | 65.0 (19–87) | 5/38 |
| Not
histologically proven | 7 | 5/2 | 68.0 (49–79) | 0/7 |
| Infection | 32 | 12/20 | 43.0 (20–78) | 14/18 |
|
Lymphadenopathy | 23 | 9/14 | 38.0 (20–76) | 10/13 |
|
Gastrointestinal | 7 | 2/5 | 55.0 (27–78) | 2/5 |
|
Others | 2 | 1/1 | 63.0 (56–70) | 2/0 |
| Non-infectious
inflammation | 18 | 11/7 | 54.1 (24–80) | 5/13 |
|
Autoimmune | 10 | 6/4 | 61.8 (28–80) | 2/8 |
|
Allergy | 3 | 1/2 | 47.7 (24–71) | 2/1 |
|
Others | 5 | 4/1 | 60.6 (28–80) | 1/4 |
| Miscellaneous | 15 | 7/8 | 65.1 (35–85) | 1/14 |
The histological subtypes of aggressive or indolent
lymphomas and other lymphoid neoplasms are listed in Table II. Among patients diagnosed with
non-lymphoid solid neoplasms, the most common types of tumors were
of gastrointestinal or urogenital origin, followed by primary
unknown carcinomas, non-epithelial tumors, abdominal lymph node
enlargement, and splenic or bone tumors without histological
confirmation.
 | Table II.Histological diagnosis of patients
with lymphoid neoplasms. |
Table II.
Histological diagnosis of patients
with lymphoid neoplasms.
| Category | Diagnosis | M/F | Mean age (range),
years |
|---|
| Aggressive
lymphomas | Diffuse large
B-cell lymphoma | 41/27 | 66 (24–90) |
|
| Intravascular large
B-cell lymphoma | 1/2 | 66 (69–76) |
|
| Mantle cell
lymphoma | 4/1 | 72 (46–83) |
|
| Follicular
lymphoma, grade 3A | 3/1 | 62 (54–81) |
|
| Classical Hodgkin's
lymphomas | 4/0 | 46 (21–59) |
|
| Peripheral T-cell
lymphoma | 2/0 | 57 (50–64) |
|
| Angioimmunoblastic
T-cell lymphoma | 3/1 | 77 (71–79) |
|
| ATLL | 0/1 | 60 |
|
| NK/T-cell
lymphoma | 4/0 | 49 (26–71) |
|
|
Methotrexate-related LPD | 0/2 | 58 (55–61) |
| Indolent
lymphomas | Follicular
lymphoma, grade 1–2 | 3/5 | 62 (43–77) |
|
| MALT type
lymphoma | 3/9 | 63 (32–82) |
|
| Nodal marginal zone
B-cell lymphoma | 1/0 | 32 |
|
| Lymphoplasmacytic
lymphoma | 1/0 | 62 |
|
| Cutaneous T-cell
lymphoma | 1/0 | 68 |
| CLL | B-CLL | 1/4 | 70 (54–83) |
| Plasma cell
disorders | Multiple
myeloma | 1/1 | 64 (59–68) |
|
| Plasmacytoma | 1/0 | 56 |
|
| POEMS
syndromea | 0/1 | 48 |
|
| AL-amyloidosis | 1/0 | 70 |
|
| Light chain
deposition disease | 0/1 | 70 |
|
| IgM-MGUS | 1/1 | 63.5 (51–76) |
Comparison of each parameter in
patients with lymphoid tumors or other diagnoses
The sIL2R levels and other parameters were compared
between the two groups: Malignant lymphomas, including CLL and
plasma cell disorders (ML group) and other diagnoses (other group)
(Table III). The median age of the
patients in the ML group was significantly higher compared with
that in the other group (67 years vs. 60 years, respectively;
P=0.02). The median WBC count in the ML group was similar to that
of the other group (4,570/µl vs. 4,680/µl). The
median serum CRP level in the ML group was somewhat higher compared
with that of the other group, but the difference was not
statistically significant (0.4 mg/dl vs. 0.2 mg/dl, respectively;
P=0.066). The median LDH level in the ML group was significantly
higher compared with that in the other group (217 U/l vs. 182 U/l,
respectively; P<0.01). The median serum sIL2R level in the ML
group was significantly higher compared with that in the other
group (920 U/ml vs. 520 U/ml, respectively; P<0.001). Similar
results were observed if patients with CLL and plasma cell
disorders were extracted from the lymphoma group (data not
shown).
 | Table III.Comparison of characteristics between
the ML and other groups. |
Table III.
