Male breast cancer is a rare entity that shares many
overlapping features with female breast cancer (1). Although female breast cancer has been
extensively studied, far less is known about male breast cancer. As
with women, the incidence of breast cancer in men increases with
age and males are typically diagnosed 5 to 10 years later than
females (2-8).
Furthermore, the incidence of male breast cancer seems to be
increasing (9). Family history of
breast cancer appears to play an important role in the development
of male breast cancer (10). For
example, men with a family history of breast cancer in a female or
male relative have two to three times the risk of developing breast
cancer themselves (11-13).
BRCA2 mutations are well described as causal factors for male
breast cancer. Multiple studies have demonstrated that 4-15% of men
with breast cancer carry deleterious BRCA2 mutations (14-16).
BRCA1 mutations are less commonly seen with <5% of male breast
cancer patients harboring the mutation (14,16-18).
Other genes have also been implicated in male breast cancer risk
including mutations in PTEN tumor suppressor gene (Cowden
syndrome), TP53 (Li-Fraumeni syndrome), PALB2, and mismatch repair
genes (Lynch syndrome) (19-21).
Other risk factors for male breast cancer include androgen/estrogen
imbalance and environmental exposures (10). Histologically, 85-90% of males
present with invasive ductal carcinomas (22,23).
Since males lack acini and lobules in the normal male breast
lobular carcinoma is rare in male breast cancer (9,24). Other
histologic variants are rare but have been observed (25). Over 80% of male breast cancer is
hormone positive with some series showing estrogen (ER) positivity
as high as 99% (10,23). Rates of human epidermal growth factor
receptor (HER2) overexpression in male breast cancer have been
variable in different studies ranging from 2 to 45% (26-30).
Cardoso et al (23) conducted
immunohistochemistry evaluations of male breast cancer patients and
found 42% luminal A-like, 42% luminal B-like, 8.7% HER2 positive,
and 0.3% triple negative expression amog male breast cancer
patients.
Prospective randomized trials in the treatment of
male breast cancer are lacking due to the rarity of this entity.
Furthermore, little data exists on the activity of CDK 4/6
inhibitors in the treatment of hormone positive metastatic breast
cancer in male patients. In this report we describe the first known
case of a male patient treated with second line Abemaciclib,
Lupron, and Fulvestrant producing a dramatic and durable complete
remission. This is the first known case of a male achieving
complete remission on a CDK 4/6 inhibitor.
We present a case of a 39 year old male with no past
medical history who initially palpated a mass in his left breast in
March 2015. A diagnostic mammogram and left breast ultrasound
showed an irregular mass measuring 9x7x7 mm in the outer left
breast at 3 o'clock suspicious for malignancy. In March 2015 he
underwent left mastectomy with pathology demonstrating grade II
