Introduction
Despite the large-scale screening programs in
developed countries, cervical cancer remains a major health concern
in the United States, accounting for >12,800 and 4,200 new
diagnoses and deaths, respectively, in 2017(1). In addition, cervical cancer is the
second most common cause of death from cancer among women aged
20-39 years (2). Early cervical
cancer (ECC), which can be treated with radical hysterectomy, has
been reported to have 5-year survival rates of 88-97% after surgery
(3,4).
Various types of conventional radical surgery, such
as radical hysterectomy, trachelectomy, and parametrectomy, remain
the standard treatment for ECC (International Federation of
Gynecology and Obstetrics stage I-IIA) (5,6).
According to the Querleu and Morrow classification, conventional
radical hysterectomy (CRH) means type C2 hysterectomy. Following
this procedure, the hypogastric nerve (sympathetic nerve), the
pelvic splanchnic nerve (parasympathetic nerve), and the vesical
branch of the pelvic plexus (both sympathetic and parasympathetic
nerves) are damaged during the dissection of the uterosacral
ligament, vesicouterine ligament and parametrium. These injuries
are the leading cause of postoperative pelvic dysfunction,
typically including bladder, sexual and colorectal dysfunction
(7,8).
As ECC has a high 5-year survival rate, the
long-term quality of life of the patients is important. It is also
important how quickly the pelvic dysfunction is restored. The
concept of ‘nerve-sparing’ surgery was first described by Höckel
et al (9) in 1998 as part of
an effort to improve the oncological outcome of radical
hysterectomy by extending the resection of parametrial tissue
without further impairing pelvic autonomic nerve functions. Since
then, this surgical procedure has been actively performed and
studied, mainly by the Japanese research group (9). However, nerve-sparing radical
hysterectomy (NSRH) remains controversial in gynecological
oncology. Although this technique may have a positive impact on the
quality of life of the patients, the heterogeneity of the technique
itself is substantial and there is ongoing debate regarding the
oncological outcome (3,4,10).
Although five systematic reviews with meta-analyses
and three randomized controlled trials (RCTs) have been published
to date, they are not sufficient to verify the efficacy and safety
of NSRH in ECC (4,10-16).
In the present systematic review and meta-analysis, pooled data may
provide evidence regarding both comparative effectiveness and
safety between NSRH and CRH in ECC. The aim was to review the
currently available relevant literature and compare morbidity,
pelvic dysfunction and oncological outcomes between the two
surgical methods.
Materials and methods
Search strategy
The present systematic review and meta-analysis
followed the recommendations of Preferred Reporting Items for
Systematic Reviews and Meta-analysis (PRISMA) guidelines (17). For this meta-analysis, the Cochrane
Central Register of controlled Trials (CENTRAL), MedLine and Embase
were searched for relevant studies published between 2000 and 2018,
using the terms ‘cervical AND cancer OR malignancy OR carcinoma’
AND ‘nerve AND sparing’ AND ‘radical AND hysterectomy’.
Study selection and inclusion
criteria
Published articles were included if they met the
following criteria: i) Studies reporting data of patients affected
by cervical cancer; ii) English language studies; iii) series
including ≥20 patients and iv) studies reporting data of patients
with comparison of clinical outcomes between NSRH and CRH. If there
were duplicate studies, the studies that were published first or
provided more information were included.
Data extraction
Two authors (KHY, KSJ) independently extracted the
data, and disagreements were re-evaluated by two other authors
(YJH, CSE). Two reviewers (KHY, KSJ) worked independently, and
examined the potential eligibility of all studies retrieved from
the databases based on selection and exclusion criteria. The third
and fourth reviewers (YJH, CSE) resolved inconsistencies between
the first two reviewers through consensus and discussion. The data
investigated were perioperative outcomes, quality of life
indicators, progression-free survival (PFS) and overall survival
(OS).
Quality assessment
The questionnaire for methodological quality of the
Cochrane Collaboration's Risk of Bias assessment tool was answered
for each article to determine the risk of bias. The level of bias
of the included studies was assessed based on the Cochrane
Collaboration system (18).
Furthermore, the quality of outcomes was rated using the Grading of
Recommendations, Assessment, Development and Evaluation system
(19). The meta-analyses were
conducted using Review Manager software, version 5.3(20), which is designed for conducting
Cochrane reviews.
Statistical analysis
Dichotomous outcomes eligible in each study are
demonstrated as risk ratio (RR) with estimated 95% confidence
interval (CI). Continuous outcomes are shown as the weighted mean
difference (WMD) with 95% CI, which were calculated from mean,
standard deviation (SD), P-value and sample size in each study.
Heterogeneity was assessed using Higgins I2, evaluating
the percentage of total variation across studies that was due to
heterogeneity rather than chance. Thus, an I2 of >50%
was considered to reflect substantial heterogeneity, and thereby
the random effects model using the DerSimonian and Laird method was
used. The fixed effects model, using the Mantel-Haenszel method,
was employed when I2 was ≤50%, indicating no
heterogeneity. The Cochrane Review software (Review Manager version
5.3) was used in order to assess the heterogeneity of the included
studies and to evaluate pooled results of the included
investigations. The level of heterogeneity was studied for each
comparison. On the basis of the level of heterogeneity, the random
and fixed effects models were used to compare outcomes between
groups. Random and fixed effects models were used as appropriate.
Forest plots were created for each comparison and RR, WMD and 95%
CI are presented; P-values <0.05 were considered to indicate
statistically significant differences.
