Introduction
In patients with diffuse large B cell lymphoma
(DLBCL), rituximab-based chemotherapy regimens can achieve superior
long-term progression-free survival (PFS) and overall survival (OS)
rates relative to regimens that do not contain rituximab (1). However, even in the rituximab era,
approximately 10–15% of patients with DLBCL treated with rituximab
plus cyclophosphamide, doxorubicin, vincristine, and prednisone
(R-CHOP) fail to achieve complete remission (CR) (2,3). Such
patients can often be treated with salvage chemotherapy regimens
and subsequent autogenous stem cell transplantation (ASCT)
(4,5). To date, there has been no definite
consensus on salvage therapy in this patient population and little
information on the ideal treatment regimen, with data from
randomized trials of salvage therapy failing to reveal significant
differences between regimens (5).
Two randomized clinical studies have compared treatment regimens
prior to ASCT. In the Collaborative Trial in Relapsed Aggressive
Lymphoma (CORAL) study, R-ICE (rituximab, ifosfamide, carboplatin,
etoposide) was compared with R-DHAP (rituximab, cytosine
arabinoside, cisplatin, dexamethasone), followed by ASCT with or
without rituximab maintenance, and no difference was identified
between the regimens (4). In the
LY02 study, R-DHAP was compared with R-GDP (rituximab, gemcitabine,
dexamethasone, cisplatin) before ASCT. Although no difference in OS
was reported, R-GDP was associated with fewer AEs, improved quality
of life (QOL), and less-frequent hospitalization (5). In a recent patient-level analysis of
outcomes of refractory DLBCL from two large randomized trials and
two academic databases (SCHOLAR-1) (6), an objective response rate of 26% (CR
7%) to the next line of therapy was demonstrated, with a median OS
of 6.3 months. This analysis also showed that a poor outcome was
associated with relapsed and refractory DLBCL. The results from
these previous studies indicate a clear need for novel therapies to
improve outcomes in this patient population.
We therefore sought to determine the safest and most
effective regimen in the clinical setting. Furthermore, with
current treatment trends, we determined which regimens are changing
from administration in an inpatient to an outpatient setting.
Patients and methods
Patients
This retrospective analysis was conducted at Kansai
Medical University Hospital and Kansai Medical University Medical
Center. Among 530 patients diagnosed with DLBCL from April 2002 to
November 2017, 131 relapsed and refractory patients who received
salvage therapy were enrolled in this study. Primary treatment
included R-CHOP or R-CHOP-like regimens. Tumor responses were
assessed according to the classification of the International
Workshop to Standardize Response Criteria for Non-Hodgkin's
Lymphoma 1999. Relapse was defined as emerging new sites or
enlarging sites and refractory disease was defined as
progressive/stable disease during first-line treatment. Performance
status was evaluated by an Eastern Cooperative Oncology Group
(ECOG) performance status score. To predict prognosis, we used both
the international prognostic index (IPI) and the National
Comprehensive Cancer Network-International Prognostic Index
(NCCN-IPI). Although IPI was the most powerful prognostic scale
before the advent of rituximab, its power has reduced (7). NCCN-IPI has been reported more accurate
than older IPI in rituximab era (8).
However, NCCN-IPI has not been evaluated enough in Japan, thus, we
used both scales.
Statistical analysis
Progression-free survival 2 (PFS2) was defined as
the period from the start of initial treatment to the second
exacerbation or death after second-line treatment. OS was
calculated as the time from diagnosis until the time of death or
the last clinical follow-up.
Survival curves were generated using the
Kaplan-Meier method, and differences were evaluated using the
log-rank test. Multivariate Cox-proportional hazards models were
used to determine whether baseline characteristics were associated
with PFS2 and OS. All statistical tests were two-sided, statistical
significance was defined as P<0.05, and 95% confidence intervals
(CIs) were calculated. All statistical analyses were performed
using EZR (Saitama Medical Center, Jichi Medical University,
Saitama, Japan), a graphical user interface for R version 2.13.0
(The R Foundation). Specifically, EZR is a modified version of R
Commander (version 1.6–3) that adds statistical functions
frequently used in biostatistics (9). Toxicity was evaluated according to the
Common Terminology Criteria for Adverse Events (AEs) (CTCAE 4.0,
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES, National Institutes
of Health, National Cancer Institute, [http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40]).
This study was conducted in accordance with the ethical principles
of the Declaration of Helsinki, and was approved by the
institutional review board of Kansai Medical University.
