Introduction
Currently, there is no established methodology for
the treatment of progressive, relapsed or metastatic in oral
squamous cell carcinoma (OSCC). Cetuximab is a promising
chemotherapeutic agent for treating OSCC that specifically binds to
the epidermal growth factor receptor (EGFR). Cetuximab has been
demonstrated to be associated with radiation therapy to improve
local disease control rates and patient survival rates in locally
advanced squamous cell carcinoma of the head and neck. In addition,
treatment with cetuximab in conjunction with platinum drugs and
fluorouracil was found to improve the survival of patients with
recurrent or metastatic squamous cell carcinoma of head and neck
(SCCHN) (1–4). However, there is no established
predictor of treatment response in OSCC. Since the tumor response
cannot be accurately predicted, patients are administered cetuximab
regardless of tumor sensitivity. Moreover, cetuximab can result in
infusion reactions in addition to other serious adverse events.
Thus, the identification of predictors of treatment response in
OSCC patients is essential to avoid ineffective drug administration
and the risk associated with patients. Chemosensitivity tests used
in the fields of colon cancer, stomach cancer and breast cancer are
said to be effective for personalized medicine (5). In 1995, Kobayashi developed the CD-DST
method, which is a chemical sensitivity test that combines a
three-dimensional (3D) cell culture, serum-free culture, and an
image-based colorimetric assay. The CD-DST method is used in many
fields by overcoming many of the problems in chemical sensitivity
tests that have been conducted so far (5,6). Since
CD-DST uses a micro-3D culture, it can be used to evaluate activity
against OSCCs, as well as other types of cancer with a low tumor
volume. Further, its use in personalized medicine initiatives to
evaluate cytotoxic anticancer drug sensitivity of head and neck
cancer has been described (7). In
this study, we investigated whether the effectiveness of
sensitivity testing in clinical specimens using cetuximab, a
molecularly targeted drug, can be determined. The cut-off value of