infiltrating ductal carcinoma, 1.6 cm tumor with extensive
lymphovascular invasion, five of five lymph nodes positively
involved, and margins negative. The invasive component was estrogen
receptor 58% positive, progesterone receptor 7% positive, human
epidermal growth factor receptor 1+ not overexpressed/negative
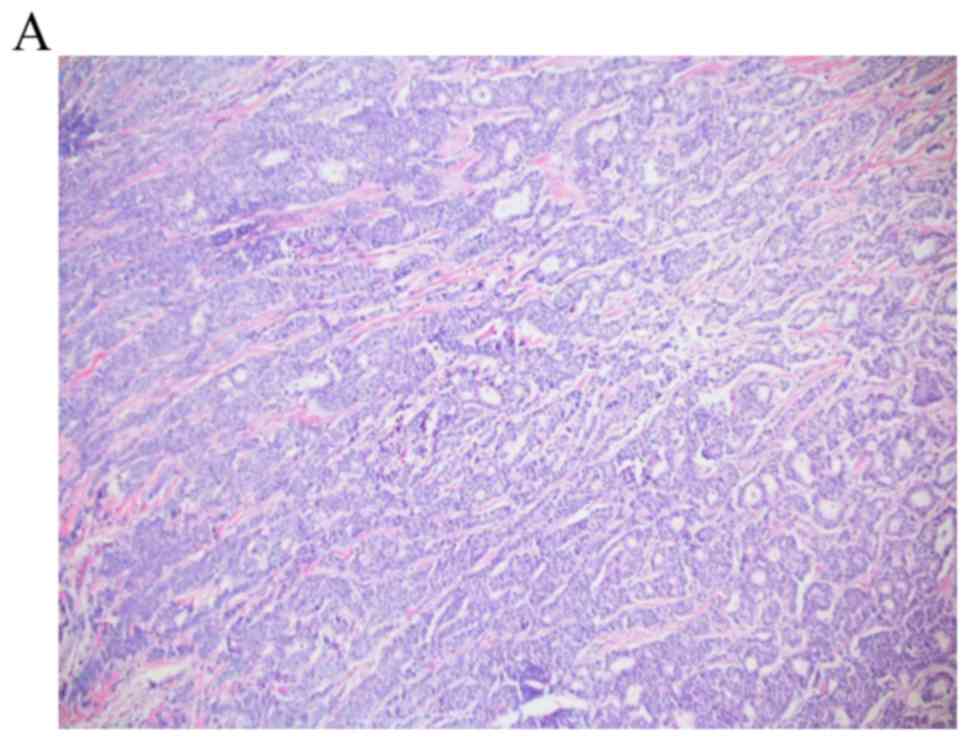
(Fig. 1). Of note, a computed
tomography scan (CT) of the chest abdomen pelvis and a bone scan
were performed and negative for metastatic disease. The patient was
staged as pT1cN2aMx stage IIIA. He was treated with adjuvant
chemotherapy with Adriamycin and Cyclophosphamide followed by
Paclitaxel then radiation therapy to the chest wall and regional
lymphatics (left supraclavicular fossa 5,000 cGy, left chest wall
5,000 cGy, left scar boost 1,000 cGy) ending December 2015. In
December 2015 the patient was started on Tamoxifen 20 mg orally
daily and was doing well until a restaging MRI in April 2017
identified a solitary metastatic lesion to the sternum. No biopsy
was performed at this time. He received palliative radiation (4,000
cGy) to the sternal lesion which was completed in June 2017. A
follow-up CT chest abdomen and pelvis October 2017 showed numerous
bilateral pulmonary nodules suspicious for metastatic disease. His
local team switched him to Anastrazole in June 2017. Patient
presented for initial consultation to our facility October 2017
where a biopsy to confirm metastatic disease and to obtain genomic
information was requested. Patient underwent video assisted
thoracoscopy and wedge resection of two pulmonary nodules in left
upper and lower lobes November 2017. Pathology was consistent with
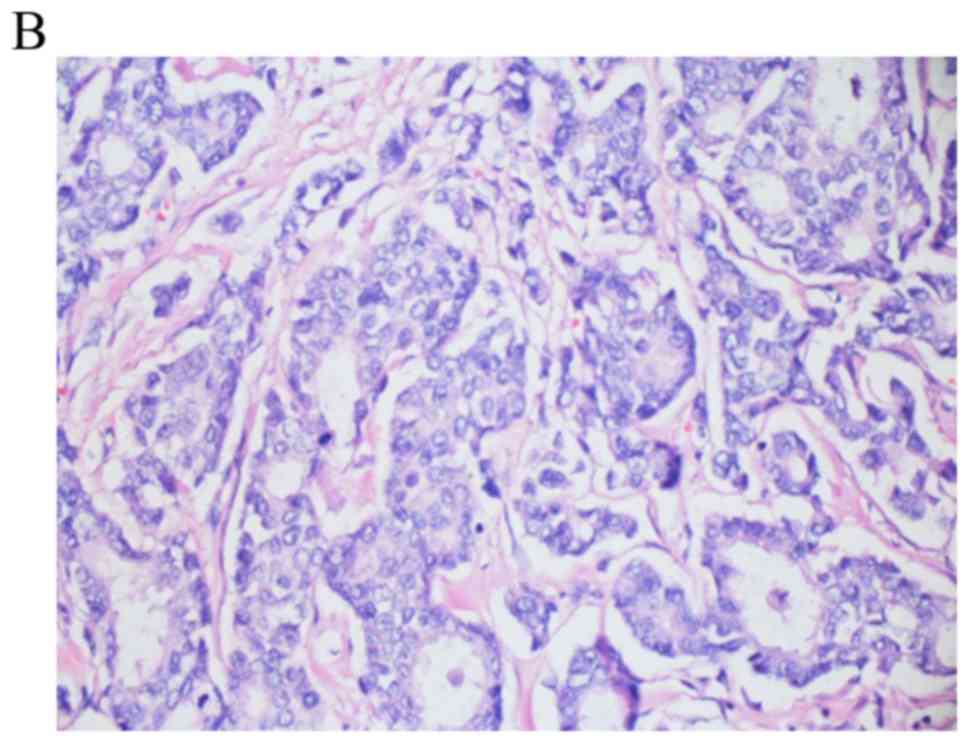
metastatic adenocarcinoma compatible with breast primary (Fig. 2). Genomic testing on the lung biopsy
specimen revealed PIK3CA amplification, GATA 3 mutation, stable
microsatellites, and a low tumor mutational burden. Genetic testing
revealed absence of deleterious mutations for the BRCA1 or BRCA2
genes. In November 2017 a baseline 18F-fluorodeoxyglucose-positron
emission tomography computed tomography (FDG-PET CT) was performed
post wedge resection showing metastatic disease to subcarinal lymph
node, left hilum, and osseous metastatic disease involving the 5th
cervical vertebral body, 2nd lumbar vertebral body, the ninth right
rib (Fig. 3). Baseline labs: CA 15-3
was 45.2 U/ml (0.0-35.0 U/ml), CA 27.29 was 60 U/ml (<38 U/ml),
complete blood count with white blood cell (wbc) count 5.2 K/µl,
hemoglobin 17.2 g/dl, platelet count 134 K/µl (150-450 K/µl),
absolute neutrophil 2.15 K/µl, complete metabolic panel was normal
except for elevated aspartate aminotransferase (AST) 69 U/l (17-59
U/l), alanine aminotransferase 135 U/l (21-72 U/l), and
testosterone level 1,240 ng/dl (132-813.0 ng/dl).
The patient was initiated on Abemaciclib 150 mg
orally twice daily, Fulvestrant 500 mg intramuscular injection days
1, 15, 29 then monthly there after, and Leuprolide 7.5 mg
intramuscular injections every month in November 2017.
Additionally, he was given Denosumab 120 mg subcutaneously every