Comparison of characteristics between
the ML and other groups.
|
| ML group | Other group |
|
|---|
|
|
|
|
|
|---|
|
| Median (range) | Mean ± SD | Median (range) | Mean ± SD | P-value |
|---|
| Age, years | 67 (21–90) | 64.2±13.3 | 60 (19–87) | 57.4±18.9 | 0.02 |
| WBC count
(cells/µl) | 4,570
(1,950–84,590) | 8,136±9,745 | 4,680
(1,920–17,470) | 6,753±3,008 | 0.89 |
| C-reactive protein
(mg/dl) | 0.40 (0–17.3) | 2.12±3.59 | 0.20 (0–27.1) | 1.99±4.40 | 0.07 |
| Lactate
dehydrogenase (U/l) | 217 (97–1,269) | 295±219 | 182 (99–4435) | 308±565 | <0.01 |
| sIL2R (U/ml) | 920
(159–58,089) | 3,240±7,312 | 520
(150–4,433) | 786±727 | <0.01 |
sIL2R levels in each category and
subcategory
The comparison between serum sIL2R levels in the ML
and the other groups is presented in Fig. 1A. The serum sIL2R levels in each of
the disease categories are shown in Fig.
1B. Although aggressive lymphomas were associated with the
highest sIL2R levels, indolent types of lymphomas appeared to have
sIL2R levels similar to those of other disease categories,
including non-hematological tumors and infection, among others.
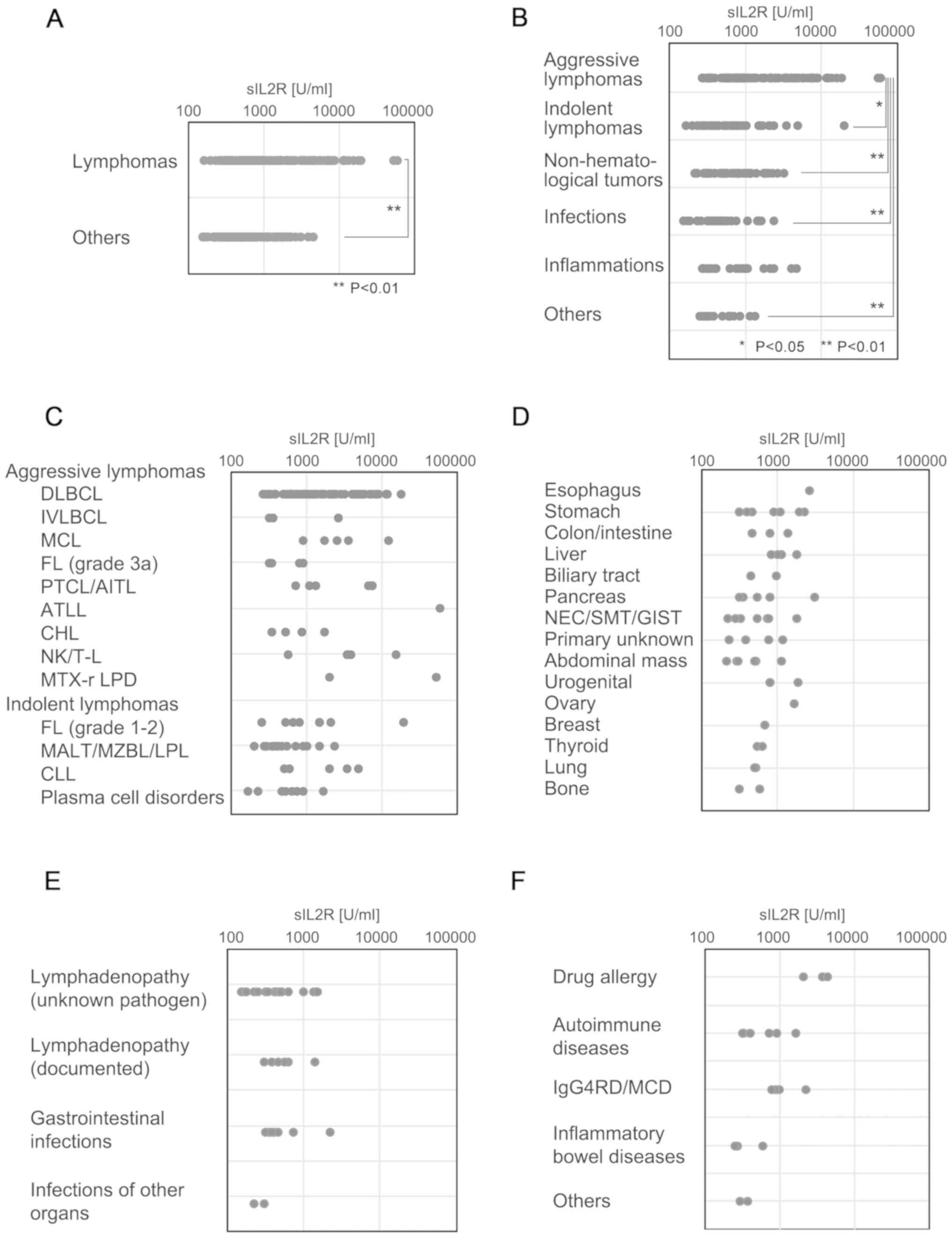 | Figure 1.sIL2R levels in each category and
subcategory. Dot plot of serum sIL2R levels in each diagnosis
category. (A) Comparison of the serum sIL2R levels in the lymphoma
group and the non-lymphoid group. (B) Serum sIL2R levels for each
disease category, including aggressive or indolent types of
lymphomas, CLL, plasma cell disorders including plasma cell
myeloma, infection, non-infectious inflammation, and others. (C)
Serum sIL2R levels of each of the detailed subtypes of aggressive
lymphomas, indolent lymphomas, CLL and plasma cell disorders,
including plasma cell myeloma. (D) Serum sIL2R levels in various
non-lymphoid tumors. (E) Serum sIL2R levels in various infectious
diseases. (F) Serum sIL2R levels in various inflammatory diseases.