T/C% for assessing the efficacy of drug regimens including
cisplatin (CDDP) and 5-fluorouracil (5-FU) in the presence or
absence cetuximab in the CD-DST method was set to 40%.
Patients and methods
Patients
Twenty-five OSCC patients who consented to
participate in the study from October 2013 to December 2017 in
Nippon Dental University at Oral and Maxillofacial Surgery, Niigata
Hospital (Table I). The Ethics
Committee of The Nippon Dental University School of Life Dentistry
at Niigata (Niigaka, Japan) approved the present study (approval
no. ECNG-H-119).
 | Table I.Patient characteristics (n=25). |
Table I.
Patient characteristics (n=25).
| Characteristics | No. of patients
(%) |
|---|
| Sex |
|
| Male | 15 (60.0) |
|
Female | 10 (40.0) |
| Age, mean, range | 70.6, 37–93 |
| Histology (Squamous
cell carcinoma) |
|
| Differentiation | 19 (76.0) |
|
Moderately differentiated | 5 (20.0) |
| Poorly
differentiated | 1 (4.0) |
| Stage |
|
| I | 3 (1.2) |
| II | 9 (36.0) |
| III | 3 (12.0) |
| IV | 10 (40.0) |
| Primary site |
|
|
Tongue | 9 (36.0) |
| Buccal
mucosa | 6 (24.0) |
| Oral
floor | 2 (8.0) |
|
Gingival | 6 (24.0) |
| Hard
palate | 2 (8.0) |
| Resection mode |
|
|
Biopsy | 20 (80.0) |
|
Surgery | 5 (20.0) |
| Sample site |
|
| Neck
metastasis | 1 (4.0) |
|
Primary | 24 (96.0) |
| CD-DST |
|
|
Success | 21 (84.0) |
|
Failure | 4 (16.0) |
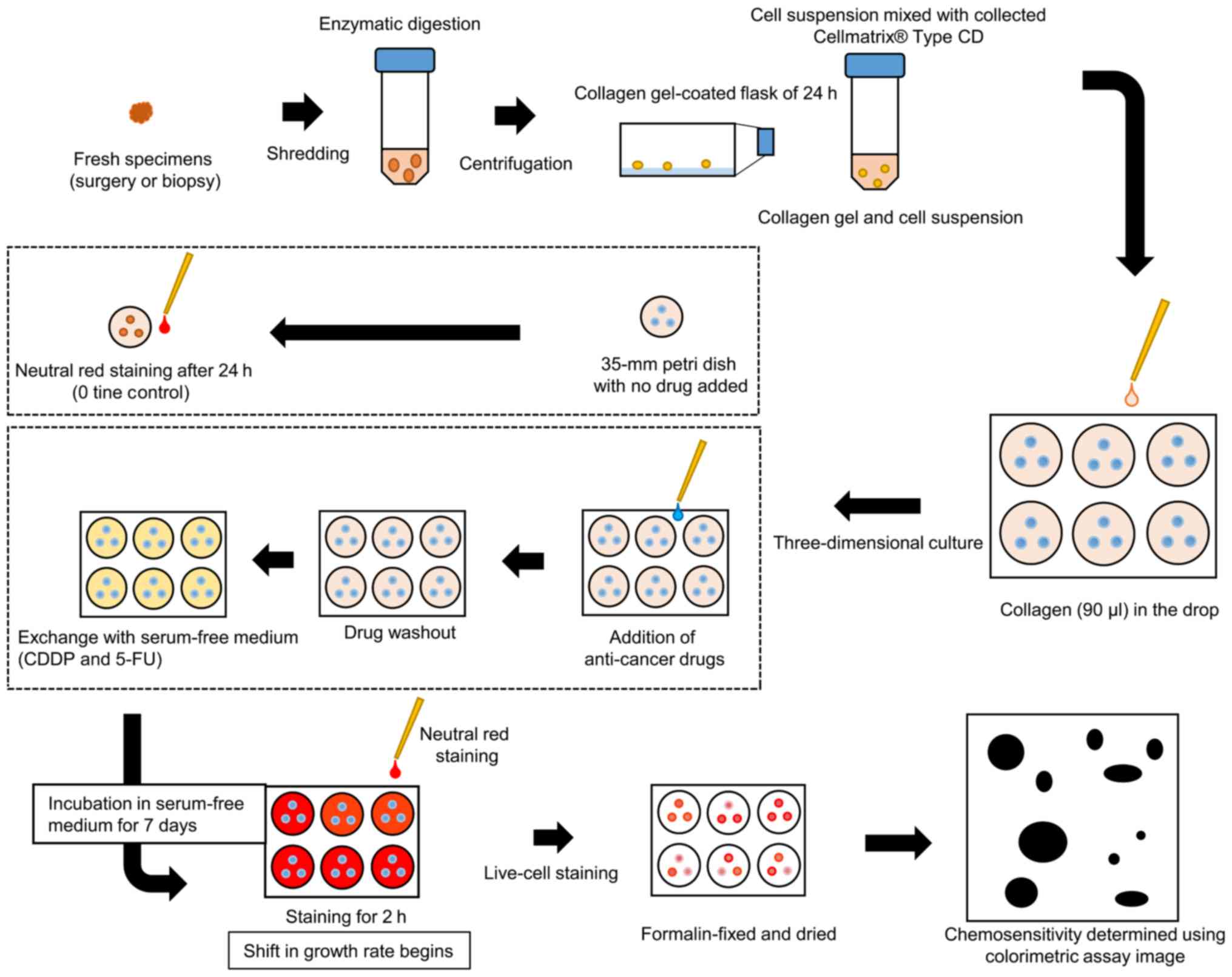
CD-DST was performed on 25 primary lesions and one
metastatic lymph node; 5–7-mm2 tissues (approximately
0.25–0.5 mg) were collected mainly from the area surrounding the
hardened part of the tumor. CD-DST was performed in accordance with
the methods described by Kobayashi et al (6,8), using a
human tumor cell primary culture system kit (Primaster®;
Kurabo Industries Ltd., Osaka, Japan). Briefly, the samples were
treated with Dispersion Enzyme Cocktail EZ (Primaster®
reagent). The samples comprised of cell suspensions were
transferred into collagen gel-coated flasks (CG flasks, a
Primaster® device) and incubated overnight in
pre-culture medium, PCM-1 (Primaster® content). The
collagen gel was digested with EZ to obtain viable cancer cells.