Results
Patient characteristics
Clinical characteristics of the 131 enrolled
patients (median age, 68 years; age range, 35–87 years; 54% male)
are shown in Table I. PS over 2 was
7%. Stage I was 11%, Stage II was 16%, Stage III was 18%, and Stage
IV was 55%. Using the IPI, 22% of patients were classified as being
at low risk, 18% as low-intermediate (LI) risk, 22% as
high-intermediate (HI) risk, and 38% as high risk. In comparison,
under the National Comprehensive Cancer Network (NCCN)-IPI, 7% of
patients were classified as being at low risk, 40% as LI, 50% as
HI, and 3% as high risk. The median follow-up period was 33.6
months (range, 1.9–155.9 months).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | Number (%) |
|---|
| Number of
patients | 131 |
| Median age, years
(range) | 68 (35–87) |
| Male | 71 (54) |
| PS ≥2 | 9 (7) |
| Stage |
|
| I | 14 (11) |
| II | 21 (16) |
| III | 24 (18) |
| IV | 72 (55) |
| IPI |
|
| Low | 29 (22) |
|
Low-int | 23 (18) |
|
High-int | 29 (22) |
| High | 50 (38) |
| NCCN-IPI |
|
| Low | 10 (7) |
|
Low-int | 52 (40) |
|
High-int | 65 (50) |
| High | 4 (3) |
Salvage regimens
The most common salvage regimen was R-DeVIC