sIL2R, soluble interleukin-2 receptor; CLL, chronic lymphocytic
leukemia; DLBCL, diffuse large B-cell lymphoma; IVLBCL,
intravascular diffuse large B-cell lymphoma; MCL, mantle cell
lymphoma; FL, follicular lymphoma; AITL, angioimmunoblastic T-cell
lymphoma; PTCL, peripheral T-cell lymphoma; ATLL, adult T-cell
leukemia/lymphoma; CHL, classical Hodgkin's lymphoma; NK/T-L,
natural killer/T-cell lymphoma; MTX-r LPD, methotrexate-related
lymphoproliferative disorders; MALT, extranodal mucosa-associated
lymphoid tissue type lymphoma; MZBL, nodal marginal zone B-cell
lymphoma; LPL, lympho-plasmacytic lymphoma; NEC, neuroendocrine
carcinoma; SMT, submucosal tumor; GIST, gastrointestinal stromal
tumor; IgG4-RD, IgG4-related disease; MCD, multicentric Castleman's
disease. |
The ML group was subcategorized into aggressive or
indolent histological types, CLL and plasma cell disorders,
including plasma cell myeloma. Aggressive lymphomas exhibited the
highest sIL2R levels, followed by indolent lymphomas, CLL and
plasma cell disorders (Fig. 1C). One
patient with ATLL (acute type) had the highest levels of sIL2R
(58,089 U/ml). The sIL2R level in patients with aggressive T/NK
lymphomas except ATLL (median, 5,080 U/ml; range, 556–15,087 U/ml)
was significantly higher (P<0.002) compared with that in
patients with aggressive B-cell lymphomas (median, 1,046 U/ml;
range, 257–17,709 U/ml).
The sIL2R levels in various non-lymphoid solid
tumors are shown in Fig. 1D. Of
note, 2 of 3 patients with extremely high sIL2R levels (>2,000
U/ml) had advanced tumors with splenic involvement. The sIL2R
levels in various infectious diseases are shown in Fig. 1E. Mild or moderate increases in sIL2R
levels were observed. The sIL2R levels in various inflammatory
diseases are shown in Fig. 1F. Of
note, 3 of 4 patients with sIL2R levels >2,000 U/ml had a drug
allergy or another IgG4-related disease (Mikulicz disease).
Chronological increase of sIL2R levels
in relation to disease progression
In our cohort, 13 patients with diffuse large B-cell
lymphoma had their sIL2R levels re-measured prior to treatment
(interval range, 19–168 days; median, 27 days). The evaluation of
sIL2R levels (median, 2,685 U/ml) revealed that they had increased
from the initial measurement (median, 1,156 U/ml), consistently
with disease progression (P=0.0198).
Diagnostic value of serum sIL2R level
for the diagnosis of malignant lymphoma
Patients in the ML and other groups were divided
into sIL2R increase-positive and -negative populations by adjusting
the cutoff value of the sIL2R level 500–5,000 U/ml by every 500 or
1,000 U/ml (Table IV). The Sn
decreased from 47.4 to 15% by incrementing the point of the cutoff
value, whereas the Sp increased from 76.5 to 100%. When the Sp was
80%, the threshold level of sIL2R was 1,104 U/ml, at which point ML
was suspected. When the cutoff value was increased up to 1,500
U/ml, the Sp, odds ratio, and positive likelihood ratio (LR+) were
elevated to 87%, 4.28, and 2.997, respectively. Even when the
cutoff value was adjusted to 2,000 U/ml, the Sp, odds ratio and LR+
were elevated to 93.2%, 17.07 and 5.058, respectively.
 | Table IV.Diagnostic characteristic in each
cut-off level of sIL2R for the diagnosis of ML. |
Table IV.