Type I collagen, F-12 medium 10× concentrate, and reconstitution
buffer were mixed in ice water at a 8:1:1 ratio
(Primaster® content). The cancer cell suspension
(1×105 cells/ml) was then combined with the to the
collagen solution. After 1 h, DF medium supplemented with 10% fetal
bovine serum was added to each well; the plates were placed in a
CO2 incubator overnight at 37°C. The following final
concentrations of the anticancer drugs were added: 0.5 µg/ml
cisplatin (CDDP; Randa Injection 50 mg/100 ml; Nippon Kayaku,
Tokyo, Japan) (9); and 0.7 µg/ml
fluorouracil (5-FU injection 250 Kyowa®; Kyowa Hakko,
Tokyo, Japan) (9). The cancer cells
were incubated for 24 h. In addition, cetuximab
(Erbitux® Injection 100 mg/20 ml; Merck Serono, Tokyo,
Japan) was added at 250 µg/dl and incubated for 144 h as reported
by Ryuki et al (10)
(Table II). After removing the
medium containing the 5-FU and CDDP anticancer agents, each well
was washed twice with 3 ml of Hanks' Balanced Salt Solution and
covered with 4 ml PCM-2 medium (Primaster® serum-free
medium). When cetuximab was further added, it was incubated for 6
days after cetuximab addition. After the incubation, a final
concentration of 50 µg/ml neutral red solution was added to each
well. Surviving colonies in the collagen gel droplets were stained
for 2 h and subsequently fixed in 10% neutral buffered formalin.
After fixing, the samples rinsed with water and permitted to
air-dry before being quantified by an optical density image
analysis using Primage System® (Solution Systems, Tokyo,
Japan) (Fig. 1). In vitro
sensitivity was expressed as the T/C ratio of the optical density,
where T represents the treated samples and C represents the
controls; a T/C ratio of <50% was regarded as in vitro
chemosensitivity. A tumor cell colony volume ratio (tumor growth
rate) at 0 time was calculated using the control group; a value of
less than 0.8 was regarded as unsuccessful culture (low growth
rate), regardless of the tumor cell colony volume in the control
group.
 | Table II.Anticancer agents and concentrations
used in the CD-DST. |
Table II.
Anticancer agents and concentrations
used in the CD-DST.
| Agents | Concentration
(µg/ml) | Time (h) |
|---|
| CDDP | 0.5 | 24 |
| 5-FU | 0.8 | 24 |
| Cetuximab | 250 | 144 |
Statistical analysis
Fisher's exact test was used to determine
differences between groups. A value of P<0.05 was considered
statistically significant (Ekuseru-Toukei 2015, Social Survey
Research Information Co., Ltd.).
Results
Evaluation success rate using clinical
specimens
CD-DST of cetuximab against OSCC had an overall
evaluation success rate of 84.0% (21 of 25 cases), and could be
applied to all anticancer agents used in the present study. The
evaluation success rate was 83.3% (20 of 24 cases) for primary
lesions and 100% (1 of 1 case) for metastatic lymph nodes (Table III). The causes of unsuccessful
CD-DST were insufficient tumor cells (growth rate less than 0.8) in
2 cases and bacterial contamination in 2 cases. The mean tumor
growth rate was 6.13±4.82. In addition, no significant difference
was observed between sensitivity to various anticancer agents,
stage, or histological differentiation (n.s.).
 | Table III.Evaluation of the success rate of the
CD-DST using oral squamous cell carcinoma clinical specimens. |
Table III.
Evaluation of the success rate of the
CD-DST using oral squamous cell carcinoma clinical specimens.
| Sample site | Number of
samples | Evaluable cases | Evaluation success
rate, % |
|---|
| Primary lesion | 24 | 20 | 83.3
(20/24)a |
| Metastatic lymph
node | 1 | 1 | 100
(1/1)a |
| Total | 25 | 21 | 84.0
(21/25)a |
Comparison between in vitro efficacy
rate and clinical response rate to various anticancer drugs
We compared the in vitro efficacy rate and
clinical response rate of representative anticancer drugs used for
clinical treatment of OSCC (2,11)
(Table IV). PF's in vitro
efficacy rate was 23.8%, which was similar to its clinical response
rate of 20–30% (2,11). In addition, the in vitro
efficacy rate of single agent cetuximab was 11.1%, which was
similar to its clinical response rate of 13%. However, PF +
cetuximab had an in vitro efficacy rate of 52.0% versus a
clinical response rate of 36.0% (2).
 | Table IV.In vitro sensitivity and
clinical efficacy rates of individual drugs against oral squamous
cell carcinoma. |
Table IV.