month for prevention of skeletal related events. The patient
tolerated treatment well with grade 1 fatigue, grade 1 hot flashes,
grade 3 diarrhea mitigated by Loperamide and resolved. Testosterone
levels appropriately suppressed <50 ng/dl. Patient also had
transient grade 2 thrombocytopenia which resolved spontaneously and
persistent grade 2 neutropenia. Follow-up PET CT February 2018
showed resolution of the hypermetabolic osseous metastatic foci
with sclerosis at prior locations also there was resolution of the
previously described abnormal metabolic activity in the left hilar
and subcarinal mediastinal regions. Patient's subsequent PET CT
imaging every 3 months remained negative with last PET CT June
2019. Patients tumor markers normalized in December 2017 with
episodic mild flare up in CA 27.29. Last tumor markers over past 10
months remained negative June 2019. Patient is clinically
asymptomatic and developed a grade 3 neutropenia in October 2018
requiring dose reduction of Abemaciclib to 100 mg po BID. So far
the patient remains in a durable complete remission for 18 months
on this treatment regimen.
Due to the rarity of male breast cancer, treatment
approaches used for female breast cancer patients in the metastatic
setting are often applied to males with metastatic breast cancer.
Given that most males with metastatic breast cancer are hormone
positive, hormonal therapy is often the first approach in the
absence of visceral crisis (31).
Tamoxifen is considered standard of care frontline therapy for
males with metastatic disease (32,33).
Luteinizing hormones-releasing hormone agonists with or without
anti-androgens have been shown to be effective in male breast
cancer (34-36).
Aromatase inhibitors have shown clinical activity in male breast
cancer with increased clinical benefit observed with the addition
of a GnRH analogue (37). Data
regarding the role of Fulvestrant are limited. One pooled analysis
of 23 male patients receiving Fulvestrant in the first, second, or
third line setting reported a partial response rate of 26% and an
additional 48% had stable disease (38). Resistance to hormonal therapy in the
metastatic setting is common and most patients will eventually
experience progression of disease (39). Research into the mechanisms of
resistance to endocrine therapy had shed light on cell cycle
regulation, particularly the cyclin-dependent kinases (CDKs). The
CDKs play an important role in regulating cell-cycle progression
(40).
The cyclin-dependent kinases, CDK4 and CDK6, are
responsible for regulating the cell cycle by initiating the
transition of cells through the G1 restriction point (41). A common feature in human cancers is
the dysregulation and aberrant activation of CDK4 and 6 therefore
promoting cell cycle progression (42,43).
Inhibition of CDK4 and CDK6 seems like a rational therapeutic
target to prevent the progression of tumor cells through the G1
restriction point. Various preclinical studies have been conducted
and support CDK4 and CDK6 as potential tumor targets (22,44-46).