Diagnostic characteristic in each
cut-off level of sIL2R for the diagnosis of ML.
| Cut-off sIL2R level
(U/ml) | ML/other | Sn | Sp | PPV | NPV | Accuracy | LR+ | LR- | OR |
|---|
| ≥500 | 105/61 | 0.79 | 0.47 | 0.63 | 0.66 | 0.64 | 1.4 | 0.45 | 3.32 |
| <500 | 28/54 |
|
|
|
|
|
|
|
|
| ≥1,000 | 63/27 | 0.47 | 0.77 | 0.70 | 0.56 | 0.61 | 2.02 | 0.69 | 2.93 |
| <1,000 | 70/88 |
|
|
|
|
|
|
|
|
| ≥1,500 | 52/15 | 0.39 | 0.87 | 0.78 | 0.55 | 0.61 | 3.00 | 0.70 | 4.28 |
| <1,500 | 81/100 |
|
|
|
|
|
|
|
|
| ≥2,000 | 46/8 | 0.35 | 0.93 | 0.85 | 0.55 | 0.62 | 4.97 | 0.70 | 7.07 |
| <2,000 | 87/107 |
|
|
|
|
|
|
|
|
| ≥2,500 | 37/4 | 0.28 | 0.97 | 0.90 | 0.54 | 0.60 | 8.00 | 0.75 | 10.7 |
| <2,500 | 96/111 |
|
|
|
|
|
|
|
|
| ≥3,000 | 35/3 | 0.26 | 0.97 | 0.92 | 0.53 | 0.59 | 10.1 | 0.76 | 13.3 |
| <3,000 | 98/112 |
|
|
|
|
|
|
|
|
| ≥4,000 | 26/1 | 0.20 | 0.99 | 0.96 | 0.52 | 0.57 | 22.5 | 0.81 | 27.7 |
| <4,000 | 107/114 |
|
|
|
|
|
|
|
|
| ≥5,000 | 20/0 | 0.15 | 1.00 | 1.00 | 0.50 | 0.54 | Inf | 0.85 | – |
| <5,000 | 113/115 |
|
|
|
|
|
|
|
|
The ROC curve for prediction of lymphoma by sIL2R is
shown in Fig. 2. The area under the
curve (AUC) was 0.695. The curve was nearest to the left corner of
the plot when the threshold was 1,946 U/ml, at which point the Sn
and Sp were 34.6 and 93.2%, respectively. The positive predictive
value (PPV) and negative predictive value (NPV) at this threshold
were 85.2 and 55.6%, respectively. This threshold was considered
appropriate, as the Sp declined rapidly when a threshold of
<1,946 was applied. Age and LDH levels appeared to contribute to
the diagnosis of ML. By contrast, WBC and CRP did not appear to be
predictive of the presence of ML. The ROC curve was nearest to the
left corner of the plot when the thresholds were as follows: Age 46
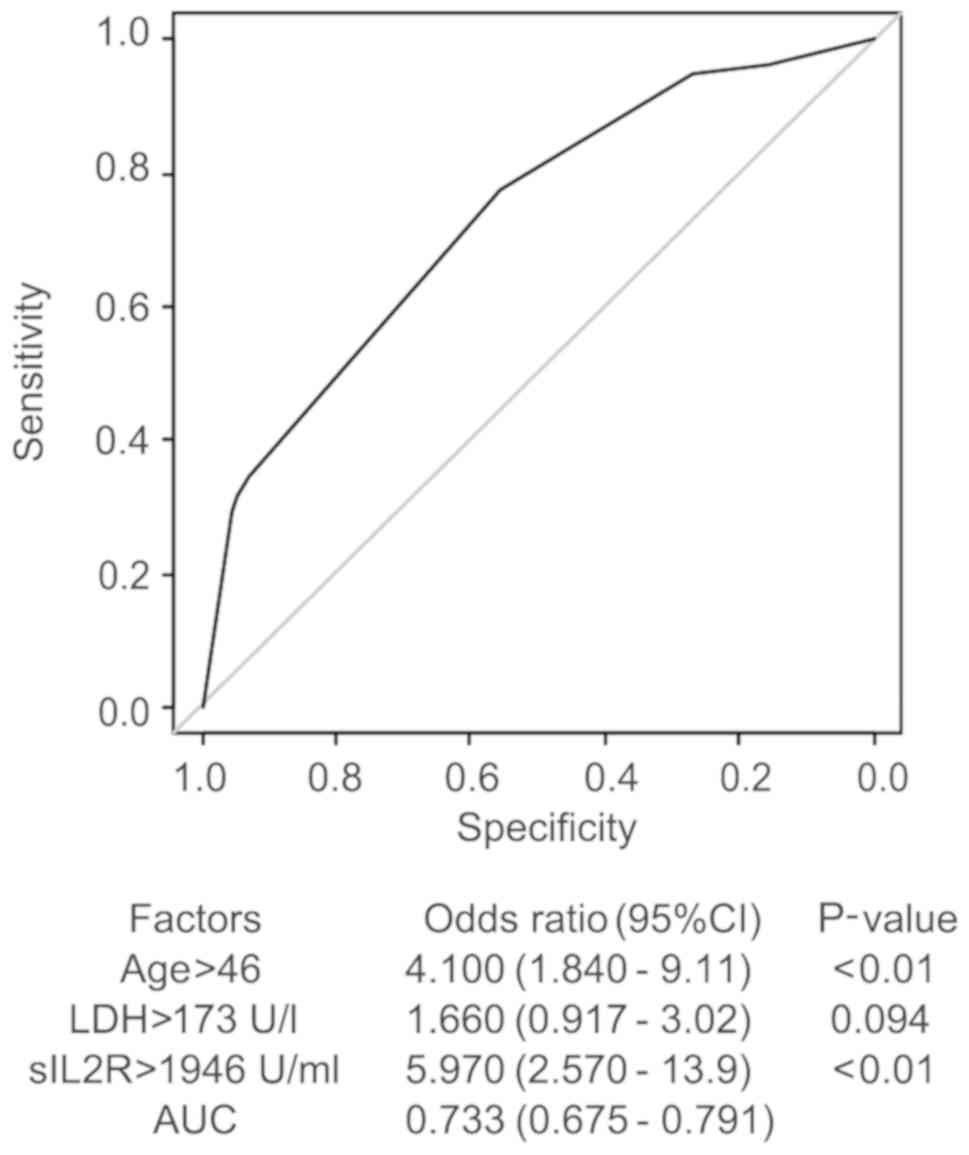
years, LDH 173 U/l, and sIL2R 1,946 U/ml (Fig. 2).