In vitro sensitivity and
clinical efficacy rates of individual drugs against oral squamous
cell carcinoma.
| Efficacy rate | PF | Cetuximab, 250
µg | PF + Cetuximab |
|---|
| CD-DST in
vitro efficacy rate, % | 23.8
(5/21)a | 11.1
(2/18)a | 52.3
(11/21)a |
| Clinical efficacy
rate, % | 20.0–30.0
(2,11) | 13.0 (2) | 36.0 (2) |
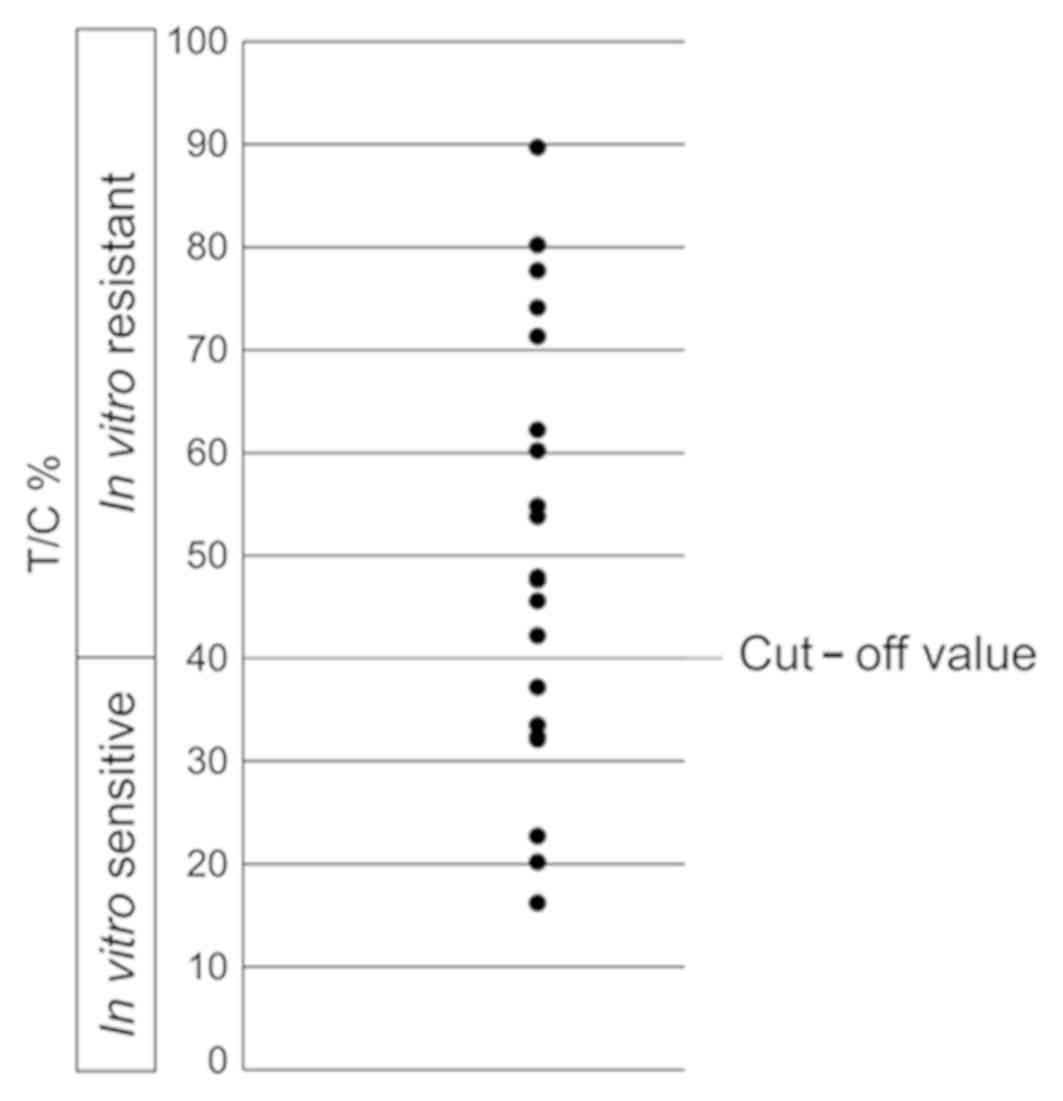
Determination of cut-off value of PF +
cetuximab in CD-DST
In anticancer drug sensitivity testing, conducted
using the CD-DST method in the present study, the in vitro
efficacy rate of PF + cetuximab was 52.3% at a cut-off value of
50%, which was higher than the clinical response rate. The cut-off
value calculated with CD-DST data using PF + cetuximab in 21
samples and the clinical response rate (2) of PF + cetuximab, the cut-off value was
40%, which was similar to the clinical response rate of 33.3%
(Fig. 2).