(rituximab, etoposide, dexamethasone, ifosfamide, carboplatin)
(42%), followed by R-ESHAP (rituximab, etoposide, solumedrol,
cytarabine, cisplatin) (23%) (Fig.
1). Other aggressive regimens were administered to 12% of
patients, and included R-CHASE (rituximab, cyclophosphamide,
cytosine arabinoside, etoposide, dexamethasone) (n=5), rituximab
plus methotrexate-based treatment (n=5), R-CHOP-based treatment
(n=3), R-GDP (rituximab, gemcitabine, cisplatin, dexamethasone)
(n=2), and R-EPOCH (rituximab, etoposide, vincristine, doxorubicin,
cyclophosphamide, prednisolone) (n=1). Finally, 23% of patients
underwent palliative therapy such as radiation, rituximab
monotherapy, oral etoposide, or oral prednisolone.
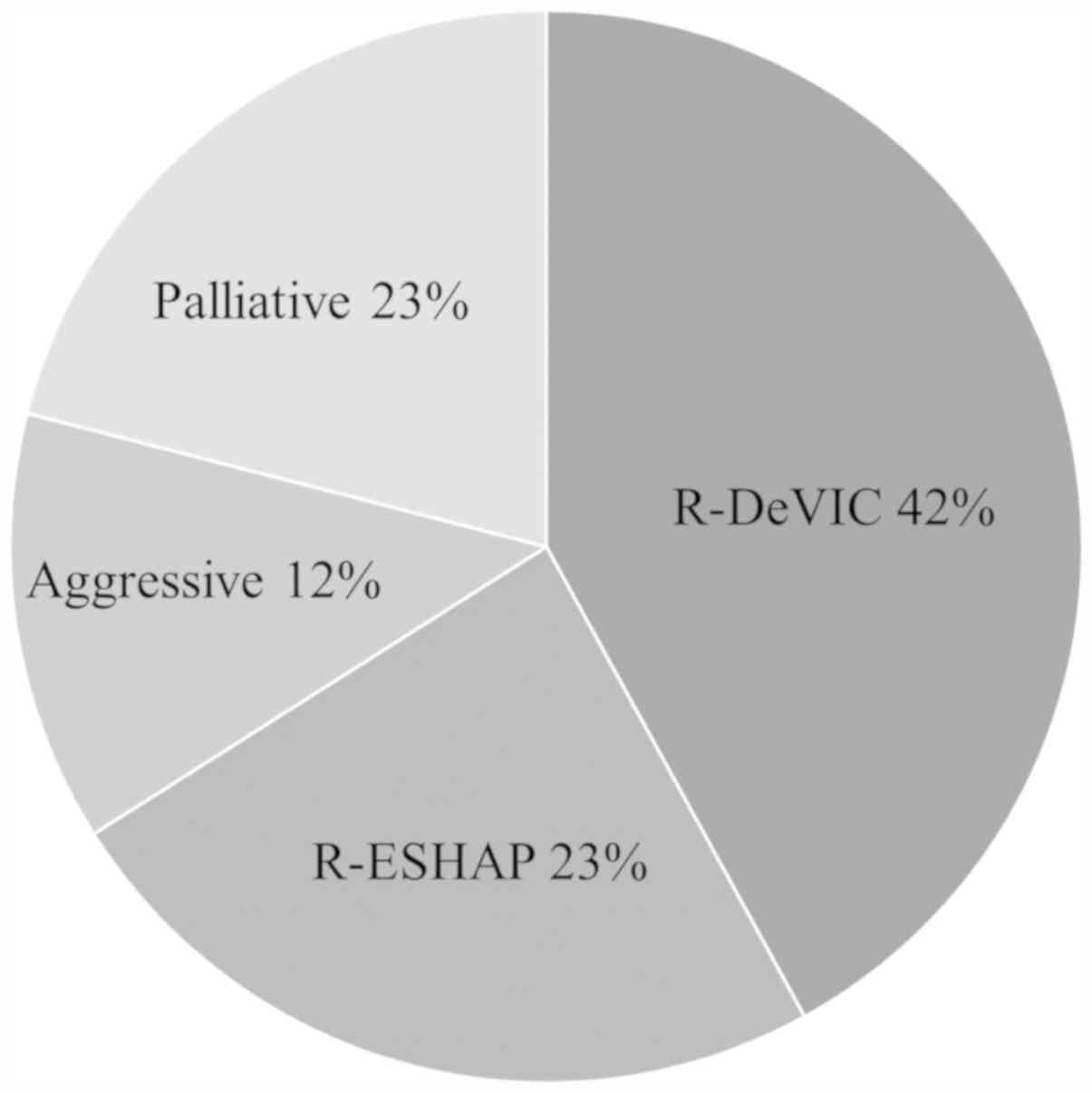 | Figure 1.Percentages of salvage therapies. The
aggressive regimen was applied in 12% of cases, and included
R-CHASE (n=5), rituximab plus methotrexate-based (n=5), R-CHOP like
(n=3), R-GDP (n=2) and R-EPOCH (n=1). Palliative therapy included
radiation only, rituximab monotherapy, oral etoposide and oral
prednisolone. R-CHASE, rituximab, cyclophosphamide, cytosine
arabinoside, etoposide, dexamethasone; R-GDP, rituximab,