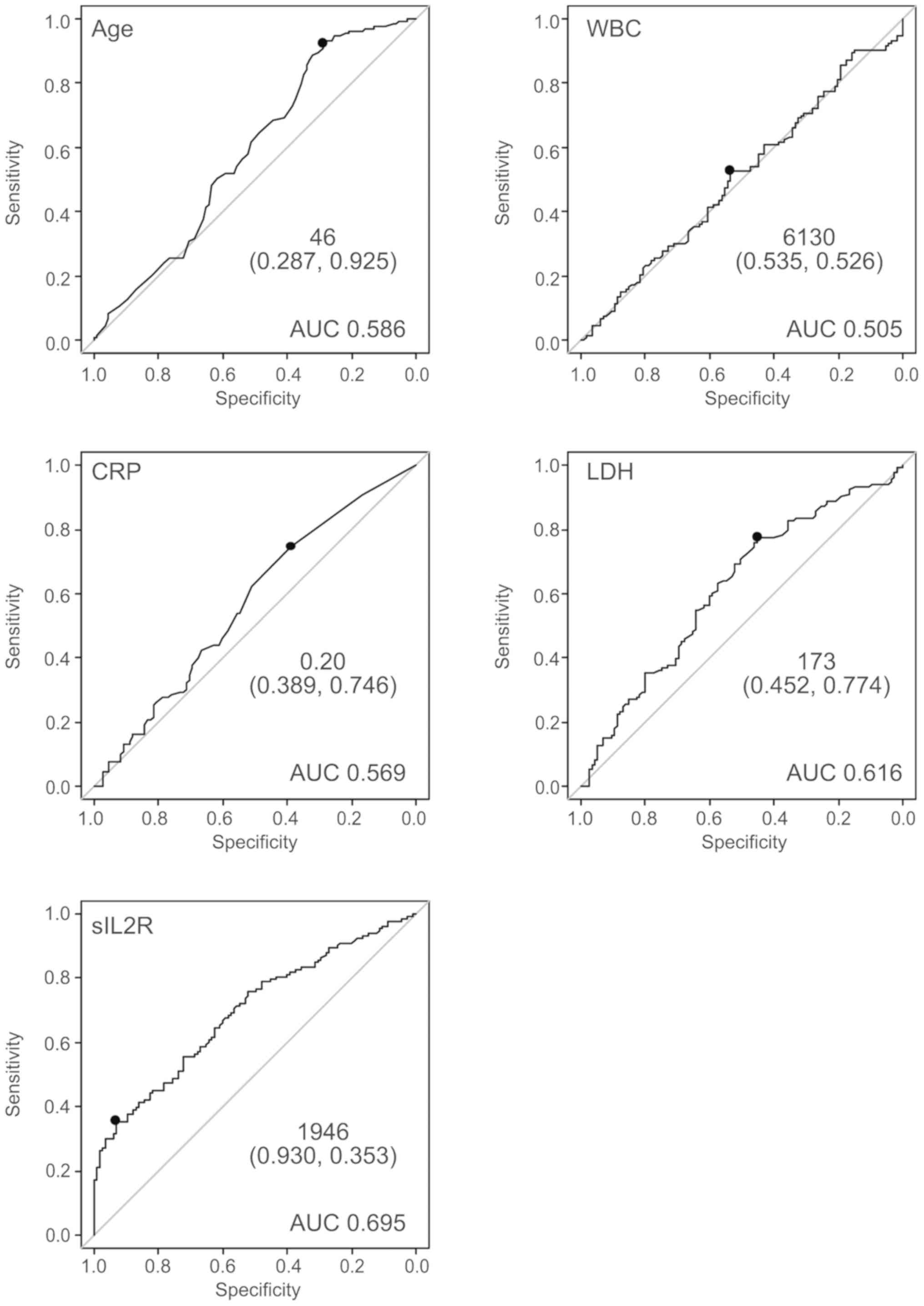 | Figure 2.The ROC curve for prediction of
lymphoma by several clinical parameters. The ROC curve for
prediction of lymphoma by several clinical parameters (age, WBC
count, CRP, LDH, sIL2R). WBC and CRP did not appear to contribute
to the diagnosis of ML. By contrast, age, LDH, and sIL2R appeared
to be of diagnostic value for the presence of ML. ROC, receiver
operating characteristic; WBC, white blood cell; CRP, C-reactive
protein; LDH, lactate dehydrogenase; sIL2R, soluble interleukin-2
receptor; AUC, area under the ROC curve; ML, malignant
lymphoma. |
Risk factors in the diagnosis of
malignant lymphoma by multivariate analysis
The multivariate analysis of the serum sIL2R level
and several factors (age, sex, presence of fever, WBC count, LDH
and sIL2R) was performed using the multivariate logistic regression
model. The AUC was 0.695 for sIL2R alone, and increased to 0.733 by
including age >46 years an LDH >173 U/l with the sIL2R cutoff
value at 1,946 U/ml (Fig. 3). The
adjusted odds ratio of the sIL2R level was 5.97.
This analysis suggests that the risk factors for the
diagnosis of ML are age >46 years, LDH >173 U/l, as well as
sIL2R >1,946 U/ml. However, the diagnostic value of higher age
[Sn 0.17, Sp 0.287, PPV 0.598, NPV 0.750, accuracy 0.625, LR+
1.286, negative likelihood ratio (LR-) 0.288] and LDH (Sn 0.767, Sp
0.452, PPV 0.617, NPV 0.627, accuracy 0.621, LR+ 1.400, LR- 0.515)
was relatively small compared with that of higher levels of sIL2R
(Sn 0.346, Sp 0.930, PPV 0.852, NPV 0.552, accuracy 0.617, LR+