Discussion
Recently, the molecular targeting drug cetuximab has
been used in combination with conventional anticancer agents for
relapsed and metastatic SCCHN (12,13).
Cetuximab competitively binds to EGFR expressed on the tumor and
shows an antitumor effect by suppressing signal transduction
(14,15). Specific biomarkers in anti-tumor
effects have resulted in elimination of ineffective agents for
chemotherapy, development of personalized chemotherapy, improvement
of treatment response, and improvement of patients' QOL. However,
biomarkers for cetuximab in the OSCC have not been defined. As a
result, personalized therapy for molecular targeted therapies was
not currently available to patients with OSCC. Therefore,
predicting the therapeutic response of cetuximab in OSCC patients
can avoid ineffective drug administration and can avoid the patient
adverse events associated therewith.
The CD-DST we performed in the present study has a
potentially high success rate, based on initial culture, needs a
small number of cells, and uses serum-free culture medium and image
colorimetry to eliminate the influence of fibroblasts. The CD-DST
has the advantage of using an anticancer drug concentration
comparable to the clinical therapeutic dose (5,6,9,10).
In fact, the primary measurement success rate of the
CD-DST was reported to be 87.5% in colorectal cancer, 79.2% in lung
cancer, and 84.3% in breast cancer (16–18), and
it shows a high clinical efficacy prediction rate of 91% (5). Similarly, in sensitivity testing using
cisplatin, fluorouracil, or docetaxel in OSCC, as we reported
earlier, the primary culture success rate was 81.8% (19). In the present study, we conducted
anticancer drug sensitivity testing, including cetuximab specimens
of OSCC cases, with a success rate of 83.3% (20 of 25 cases) in
primary tumors, 100% (1 out of 1 case) in metastatic lymph nodes,
and 84.0% overall (21 of 25 cases). This result suggested that the
CD-DST method could also evaluate anticancer drugs including
cetuximab in OSCC.
Kobayashi et al (5,6) reported
a statistically significant correlation between in vitro
efficacy in CD-DST and clinical response of breast cancer, gastric
cancer, colorectal cancer, and lung cancer. In the present study on
OSCC, the 23.8% in vitro efficacy rate of PF therapy was
comparable to the previously reported 20.0–30.0% clinical response
rate to preoperative PF chemotherapy (2,11).
Further, the 11.1% in vitro efficacy rate of cetuximab
therapy in the present study was comparable to the reported
clinical response rate of 13.0% (2).
Ryuki et al (10) reported a
concentration of cetuximab alone in the CD-DST method using a human
oral cancer cell line of 250 µg/ml. We used cetuximab alone at 250
µg/ml based on the above study. We found the in vitro
efficacy was consistent with the clinical response rate. However,
when the CD-DST method was performed with PF + cetuximab (250
µg/ml), the in vitro response rate was 52.3%, which was
higher than the clinical response rate of 36.0%. The in
vitro efficacy rate after changing the cut-off value from 50 to
40% was 33.3% (7 out of 21), similar to the clinical response rate
of 36.0%. The sensitivity of CD-DST for PF + cetuximab in oral
cancer was found to be high when T/C% was less than 40%, and lower
when it was more than 40%.