Subsequently three CDK4/6 inhibitors have been approved for use in
patients with metastatic breast cancer in the first or second line
setting: Palbociclib (PD-0332991; Pfizer), Ribociclib (LEE011;
Novartis), and Abemaciclib (LY2835219; Lilly). Palbociclib was the
first FDA approved CDK 4/6 inhibitor in combination with Letrozole
as initial therapy for postmenopausal women with advanced hormone
positive, HER2 negative metastatic breast cancer based on the
results from the phase II PALOMA-I clinical trial (47). In PALOMA-I, patients who received
Palbociclib and Letrozole experienced a roughly doubling of the
progression free survival compared to treatment with Letrozole
alone (47). These results were
later confirmed in the randomized phase III study PALOMA-II
(48). In the second line setting,
Palbociclib was paired with Fulvestrant vs. Fulvestrant alone in
patients with metastatic hormone positive HER2 negative breast
cancer who had progressed on prior endocrine therapy in the PALOMA
III randomized phase III trial (49). The study also included pre and
perimenopausal females who were required to take Goserelin
(49). The combination of
Palbociclib and Fulvestrant produced a significant 9.2 month
progression free survival compared with 3.8 in the Fulvestrant and
placebo arm (49). Abemaciclib is an
inhibitor of CDK4 and CDK6 and in enzymatic assays is 14 times more
potent against CDK4/cyclin D1 than CDK6/cyclin D3(50). Fujiwara et al (51) conducted a phase 1 study of
single-agent Abemaciclib in Japanese patients with advanced
metastatic solid tumors where 5/12 (41.6%) patients were males.
They concluded that single agent Abemaciclib demonstrated antitumor
activity as a single agent and had an acceptable safety profile
(51). In another phase I study,
Abemaciclib as a single agent demonstrated antitumor activity in
patients with several cancers with an ORR of 26% in patients with
hormone refractory hormone positive metastatic breast cancer
(52). Based on the single agent
activity observed with Abemaciclib, the phase II MONARCH 1 study
was launched (53). In this open
label phase II single arm trial, women with hormone positive HER2
negative metastatic breast cancer who had progressed on or after
prior endocrine therapy and had 1 or 2 prior chemotherapy regimens
in the metastatic setting were enrolled (53). In this study patients who received
single agent administered on a continuous schedule had an overall
response rate of 19.7% with a median progression free survival of 6
months (53). Based on the results
of MONARCH-I, the U.S. Food and Drug Administration approved
Abemaciclib to be used alone to treat women and men diagnosed with
hormone positive HER2 negative metastatic breast cancer that has
progressed after hormone therapy and prior chemotherapy in the
metastatic setting. Abemaciclib was also studied in the randomized
phase III trial MONARCH 2, where Abemaciclib and Fulvestrant vs.
Abemaciclib and placebo were studied in patients with hormone
positive HER2 negative metastatic breast cancer who had progressed
on prior endocrine therapy (54).
The combination of Abemaciclib and Fulvestrant yielded a
significantly improved PFS of 16.4 months compared with 9.3 months
in the Fulvestrant and Placebo arm (54). Data regarding treatment responses to
CDK 4/6 inhibitors in males is extremely limited. The first
reported response in males was demonstrated in 2016 by S. Sorcher
where a male with metastatic breast cancer achieved a partial
response to Palbocliclib and Letrozole in the fifth line setting
(55). The second known report by
Castrellon et al (56)
demonstrated a case of a male with metastatic breast cancer to lung
and bone who achieved partial response to CDK 4/6 therapy with
Palbociclib and Fulvestrant. Here we report the first male patient
with metastatic breast cancer to achieve complete remission on a
CDK 4/6 inhibitor. Given the lack of randomized controlled trials
in male breast cancer treatment decisions are often extrapolated
from data derived from female breast cancer trials. The standard of
care for females with metastatic hormone positive HER2 negative
metastatic breast cancer who progress on endocrine therapy is
treatment with CDK 4/6 inhibitor with Fulvestrant. Our patient was
treated as per MONARCH-II protocol given the significant benefit of
the addition of Abemaciclib to Fulvestrant compared with
Fulvestrant alone (54).