gemcitabine, cisplatin, dexamethasone; R-EPOCH, rituximab,
etoposide, vincristine, doxorubicin, cyclophosphamide,
prednisolone; R-DeVIC, rituximab, etoposide, dexamethasone,
ifosfamide, carboplatin; R-ESHAP, rituximab, etoposide, solumedrol,
cytarabine, cisplatin. |
Survival
Median OS by regimen was 45.7 (30.7–75.8) months for
R-DeVIC, 41.8 (23.4–92.3) months for palliative therapy, 29.4
(14.1–98.1) months for R-ESHAP, and 28.5 (13.5-not applicable)
months for aggressive regimens (P=0.937; Fig. 2).
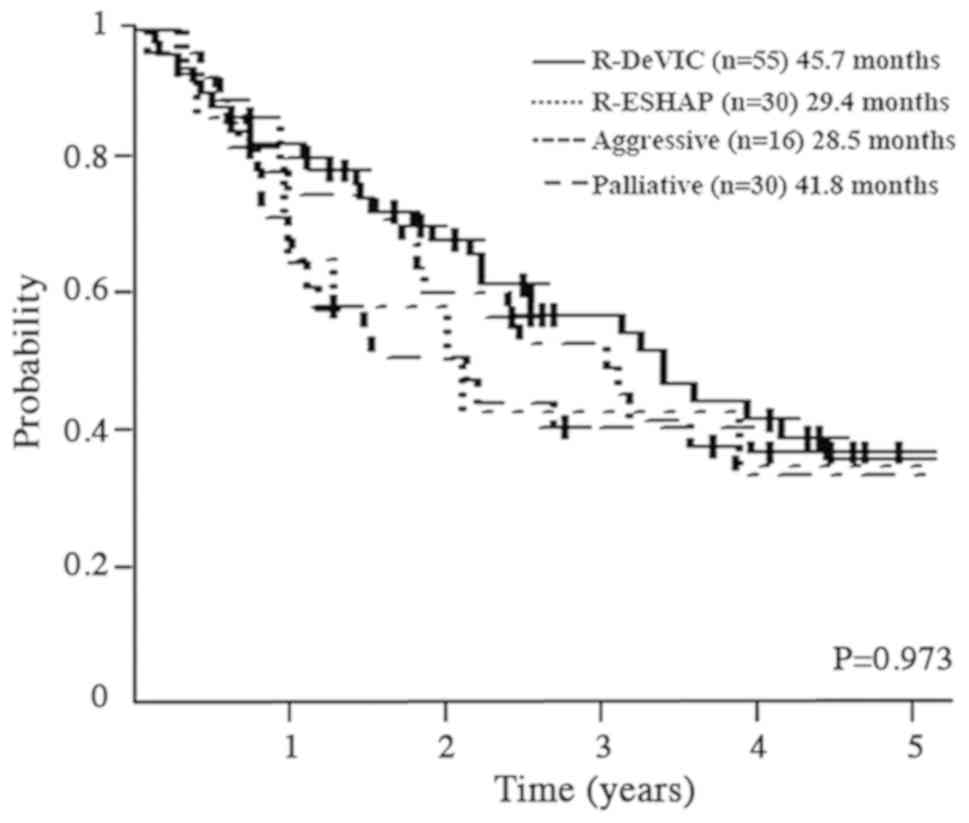 | Figure 2.Survival of patients treated using
different regimens. R-DeVIC, rituximab, etoposide, dexamethasone,
ifosfamide, carboplatin; R-ESHAP, rituximab, etoposide, solumedrol,
cytarabine, cisplatin. |
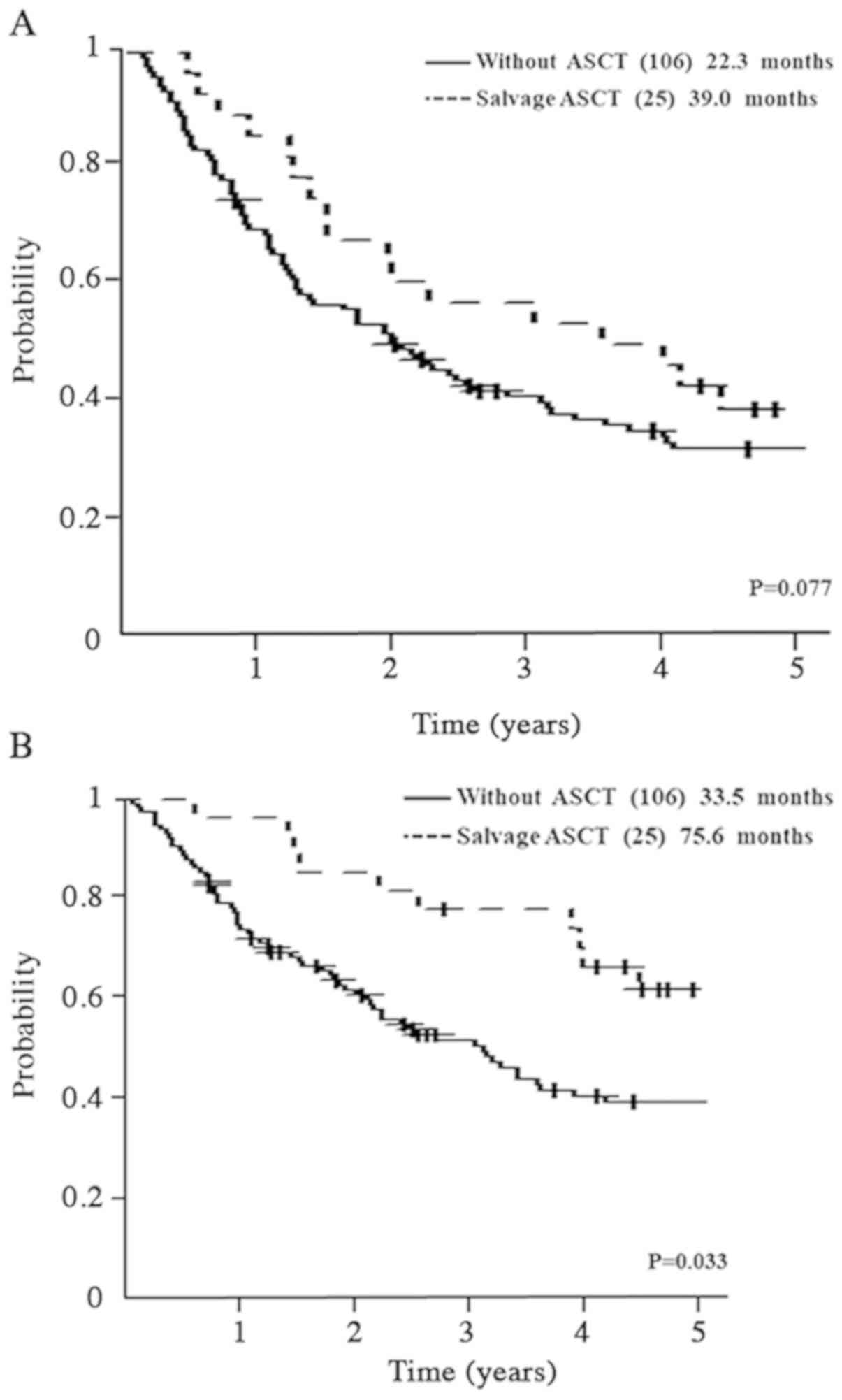
Outcome of salvage ASCT
Twenty-five patients underwent ASCT, and had PFS2 of
39.0 (19.2–56.6) months compared with 22.3 (15.2–29.0) months in
patients who did not undergo ASCT (P=0.077; Fig. 3A). OS was 75.6 (51.8-not applicable)
months in patients undergoing ASCT vs. 33.5 (25.6–45.6) months in
patients who did not undergo ASCT (P=0.033; Fig. 3B).