We previously performed CD-DST in a recurrent
postoperative course of hard palate cancer (T2N2 cM0: Stage IVA)
and compared the results to those of sensitivity testing of PF +
cetuximab and its clinical efficacy (20). In the same study, the T/C% of the
CD-DST method was found to represent high sensitivity at 32.4%, and
the clinical efficacy of PF + cetuximab was consistent with the
partial response (20). The reported
T/C% is 32.4%, which is less than 40% of the cut-off value we
determined in the present study, and can be considered to represent
high sensitivity. Further, because it is consistent with clinical
efficacy, the cut-off value of 40% determined in the present study
appears appropriate. In addition, the antitumor effects of
cetuximab include antibody-dependent cell-mediated cytotoxicity
(ADCC) activity in addition to its inhibitory effect on EGFR
signaling (21). Although it is
challenging to evaluate ADCC activity in vitro, a
comprehensive evaluation was possible in the present study because
the drug effect was assessed based on results of CD-DST and
clinical response rate.
The mechanism by which the antitumor effect is
enhanced has not been demonstrated when cetuximab is used in
combination with conventional anticancer agents. It has been
suggested that the CD-DST method can contribute to the development
of individualized chemotherapy by predicting the treatment effect
when the anticancer drug is administered with cetuximab in the
upper part. Cetuximab alone and the effect of adding cetuximab to
combination therapy can be evaluated.
Chemotherapy, including molecularly targeted drugs,
can increase the physical and economic burden on patients and
affect their quality of life. Therefore, it was suggested that the
application of CD-DST method could become an increasingly important
test method in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KS and SH have made substantial contributions to the
conception and design of the study, and the acquisition, analysis
and interpretation of data. HT and AT were involved in drafting the
manuscript and critically revised it for important intellectual
content. HT and AT made substantial contributions to the conception
and design of the study, and the acquisition, analysis and
interpretation of data.
Ethics approval and consent to
participate
The patients consented to participate in the study.
The Ethics Committee of The Nippon Dental University School of Life
Dentistry at Niigata (Niigata, China) approved the present study
(approval no. ECNG-H-119).
Patient consent for publication
Written informed consent was obtained from the
patients.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ADCC
|
antibody-dependent cell-mediated
cytotoxicity
|
|
CG
|
collagen gel-coated
|
|
EGFR
|
epidermal growth factor receptor
|
|
OSCC
|
oral squamous cell carcinoma
|
|
SCCHN
|
squamous cell carcinoma of the head
and neck
|
References
|
1
|
Bonner JA, Harari PM, Giralt J, Azarnia N,
Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vermorken JB, Mesia R, Rivera F, Remenar
E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol
D, et al: Platinum-based chemotherapy plus cetuximab in head and
neck cancer. N Engl J Med. 359:1116–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rivera F, García-Castaño A, Vega N,
Vega-Villegas ME and Gutiérrez-Sanz L: Cetuximab in metastatic or
recurrent head and neck cancer: The EXTREME trial. Expert Rev
Anticancer Ther. 9:1421–1428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoshino T, Hasegawa Y, Takahashi S, Monden
N, Homma A, Okami K, Onozawa Y, Fujii M, Taguchi T, de Blas B, et
al: Platinum-based chemotherapy plus cetuximab for the first-line
treatment of Japanese patients with recurrent and/or metastatic
squamous cell carcinoma of the head and neck: Results of a phase II
trial. Jpn J Clin Oncol. 43:524–531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kobayashi H, Tanisaka K, Doi O, Kodama K,
Higashiyama M, Nakagawa H, Miyake M, Taki T, Hara S, Yasutomi M, et
al: An in vitro chemosensitivity test for solid human tumors
using collagen gel droplet embedded cultures. Int J Oncol.