Furthermore, Abemaciclib is the only CDK 4/6 inhibitor with an FDA
approval in males and it has been previously studied in male cancer
patients (51,52). It should be noted however among the
three FDA approved CDK 4/6 inhibitors (Abemaciclib, Palbociclib,
Ribociclib) no head to head trials have been performed therefore no
superior agent has been identified in cancer patients. The relative
favorable side effect profile and response seen in this patient
utilizing the combination of Fulvestrant, Abemaciclib and Lupron
seems encouraging and further reports of CDK4/6 drug combinations
may show responses. Identification of predictive biomarkers of
response to CDK inhibitors represents one of the most important
clinical areas of interest as CDK inhibitors have become the
accepted first line treatment in metastatic hormone receptor
positive HER2 negative breast cancer. Despite the excellent
clinical advancement afforded by CDK inhibition a significant
percent (20%) of patients will not respond to CDK inhibition.
Therefore identification of predictive biomarkers of response to
CDK inhibition is prudent. Studies are slowly emerging in this
field. Gong et al (57)
analyzed the sensitivity of 560 cell lines to the selective CDK4/6
inhibitor abemaciclib and they found that cell lines with genomic
features of D-cyclin activating features are particularly
sensitive. Clinically however no reproducible predictive biomarker
has emerged. For example in a phase II study using Palbociclib as a
single agent in advanced breast cancer assessed progression free
survival and Rb expression, KI-67, p16 loss, and CCND1
amplification. In this study there was no association between these
biomarkers and response to therapy (58). Several studies are ongoing to
elucidate potential predictive biomarkers. If more
clinicopathologic and predicitive biomarker data could be
accumulated on CDK 4/6 drug combinations in males with metastatic
hormone positive male breast cancer this would help facilitate
clinicians in selecting optimal therapeutic algorithms for
individual males with breast cancer.
Not applicable.
No funding was received.
All data generated or analyzed during the present
study are included in this published article.
DH provided clinical management for the patient and
developed their treatment protocol. DH also conceived the present
study, wrote the manuscript, reviewed the treatment of stage IV
male breast cancer and CDK 4/6 inhibitors for use in breast cancer,
and supervised the study. SJ clinically treated the patient, and
partially wrote and revised the manuscript. JS assembled
pathological images and partially wrote the manuscript. RV
assembled radiographic images and partially wrote the manuscript.
RA wrote, critically revised and approved the manuscript, and
developed the treatment protocol. All authors read and approved the
final manuscript.
The present case report was reviewed and approved by
the Ethics Committee of the Cancer Treatment Centers of
America.
The patient verbalized consent for the publication
of their information in a medical journal, which was documented in
the medical record of the patient.
The authors declare that they have no competing
interests.
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Anderson WF, Althuis MD, Brinton LA and
Devesa SS: Is male breast cancer similar or different than female
breast cancer? Breast Cancer Res Treat. 83:77–86. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Thomas DB: Breast cancer in men. Epidemiol
Rev. 15:220–231. 1993.PubMed/NCBI View Article : Google Scholar
|
|
4
|
O'Malley CD, Prehn AW, Shema SJ and Glaser
SL: Racial/ethnic differences in survival rates in a
population-based series of men with breast carcinoma. Cancer.
94:2836–2843. 2002.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Giordano SH, Buzdar AU and Hortobagyi GN:
Breast cancer in men. Ann Intern Med. 137:678–687. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cutuli B, Lacroze M, Dilhuydy JM, Velten
M, De Lafontan B, Marchal C, Resbeut M, Graic Y, Campana F and
Moncho-Bernier V: Male breast cancer: Results of the treatments and
prognostic factors in 397 cases. Eur J Cancer. 31A:1960–1964.
1995.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mabuchi K, Bross DS and Kessler II: Risk
factors for male breast cancer. J Natl Cancer Inst. 74:371–375.
1985.PubMed/NCBI
|
|
8
|
Nahleh ZA, Srikantiah R, Safa M, Jazieh
AR, Muhleman A and Komrokji R: Male breast cancer in the veterans
affairs population: A comparative analysis. Cancer. 109:1471–1477.
2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Giordano SH, Cohen DS, Buzdar AU, Perkins
G and Hortobagyi GN: Breast carcinoma in men: A population-based
study. Cancer. 101:51–57. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ferzoco RM and Ruddy KJ: The epidemiology
of male breast cancer. Curr Oncol Rep. 18(1)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ewertz M, Holmberg L, Tretli S, Pedersen
BV and Kristensen A: Risk factors for male breast cancer-a
case-control study from scandinavia. Acta Oncol. 40:467–471.
2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rosenblatt KA, Thomas DB, McTiernan A,
Austin MA, Stalsberg H, Stemhagen A, Thompson WD, Curnen MG,
Satariano W and Austin DF: Breast cancer in men: Aspects of
familial aggregation. J Natl Cancer Inst. 83:849–854.