Prognostic factors
Multivariate analysis was performed to identify risk
factors associated with PFS2 and OS. Age over 70 years, male sex,
PS>3, more than HI in IPI, and more than HI in NCCN-IPI were
evaluated. NCCN-IPI [Hazard ratio (HR)]: 2.22, 95% CI: 1.46–3.38,
P=0.0001), male (HR: 1.60, 95% CI: 1.06–2.41, P=0.024), and PS>3
(HR: 1.47, 95% CI: 1.06–2.03, P=0.022) remained as significant
factors affecting PFS2 in multivariate analysis, whereas other
factors were eliminated by backward stepwise selection. NCCN-IPI
(HR: 2.56, 95% CI: 1.56–4.20, P=0.0002), IPI (HR: 1.68, 95% CI:
1.00–2.82, P=0.049), and male (HR: 1.58, 95% CI: 1.00–2.47,
P=0.049) remained as significant factors affecting survival.
Regimen transmission to outpatient
chemotherapy
Our facilities established an outpatient
chemotherapy unit in 2014, after which patients were primarily
treated as outpatients. We analyzed the change in proportion of
regimen prior to and after 2014 (Fig.
4). Before 2014, R-DeVIC was the most commonly administered
regimen (37%), followed by R-ESHAP (31%). After 2014, R-DeVIC use
increased to 46%, whereas R-ESHAP decreased to 17%.
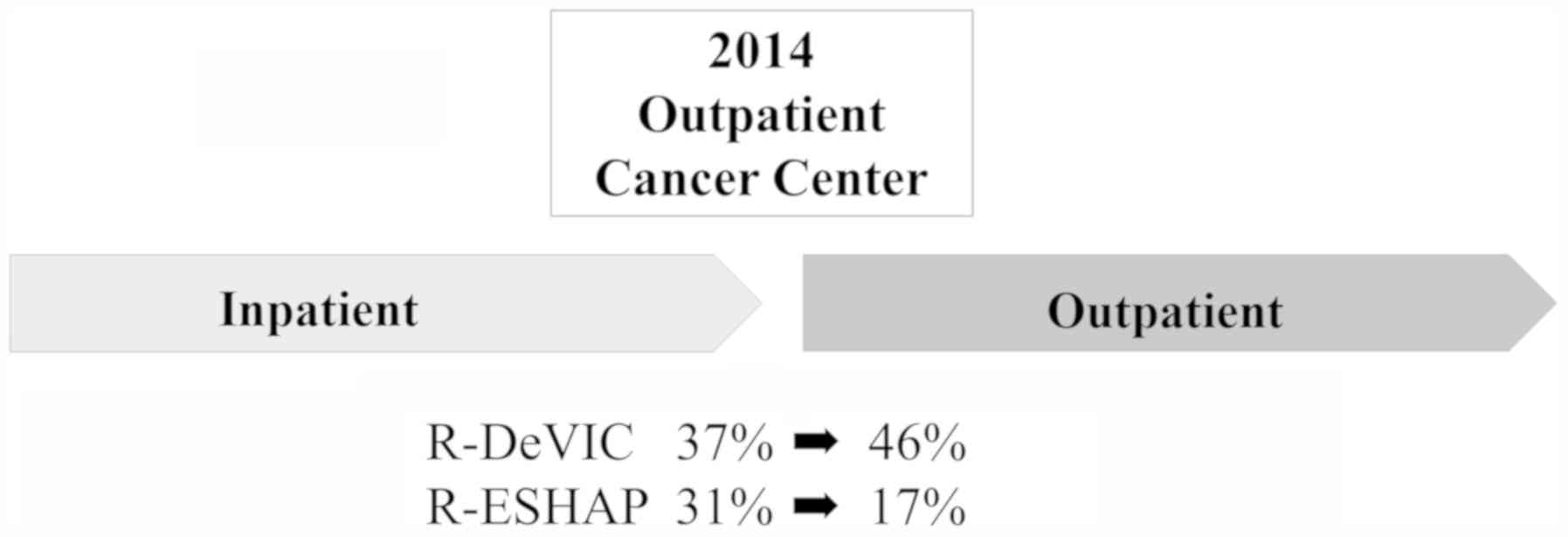 | Figure 4.Transition of regimen in the
outpatient chemotherapy unit. An outpatient chemotherapy unit was
established in 2014. Prior to 2014, R-DeVIC was the most common
regimen, followed by R-ESHAP. After 2014, R-DeVIC increased to 46%,
whereas R-ESHAP decreased to 17%. R-DeVIC, rituximab, etoposide,
dexamethasone, ifosfamide, carboplatin; R-ESHAP, rituximab,
etoposide, solumedrol, cytarabine, cisplatin. |
Comparison between R-DeVIC and
R-ESHAP
In our facilities, more than half of enrolled
patients were treated with either R-DeVIC or R-ESHAP as salvage
therapy. Thus, we compared the efficacy of these two regimens. The
treatment schedule is shown in Table
II, and patient characteristics are presented in Table III. In total, 55 patients were
treated with R-DeVIC and 30 patients were treated with R-ESHAP.