11:449–455. 1997.PubMed/NCBI
|
|
6
|
Kobayashi H: Development of a new in vitro
chemosensitivity test using collagen gel droplet embedded culture
and image analysis for clinical usefulness. Recent Results Cancer
Res. 161:48–61. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shintani S, Hino S, Nakashiro K and
Hamakawa H: Clinical trial of chemotherapy identified according to
chemosensitivity assay for oral cancer patients with unresectable
recurrent lesions. Gan To Kagaku Ryoho. 33:357–360. 2006.(In
Japanese). PubMed/NCBI
|
|
8
|
Kobayashi H, Tanisaka K, Kondo N, Mito Y,
Koezuka M, Yokouchi H, Higashiyama M, Kodama K, Doi O and Yamada M:
Development of new in vitro chemosensitivity test using collagen
gel droplet embedded culture and its clinical usefulness. Gan To
Kagaku Ryoho. 22:1933–1939. 1995.(In Japanese). PubMed/NCBI
|
|
9
|
Sakuma K, Tanaka A and Mataga I: Collagen
gel droplet- embedded culture drug sensitivity testing in squamous
cell carcinoma cell lines derived from human oral cancers: Optimal
contact concentrations of cisplatin and fluorouracil. Oncol Lett.
12:4643–4650. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ryuki T, Sakuma K and Tanaka A: Optimal
cetuximab contact concentration using a collagen gel
droplet-embedded culture drug chemosensitivity test in human oral
squamous carcinoma cell lines. Chemotherapy. 7:253–260. 2018.
|
|
11
|
Kiyota N, Tahara M, Kadowaki S, Fuse N,
Doi T, Minami H and Ohtsu A: Systemic chemotherapy with cisplatin
plus 5-FU (PF) for recurrent or metastatic squamous cell carcinoma
of the head and neck (R/M SCCHN): Efficacy and safety of a lower
dose of PF (80/800) at a single institution in Japan. Jpn J Clin
Oncol. 39:225–230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saijo N: Molecular target therapy in
cancer. Nihon Rinsho. 68:1779–1786. 2010.(In Japanese). PubMed/NCBI
|
|
13
|
Saijo N: Molecular targeted therapy for
lung cancer, recent topics. J Lung Cancer. 7:1–8. 2008. View Article : Google Scholar
|
|
14
|
Lurje G and Lenz HJ: EGFR signaling and
drug discovery. Oncology. 77:400–410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang SM, Bock JM and Harari PM: Epidermal
growth factor receptor blockade with C225 modulates proliferation,
apoptosis, and radiosensitivity in squamous cell carcinomas of the
head and neck. Cancer Res. 59:1935–1940. 1999.PubMed/NCBI
|
|
16
|
Araki Y, Isomoto H, Matsumoto A, Kaibara
A, Yasunaga M, Hayashi K, Yatsugi H and Yamauchi K: An in vitro
chemosensitivity test for colorectal cancer using collagen-gel
droplet embedded cultures. Kurume Med J. 46:163–166. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawamura M, Inoue Y, Oyama T and Kobayashi
H: Chemosensitivity test for unresectable non-small cell lung
cancer. Nihon Geka Gakkai Zasshi. 103:229–232. 2002.(In Japanese).
PubMed/NCBI
|
|
18
|
Takamura Y, Kobayashi H, Taguchi T,
Motomura K, Inaji H and Noguchi S: Prediction of chemotherapeutic
response by collagen gel droplet embedded culture-drug sensitivity
test in human breast cancers. Int J Cancer. 98:450–455. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sakuma K, Tamura R, Hanyu S, Takahashi H,
Sato H, Oneyama T, Yamaguchi A and Tanaka A: Clinical study on
collagen gel droplet-embedded culture drug sensitivity test for
multidrug combination chemotherapy and super selective
intraarterial infusion chemoradiotherapy in oral squamous cell
carcinoma. Mol Clin Oncol. 7:1021–1026. 2017.PubMed/NCBI
|
|
20
|
Sakuma K, Tamura R, Noda N, Mizutani M,
Yamaguchi A and Tanaka A: Collagen gel droplet-embedded culture
drug sensitivity testing in hard palate cancer-predicted antitumor
efficacy of cetuximab: A case report. Mol Clin Oncol. 7:637–641.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kimura H, Sakai K, Arao T, Shimoyama T,
Tamura T and Nishio K: Antibody-dependent cellular cytotoxicity of
cetuximab against tumor cells with wild-type or mutant epidermal
growth factor receptor. Cancer Sci. 98:1275–1280. 2007. View Article : Google Scholar : PubMed/NCBI
|