1991.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Casagrande JT, Hanisch R, Pike MC, Ross
RK, Brown JB and Henderson BE: A case-control study of male breast
cancer. Cancer Res. 48:1326–1330. 1988.PubMed/NCBI
|
|
14
|
Basham VM, Lipscombe JM, Ward JM, Gayther
SA, Ponder BA, Easton DF and Pharoah PD: BRCA1 and BRCA2 mutations
in a population-based study of male breast cancer. Breast Cancer
Res BCR. 4(R2)2002.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Couch FJ, Farid LM, DeShano ML, Tavtigian
SV, Calzone K, Campeau L, Peng Y, Bogden B, Chen Q, Neuhausen S, et
al: BRCA2 germline mutations in male breast cancer cases and breast
cancer families. Nat Genet. 13:123–125. 1996.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Friedman LS, Gayther SA, Kurosaki T,
Gordon D, Noble B, Casey G, Ponder BA and Anton-Culver H: Mutation
analysis of BRCA1 and BRCA2 in a male breast cancer population. Am
J Hum Genet. 60:313–319. 1997.PubMed/NCBI
|
|
17
|
Ottini L, Masala G, D'Amico C, Mancini B,
Saieva C, Aceto G, Gestri D, Vezzosi V, Falchetti M, De Marco M, et
al: BRCA1 and BRCA2 mutation status and tumor characteristics in
male breast cancer: A population-based study in italy. Cancer Res.
63:342–347. 2003.PubMed/NCBI
|
|
18
|
Sverdlov RS, Barshack I, Bar Sade RB,
Baruch RG, Hirsh-Yehezkel G, Dagan E, Feinmesser M, Figer A and
Friedman E: Genetic analyses of male breast cancer in israel. Genet
Test. 4:313–327. 2000.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ding YC, Steele L, Kuan CJ, Greilac S and
Neuhausen SL: Mutations in BRCA2 and PALB2 in male breast cancer
cases from the United States. Breast Cancer Res Treat. 126:771–778.
2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Silvestri V, Rizzolo P, Zanna I, Falchetti
M, Masala G, Bianchi S, Papi L, Giannini G, Palli D and Ottini L:
PALB2 mutations in male breast cancer: A population-based study in
central italy. Breast Cancer Res Treat. 122:299–301.
2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Boyd J, Rhei E, Federici MG, Borgen PI,
Watson P, Franklin B, Karr B, Lynch J, Lemon SJ and Lynch HT: Male
breast cancer in the hereditary nonpolyposis colorectal cancer
syndrome. Breast Cancer Res Treat. 53:87–91. 1999.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fry DW, Harvey PJ, Keller PR, Elliott WL,
Meade M, Trachet E, Albassam M, Zheng X, Leopold WR, Pryer NK and
Toogood PL: Specific inhibition of cyclin-dependent kinase 4/6 by
PD 0332991 and associated antitumor activity in human tumor
xenografts. Mol Cancer Ther. 3:1427–1438. 2004.PubMed/NCBI
|
|
23
|
Cardoso F, Bartlett JMS, Slaets L, van
Deurzen CHM, van Leeuwen-Stok E, Porter P, Linderholm B, Hedenfalk
I, Schröder C, Martens J, et al: Characterization of male breast
cancer: Results of the EORTC 10085/TBCRC/BIG/NABCG international
male breast cancer program. Ann Oncol. 29:405–417. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cardoso F, Costa A, Senkus E, Aapro M,
André F, Barrios CH, Bergh J, Bhattacharyya G, Biganzoli L, Cardoso
MJ, et al: 3rd ESO-ESMO international consensus guidelines for
advanced breast cancer (ABC 3). Ann Oncol. 28:16–23.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sharif MA, Mamoon N, Arif A and Khadim MT:
Histological and immuno-histochemical study of male breast
carcinoma in northern Pakistan. J Pak Med Assoc. 59:67–71.
2009.PubMed/NCBI
|
|
26
|
Fox SB, Rogers S, Day CA and Underwood JC:
Oestrogen receptor and epidermal growth factor receptor expression
in male breast carcinoma. J Pathol. 166:13–18. 1992.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Arslan UY, Oksüzoğlu B, Ozdemir N, Aksoy
S, Alkış N, Gök A, Kaplan MA, Gümüş M, Berk V, Uncu D, et al:
Outcome of non-metastatic male breast cancer: 118 patients. Med
Oncol. 29:554–560. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Moore J, Friedman MI, Gansler T, Gramlich
TL, Derose PB, Hunt D and Cohen C: Prognostic indicators in male
breast carcinoma. Breast J. 4:261–269. 1998.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Leach IH, Ellis IO and Elston CW:
C-erb-B-2 expression in male breast carcinoma. J Clin Pathol.