Median age was 70 years in the R-DeVIC group and 61 years in the
R-ESHAP group. The most frequent adverse events other than
hematological events (grade≥3) were febrile neutropenia (n=4 in
R-DeVIC, n=8 in R-ESHAP) followed by infection (n=1 in R-DeVIC, n=3
in R-ESHAP). Early-relapse patients comprised 44% of the R-DeVIC
group and 77% of the R-ESHAP group. PFS2 was 24.7 (15.5–39.0)
months in the R-DeVIC group and 13.8 (10.2–27.4) months in the
R-ESHAP group (P=0.643; Fig. 5A),
while OS was 45.7 (30.7–75.8) months in the R-DeVIC group and 29.4
(14.1–98.1) months in the R-ESHAP group (P=0.53; Fig. 5B).
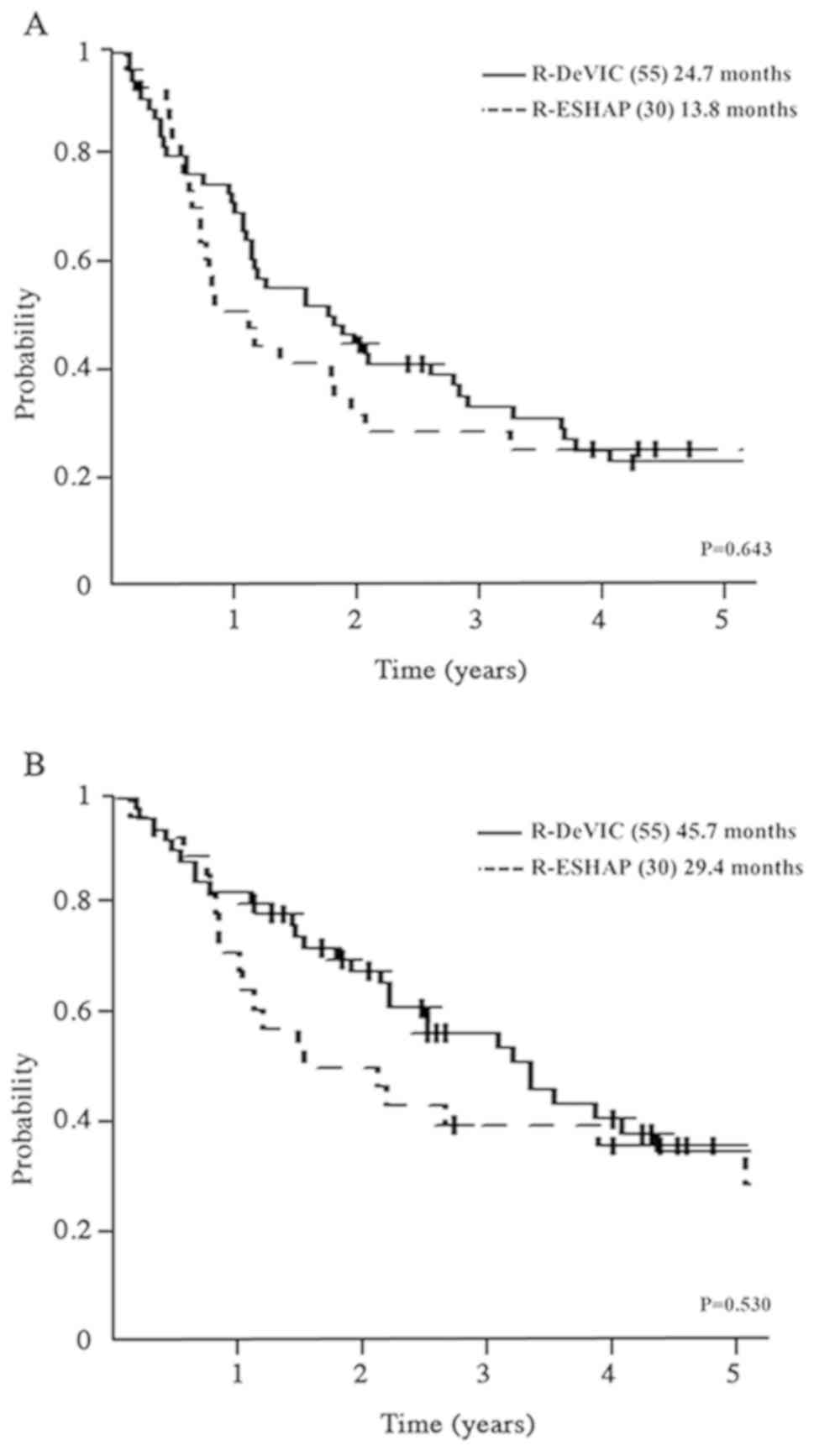 | Figure 5.Comparison of PFS and OS between
R-DeVIC and R-ESHAP. (A) Comparison of PFS between R-DeVIC and
R-ESHAP. (B) Comparison of OS between R-DeVIC and R-ESHAP. PFS,
progression-free survival; OS, overall survival; R-DeVIC,
rituximab, etoposide, dexamethasone, ifosfamide, carboplatin;
R-ESHAP, rituximab, etoposide, solumedrol, cytarabine,
cisplatin. |
 | Table II.Treatment schedule. |
Table II.