4.972, LR- 0.703). When these factors were combined with sIL2R, the
diagnostic value mildly improved (Sn 0.293, Sp 0.957, PPV 0.886,
NPV 0.539, accuracy 0.601, LR+ 6.744, LR- 0.739) compared with that
of sIL2R alone (Table V).
 | Table V.Diagnostic characteristic in each
risk group for the diagnosis of ML. |
Table V.
Diagnostic characteristic in each
risk group for the diagnosis of ML.
| Risk factors | ML/other | Sn | Sp | PPV | NPV | Accuracy | LR+ | LR- |
|---|
| Age, years |
|
>46 | 122/82 | 0.92 | 0.29 | 0.60 | 0.75 | 0.63 | 1.29 | 0.29 |
|
≤46 | 11/33 |
|
|
|
|
|
|
|
| LDH, U/l |
|
>173 | 102/63 | 0.77 | 0.45 | 0.62 | 0.63 | 0.62 | 1.40 | 0.52 |
|
≤173 | 31/52 |
|
|
|
|
|
|
|
| sIL2R, U/ml |
|
>1,946 | 46/8 | 0.35 | 0.93 | 0.85 | 0.55 | 0.62 | 4.97 | 0.70 |
|
≤1,946 | 87/107 |
|
|
|
|
|
|
|
| Number of risk
factors |
| 0 | 5/18 | 0.04 | 0.84 | 0.22 | 0.43 | 0.41 | 0.24 | 1.14 |
|
| 128/97 |
|
|
|
|
|
|
|
| 1 | 25/46 | 0.19 | 0.60 | 0.35 | 0.39 | 0.38 | 0.47 | 1.35 |
|
| 108/69 |
|
|
|
|
|
|
|
| 2 | 64/46 | 0.48 | 0.60 | 0.58 | 0.50 | 0.54 | 1.20 | 0.87 |
|
| 69/69 |
|
|
|
|
|
|
|
| 3 | 39/5 | 0.29 | 0.96 | 0.89 | 0.54 | 0.60 | 6.74 | 0.74 |
|
| 94/110 |
|
|
|
|
|
|
|
In summary, sIL2R appears to be a more useful
predictive marker compared with any other clinical parameters for
the diagnosis of lymphomas.
Discussion
The present study demonstrated the diagnostic
characteristics of sIL2R in the diagnosis of lymphomas. There have
been few reports evaluating the value of serum sIL2R levels for the
differential diagnosis of lymphoma from other conditions in the
clinical setting.