45(942)1992.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Willsher PC, Leach IH, Ellis IO, Bell JA,
Elston CW, Bourke JB, Blamey RW and Robertson JF: Male breast
cancer: Pathological and immunohistochemical features. Anticancer
Res. 17:2335–2338. 1997.PubMed/NCBI
|
|
31
|
Giordano SH: A review of the diagnosis and
management of male breast cancer. Oncologist. 10:471–479.
2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gradishar WJ, Anderson BO, Balassanian R,
Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A,
Giordano SH, et al: Invasive breast cancer version 1.2016, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
14:324–354. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
White J, Kearins O, Dodwell D, Horgan K,
Hanby AM and Speirs V: Male breast carcinoma: Increased awareness
needed. Breast Cancer Res. 13(219)2011.
|
|
34
|
Labrie F, Dupont A, Belanger A,
Lacourcière Y, Béland L, Cusan L and Lachance R: Complete response
to combination therapy with an LHRH agonist and flutamide in
metastatic male breast cancer: A case report. Clin Invest Med.
13:275–278. 1990.PubMed/NCBI
|
|
35
|
Lopez M, Natali M, Di Lauro L, Vici P,
Pignatti F and Carpano S: Combined treatment with buserelin and
cyproterone acetate in metastatic male breast cancer. Cancer.
72:502–505. 1993.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Doberauer C, Niederle N and Schmidt CG:
Advanced male breast cancer treatment with the LH-RH analogue
buserelin alone or in combination with the antiandrogen flutamide.
Cancer. 62:474–478. 1988.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zagouri F, Sergentanis TN, Azim HA Jr,
Chrysikos D, Dimopoulos MA and Psaltopoulou T: Aromatase inhibitors
in male breast cancer: A pooled analysis. Breast Cancer Res Treat.
151:141–147. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zagouri F, Sergentanis TN, Chrysikos D,
Dimopoulos MA and Psaltopoulou T: Fulvestrant and male breast
cancer: A pooled analysis. Breast Cancer Res Treat. 149:269–275.
2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lumachi F, Luisetto G, Basso SM and
Camozzi V: Endocrine therapy of breast cancer. Curr Med Chem.
18:513–522. 2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Finn RS, Aleshin A and Slamon DJ:
Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen
receptor-positive breast cancers. Breast Cancer Res.
18(17)2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lundberg AS and Weinberg RA: Functional
inactivation of the retinoblastoma protein requires sequential
modification by at least two distinct cyclin-cdk complexes. Mol
Cell Biol. 18:753–761. 1998.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Malumbres M: Cyclin-dependent kinases.
Genome Biol. 15(122)2014.PubMed/NCBI View
Article : Google Scholar
|
|
43
|
Gelbert LM, Cai S, Lin X, Sanchez-Martinez
C, Del Prado M, Lallena MJ, Torres R, Ajamie RT, Wishart GN, Flack
RS, et al: Preclinical characterization of the CDK4/6 inhibitor
LY2835219: In vivo cell cycle-dependent/independent anti-tumor
activities alone/in combination with gemcitabine. Invest New Drugs.
32:825–837. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Puyol M, Martin A, Dubus P, Mulero F,
Pizcueta P, Khan G, Guerra C, Santamaria D and Barbacid M: A
synthetic lethal interaction between K-Ras oncogenes and Cdk4
unveils a therapeutic strategy for non-small cell lung carcinoma.
Cancer Cell. 18:63–73. 2010.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Baker SJ and Reddy EP: CDK4: A key player
in the cell cycle, development, and cancer. Genes Cancer.
3:658–669. 2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Dean JL, McClendon AK, Hickey TE, Butler
LM, Tilley WD, Witkiewicz AK and Knudsen ES: Therapeutic response
to CDK4/6 inhibition in breast cancer defined by ex vivo analysis
of human tumors. Cell Cycle. 11:2756–2761. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
47
|
Finn RS, Crown JP, Lang I, Boer K,
Bondarenko IM, Kulyk SO, Ettl J, Patel R, Pinter T, Schmidt M, et
al: The cyclin-dependent kinase 4/6 inhibitor palbociclib in
combination with letrozole versus letrozole alone as first-line
treatment of estrogen receptor-positive, HER2-negative, advanced
breast cancer (PALOMA-1/TRIO-18): A randomized phase 2 study.