Treatment schedule.
|
| R-DeVIC (peripheral
vein) | R-ESHAP (central
vein) |
|---|
|
|
|
|
|---|
| Drug | Dose | Day 1 | Day 2 | Day 3 | Drug | Dose | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
|---|
| Rituximab | 375
mg/m2 | x |
|
| Rituximab | 375
mg/m2 | x |
|
|
|
|
| Ifosfamide | 15,00
mg/m2 | x | x | x | Cytarabine | 2,000
mg/m2 | x | x | x | x |
|
| Carboplatin | 300
mg/m2 | x |
|
| Cisplatin | 25
mg/m2 | x | x | x | x | x |
| Etoposide | 100
mg/m2 | x | x | x | Etoposide | 40
mg/m2 | x |
|
|
|
|
| Dexamethasone | 33 mg | x | x | x |
Methylprednisolone | 500 mg | x | x | x | x |
|
 | Table III.Comparison between R-DeVIC and
R-ESHAP. |
Table III.
Comparison between R-DeVIC and
R-ESHAP.
| Characteristics | R-DeVIC | R-ESHAP |
|---|
| Patients, n | 55 | 30 |
| Median age, years
(range) | 70 (35–84) | 61 (41–68) |
| Male sex, % | 58 | 52 |
| PS ≥2 at relapse, n
(%) | 2 (4) | 6 (19) |
| Stage at Dx, n
(%) |
|
|
| I | 6 (11) | 1 (3) |
| II | 8 (15) | 4 (13) |
| III | 13 (24) | 7 (23) |
| IV | 28 (50) | 19 (61) |
| Stage at relapse, n
(%) |
|
|
| I | 2 (5) | 5 (16) |
| II | 11 (20) | 1 (3) |
| III | 18 (33) | 9 (29) |
| IV | 24 (42) | 16 (52) |
| AE, n |
|
|
| FN | 4 | 8 |
|
Infection | 1 | 3 |
|
Ileus | 1 | 1 |
| DIC | 0 | 1 |
| Heart
disease | 0 | 1 |
| Early relapse, % | 44 | 77 |
| ASCT, % | 16 | 42 |
Discussion
In the present analysis, R-DeVIC and R-ESHAP were
used more frequently than other salvage regimens. Given that the
choice of regimen was at the physician's discretion, it is
difficult to determine the precise reason for selecting a
particular regimen. However, we suggest that several factors may
have contributed to regimen choice. First, R-ESHAP was administered
to patients with more aggressive disease progression and worse
condition, but with younger age. Physicians may have selected this
regimen with the intention of subsequent salvage ASCT, as 42% of
patients who received R-ESHAP later underwent ASCT. However,
R-ESHAP treatment was associated with more AEs compared with
R-DeVIC treatment. After 2014, the number of patients receiving
R-ESHAP decreased with the establishment of our outpatient
facility.
R-DeVIC was most commonly used regimen throughout
the present study. The decisive factor in the selection of R-DeVIC
vs. R-ESHAP is the need for hospitalization (Table II). As R-ESHAP is administered via a
central venous catheter, patients require hospitalization. However,
R-DeVIC can be given via a peripheral vein, and this type of
regimen became more common after the establishment of the
outpatient chemotherapy unit in 2014. Given that previous large
trials have shown no difference between the salvage regimens, we
suggest that the use of the outpatient unit is the likely reason
for the increase in R-DeVIC use observed in our analysis.