Nakase et al (7) reported that the sIL2R levels are
elevated in patients with hematological neoplasms and
non-hematological solid tumors compared with those in healthy
subjects. Furthermore, extremely high levels (>3,000 U/ml) are
only observed in acute leukemia or malignant lymphomas. These
findings suggest that sIL2R levels may be a useful marker for the
differential diagnosis between hematological neoplasms and
non-hematological solid tumors in patients with bulky diseases
(7). Patients with infectious
diseases were excluded from this study. Similar results were
observed in pediatric patients with leukemia, lymphoma and
malignant solid tumors (3). As
lymphoma may mimic a wide variety of diseases, the differential
diagnosis of infectious or inflammatory diseases is crucial in
patients presenting with inflammatory symptoms.
Tsujioka et al retrospectively analyzed sIL2R
levels in consecutive ML patients and compared them with those in
non-hematological diseases categorized by initial diagnosis
(8). They compared the sIL2R levels
between patients with ML and patients in the control group, which
was divided into 6 categories as follows: Autoimmune diseases,
non-hematological tumors, infections, fever of unknown origin
(FUO), lymphadenopathy, and others. They observed that sIL2R was
moderately increased in non-hematological diseases, with higher
levels at 1,500 U/ml, at which level the PPV and LR+ were also
elevated, indicating diagnostic accuracy for lymphomas.
In the present study, control groups were
categorized by final diagnosis according to the observed clinical
outcome. Our data also suggest that the diagnostic threshold of
sIL2R, in which ML is considered, is at 1,104-1,500 U/ml, at which
point Sp is elevated to 80%. In our cohort, the optimal cutoff
level of sIL2R for the diagnosis of lymphoma was 1,946-2,000 U/ml,
at which point the Sn was 35% and the Sp was 93%, strongly
suggesting the diagnosis of ML. At those levels, serum sIL2R
appears to be more useful compared with any other non-invasive
marker for the diagnosis of lymphomas.
In the present study, aggressive lymphomas exhibited
the highest serum sIL2R levels; however, indolent lymphomas
exhibited a mild elevation, similar to other disease categories.
Furthermore, among aggressive lymphomas, the sIL2R levels in
patients with T/NK lymphomas (even when ATLL was excluded) were
significantly higher compared with those in patients with
aggressive B-cell lymphomas. These findings are consistent with
previous reports (6–8).
In Japan, serum sIL2R measurement has been
introduced in clinical practice (1,4,6–8,10–12) and
has been commercially available and covered by insurance since
October 1994, for the purpose of evaluating the response after
treatment and disease monitoring after remission in patients with
ATLL and non-Hodgkin lymphomas. Furthermore, from April 2006
onwards, sIL2R has been utilized for screening as a tumor marker
when there is suspected ATLL or non-Hodgkin lymphoma. At present,
sIL2R is often used for patients with suspected lymphomas for
screenings and differential diagnosis of tumors with uncommon
presentation, lymphadenopathy with atypical course, cases with FUO,
or even unidentified complaints, as lymphoma may present with
various clinical symptoms, mimicking a number of conditions
(13), and may be considered in the
differential diagnosis of a wide variety of diseases.
It appears difficult to distinguish ML from other
conditions. However, the present study reported some evocative
findings. Extremely high sIL2R levels strongly suggest the
diagnosis of aggressive lymphoma, although common false-positive
exceptions should be considered, including advanced non-lymphoid
solid tumors with splenic involvement, drug allergies presenting
with marked inflammatory symptoms, and tuberculosis or other
infectious diseases. In addition, some common false-negative cases
include indolent lymphomas or patients with low tumor burden.
We herein attempted to evaluate sIL2R as a tumor
marker for screening and differential diagnosis using data
collected from consecutive patients with suspected lymphoma who had
their sIL2R levels measured in various clinical presentations.
Further investigations are required to evaluate the usefulness of
sIL2R in specific clinical settings to clearly determine the
subcategory of patients with clinical symptoms or characteristics
for whom sIL2R measurement may prove useful. For example, sIL2R was
found to be useful as a diagnostic marker in hemophagocytic
syndromes/hemophagocytic lymphohistiocytosis associated with
lymphomas (14–16).
In conclusion, as serum sIL2R levels may be elevated
in several pathological conditions, including inflammatory or
neoplastic diseases, they appear to be a less specific marker.
However, the findings of the present study indicate that higher
levels of sIL2R strongly suggest the presence of lymphoma, and may
thus be useful for the diagnosis of aggressive types of lymphomas,
after ruling out other false-positive conditions.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JM and HM designed the study and wrote the initial
draft of the manuscript. KA contributed to refining the figures.
KA, AW, IY and TS contributed to the acquisition, analysis and
interpretation of data. HO and MK verified the analytical methods.
All authors discussed the results and contributed to the final
manuscript.