Lancet Oncol. 16:25–35. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Finn RS, Martin M, Rugo HS, Jones SE, Im
SA, Gelmon KA, Harbeck N, Lipatov ON and Walshe JM and Walshe JM:
PALOMA-2: Primary results from a phase III trial of palbociclib (P)
with letrozole (L) compared with letrozole alone in postmenopausal
women with ER+/HER2-advanced breast cancer. J Clin
Oncol. May 11, 2017 (Epub ahead of print). doi:
10.1200/JCO.2016.34.15_suppl.507. View Article : Google Scholar
|
|
49
|
Turner NC, Ro J, Andre F, Loi S, Verma S,
Harbeck HI, Loibl S, Bartlett CH, Zhang K and Giorgetti C, et al:
PALOMA3: A double-blind, phase III trial of fulvestrant with or
without palbociclib in pre- and post-menopausal women with hormone
receptor-positive, HER2-negative metastatic breast cancer that
progressed on prior endocrine therapy. J Clin Oncol. Jan 31, 2017
(Epub ahead of print). doi: 10.1200/jco.2015.33.18_suppl.lba502.
PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lallena MJ, Boehnke K, Torres R, Hermoso
A, Amat J, Calsina B, De Dios A, Buchanan S, Du J, Beckmann RP, et
al: In-vitro characterization of Abemaciclib pharmacology in
ER+ breast cancer cell lines. Presented at Proceedings
of the 106th Annual Meeting of the American Association for Cancer
Research (AACR) Congress. (abstract 3101). 2015.doi:
10.1158/1538-7445.AM2015-3101. View Article : Google Scholar
|
|
51
|
Fujiwara Y, Tamura K, Kondo S, Tanabe Y,
Iwasa S, Shimomura A, Kitano S, Ogasawara K, Turner PK and Mori J:
Phase 1 study of abemaciclib, an inhibitor of CDK 4 and 6, as
single agent for Japanese patients with advanced cancer. Cancer
Chemother Pharmacol. 78:281–288. 2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Patnaik A, Tolaney SM, Tolcher AW, Goldman
JW, Gandhi L, Papadopoulos KP, Beeram M, Rasco DW, Hilton JF, Nasir
A, et al: Efficacy and safety of abemaciclib, an inhibitor of CDK4
and CDK6, for patients with breast cancer, non-small cell lung
cancer, and other solid tumors. Cancer Discov. 6:740–753.
2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Dickler MN, Tolaney SM, Rugo HS, Cortés J,
Diéras V, Patt D, Wildiers H, Hudis CA, O'Shaughnessy J, Zamora E,
et al: MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6
inhibitor, as a single agent, in patients with refractory
HR+/HER2- metastatic breast cancer. Clin
Cancer Res. 23:5218–5224. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Sledge GW Jr, Toi M, Neven P, Sohn J,
Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, et al:
Monarch 2: Abemaciclib in combination with fulvestrant in women
with HR+/HER2- advanced breast cancer who had
progressed while receiving endocrine therapy. J Clin Oncol.
1:2875–2884. 2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Sorscher S: A first case of male breast
cancer responding to combined aromatase inhibitor/palbociclib
therapy. Int J Cancer Clin Res. Oct 19, 2016 (Epub ahead of print).
View Article : Google Scholar
|
|
56
|
Castrellon AB, Nguyen SM, Milillo Naraine
AM, Velez M and Raez LE: Initial response to therapy with
fulvestrant and cyclin-dependent kinase 4/6 inhibitor in a male
with stage IV breast cancer. Mathews J Cancer Sci. Mar 1, 2017
(Epub ahead of print).
|
|
57
|
Gong X, Litchfield LM, Webster Y, Chio LC,
Wong SS, Stewart TR, Dowless M, Dempsey J, Zeng Y, Torres R, et al:
Genomic aberrations that activate D-type cyclins are associated
with enhanced sensitivity to the CDK4 and CDK6 inhibitor
abemaciclib. Cancer Cell. 32:761–776. 2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
DeMichele A, Clark AS, Tan KS, Heitjan DF,
Gramlich K, Gallagher M, Lal P, Feldman M, Zhang P, Colameco C, et
al: CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+
advanced breast cancer: Phase II activity, safety, and predictive
biomarker assessment. Clin Cancer Res. 21:995–1001. 2015.PubMed/NCBI View Article : Google Scholar
|