Unfortunately, our findings failed to show a
difference between R-DeVIC and R-ESHAP with respect to PFS2 and OS.
However, patients that received R-DeVIC tended to have a better
prognosis. As detailed above, R-ESHAP was selected in patients with
more aggressive disease progression and worse condition. Thus,
R-ESHAP may have been associated with a tendency toward worse
prognosis.
ASCT has been previously shown to be an effective
salvage therapy in patients with DLBCL (4,5). In the
present study, the number of patients who received ASCT was low
compared with the patient population in SCHOLAR-1 study (6). This difference may be attributable to a
higher median age in the present analysis (55 years vs. 68
years).
In multivariate analysis, NCCN-IPI was a significant
predictive factor both in PFS2 and OS. NCCN-IPI has not been
evaluated in second line treatment. NCCN-IPI might become a
promising predictive scale for DLBCL.
The present study had some limitations, including
its retrospective design, use of only two study sites, and small
sample size. Furthermore, no genetic analysis using techniques such
as fluorescence in situ hybridization (FISH) and immunostaining was
performed. Genetic analysis is carried out in clinical settings,
and its relevance to prognosis has been established. However, FISH
is not covered by insurance in Japan, and there are disparities in
immunostaining results between facilities. Thus, these evaluations
are not necessarily performed routinely in clinical practice in
Japan. We could not analyze QOL of patients although it is the
important point to evaluate. The analyze of QOL should be conducted
prospectively.
In conclusion, we were unable to identify the
optimal salvage regimen for patients with DLBCL in the present
study. However, we identified the effect of establishing an
out-patient chemotherapy unit on salvage therapy selection, and
anticipate that regimens administered via peripheral vein will
become predominant in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AN and SN were responsible for drafting the article
or revising it critically for important intellectual content. AN,
SF, AS, TN, YA, YT, RS, AK, MH, HY, KI, TI and SN made substantial
contributions to conception and design, and acquisition of data,
and analysis and interpretation of data. All authors have approved
the final version of this manuscript.
Ethics approval and consent to
participate
The present retrospective study was approved by the
Ethical Committee of Kansai Medical University (approval no.
2018063), and the requirement for written informed consent was
waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Friedberg JW: Relapsed/refractory diffuse
large B-cell lymphoma. Hematology Am Soc Hematol Educ Program.
2011:498–505. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coiffier B, Lepage E, Briere J, Herbrecht
R, Tilly H, Bouabdallah R, Morel P, Van Den, Salles G, Gaulard P,
et al: CHOP chemotherapy plus rituximab compared with CHOP alone in
elderly patients with diffuse large-B-cell lymphoma. N Engl J Med.
346:235–242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feugier P, Van Hoof, Sebban C,
Solal-Celigny P, Bouabdallah R, Fermé C, Christian B, Lepage E,
Tilly H, Morschhauser F, et al: Long-term results of the R-CHOP
study in the treatment of elderly patients with diffuse large
B-cell lymphoma: A study by the Groupe d'Etude des Lymphomes de
l'Adulte. J Clin Oncol. 23:4117–4126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gisselbrecht C, Glass B, Mounier N, Singh
Gill, Linch DC, Trneny M, Bosly A, Ketterer N, Shpilberg O, Hagberg
H, et al: Salvage regimens with autologous transplantation for
relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol.
28:4184–4190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Crump M, Kuruvilla J, Couban S, MacDonald
DA, Kukreti V, Kouroukis CT, Rubinger M, Buckstein R, Imrie KR,
Federico M, et al: Randomized comparison of gemcitabine,
dexamethasone, and cisplatin versus dexamethasone, cytarabine, and
cisplatin chemotherapy before autologous stem-cell transplantation
for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J
Clin Oncol. 32:3490–3496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Crump M, Neelapu SS, Farooq U, Van Den,
Kuruvilla J, Westin J, Link BK, Hay A, Cerhan JR, Zhu L, et al:
Outcomes in refractory diffuse large B-cell lymphoma: Results from
the international SCHOLAR-1 study. Blood. 130:1800–1808. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ziepert M, Hasenclever D, Kuhnt E, Glass
B, Schmitz N, Pfreundschuh M and Loeffler M: Standard International
prognostic index remains a valid predictor of outcome for patients
with aggressive CD20+B-cell lymphoma in the rituximab era. J Clin
Oncol. 28:2373–2380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou Z, Sehn LH, Rademaker AW, Gordon LI,
Lacasce AS, Crosby-Thompson A, Vanderplas A, Zelenetz AD, Abel GA,
Rodriguez MA, et al: An enhanced International Prognostic Index
(NCCN-IPI) for patients with diffuse large B-cell lymphoma treated
in the rituximab era. Blood. 123:837–842. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|