Ethics approval and consent to
participate
The study obtained ethical approval for the use of
an opt-out methodology due to the low risk to the patient and the
potential benefit for the patient of adequate diagnosis based on
unbiased information.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALL
|
acute lymphoblastic leukemia
|
|
ATLL
|
adult T-cell lymphoma/leukemia
|
|
AUC
|
area under the curve
|
|
CLL
|
chronic lymphocytic leukemia
|
|
CRP
|
C-reactive protein
|
|
FUO
|
fever of unknown origin
|
|
IL-2
|
interleukin 2
|
|
LDH
|
lactate dehydrogenase
|
|
LR+
|
positive likelihood ratio
|
|
LR-
|
negative likelihood ratio
|
|
NPV
|
negative predictive value
|
|
PPV
|
positive predictive value
|
|
sIL2R
|
serum soluble interleukin-2
receptor
|
|
Sn
|
sensitivity
|
|
Sp
|
specificity
|
|
ROC
|
receiver operating characteristic
|
|
WBC
|
white blood cell
|
References
|
1
|
Yasuda N, Takamatsu T, Kanoh T and Uchino
H: Serum levels of soluble interleukin 2 receptor in patients with
non-haematological disorders. Br J Haematol. 69:5731988. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rubin LA, Galli F, Greene WC, Nelson DL
and Jay G: The molecular basis for the generation of the human
soluble interleukin 2 receptor. Cytokine. 2:330–336. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bien E and Balcerska A: Serum soluble
interleukin 2 receptor alpha in human cancer of adults and
children: A review. Biomarkers. 13:1–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yasuda N, Lai PK, Ip SH, Kung PC, Hinuma
Y, Matsuoka M, Hattori T, Takatsuki K and Purtilo DT: Soluble
interleukin 2 receptors in sera of Japanese patients with adult T
cell leukemia mark activity of disease. Blood. 71:1021–1026.
1988.PubMed/NCBI
|
|
5
|
Ambrosetti A, Nadali G, Vinante F, Ricetti
MM, Todeschini G, Morosato L, de Sabata D, Bergamo Andreis IA,
Chilosi M, Semenzato G, et al: Soluble interleukin-2 receptor in
hairy-cell leukemia: A reliable marker of disease. Int J Clin Lab
Res. 23:34–37. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoshida N, Oda M, Kuroda Y, Katayama Y,
Okikawa Y, Masunari T, Fujiwara M, Nishisaka T, Sasaki N, Sadahira
Y, et al: Clinical significance of sIL-2R levels in B-cell
lymphomas. PLoS One. 8:e787302013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakase K, Tsuji K, Tamaki S, Tanigawa M,
Ikeda T, Miyanishi E and Shiku H: Elevated levels of soluble
interleukin-2 receptor in serum of patients with hematological or
non-hematological malignancies. Cancer Detect Prev. 29:256–259.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsujioka T, Kishimoto M, Kondo T, Matsuoka
A, Tasaka T, Sugihara T, Wada H and Tohyama K: The impact of serum
soluble interleukin-2 receptor levels on the diagnosis of malignant
lymphoma. Kawasaki Med J. 37:19–27. 2011.
|
|
9
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kamihira S, Atogami S, Sohda H, Momita S,
Yamada Y and Tomonaga M: Significance of soluble interleukin-2
receptor levels for evaluation of the progression of adult T-cell
leukemia. Cancer. 73:2753–2758. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Murakami S, Satomi A, Ishida K, Murai H,
Matsuki M and Hashimoto T: Serum-soluble interleukin-2 receptor
concentrations in patients with gastric cancer. Cancer.
74:2745–2748. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goto H, Tsurumi H, Takemura M,
Ino-Shimomura Y, Kasahara S, Sawada M, Yamada T, Hara T, Fukuno K,
Goto N, et al: Serum-soluble interleukin-2 receptor (sIL-2R) level
determines clinical outcome in patients with aggressive
non-Hodgkin's lymphoma: In combination with the International
Prognostic Index. J Cancer Res Clin Oncol. 131:73–79. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakashima MO, Roy DB, Nagamine M, Roullet
MR, Gabriel CA, Sood SL and Bagg A: Intravascular large B-cell
lymphoma: A mimicker of many maladies and a difficult and often
delayed diagnosis. J Clin Oncol. 29:e138–e140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tabata C and Tabata R: Possible prediction
of underlying lymphoma by high sIL-2R/ferritin ratio in
hemophagocytic syndrome. Ann Hematol. 91:63–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hayden A, Lin M, Park S, Pudek M,
Schneider M, Jordan MB, Mattman A and Chen LYC: Soluble
interleukin-2 receptor is a sensitive diagnostic test in adult HLH.
Blood Adv. 1:2529–2534. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin M, Park S, Hayden A, Giustini D,
Trinkaus M, Pudek M, Mattman A, Schneider M and Chen LYC: Clinical
utility of soluble interleukin-2 receptor in hemophagocytic
syndromes: A systematic scoping review. Ann Hematol. 96:1241–1251.
2017. View Article : Google Scholar : PubMed/NCBI
|