Results
Eligible studies
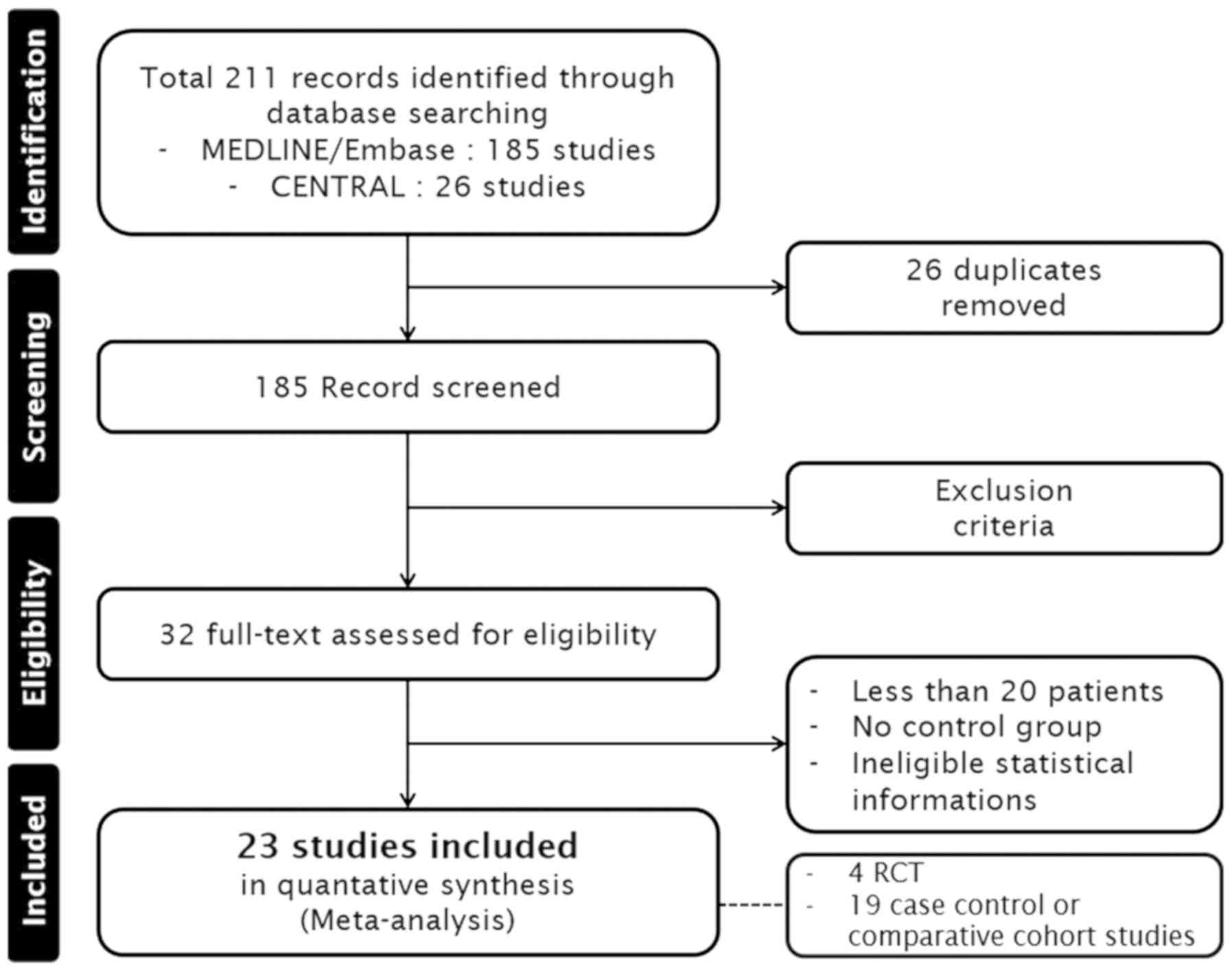
A total of 211 studies were identified, and 26
duplicated articles were excluded. In addition, 153 studies,
including non-cervical cancer (n=35), non-English language studies
(n=27), case reports and reviewed articles (n=91), were excluded.
After assessment of full-text articles for eligibility, an
additional 9 studies were excluded due to the following criteria:
Number of patients <20 (n=5), no control group (n=2), and
ineligible statistical information (n=2). Finally, 4 RCTs, 8 case
control and 11 comparative cohort studies were included in the
present meta-analysis. A total of 23 articles were selected for
data extraction. Detailed information of study acquisition may be
found in Fig. 1.
Study characteristics
A total of 23 articles were selected for inclusion
in this meta-analysis, 4 of which were RCTs and the remaining 19
were case control or comparative cohort studies. Overall, 1,769
patients were included: 912 (50.8%) and 884 (49.2%) patients had
undergone CRH and NSRH, respectively. The risk of bias was assessed
for all studies. A summary of the included studies (11,21-42)
is presented in Table I.
 | Table IMain characteristics of the included
studies |
Table I
Main characteristics of the included
studies
| First author | Year | Study design | Evaluation | Study period | Patients, n | CRH, n | NSRH, n | (Refs.) |
|---|
| Bogani | 2014 | CC | PS | 2004-2012 | 96 | 63 | 33 | (21) |
| Ceccaroni | 2012 | CC | RS | 1997-2009 | 56 | 31 | 25 | (22) |
| Chen | 2012 | RCT | PS | NS | NS | NS | NS | (12) |
| Chen | 2014 | RCT | PS | NS | NS | NS | NS | (23) |
| Ditto | 2011 | CC | NS | NS | NS | NS | NS | (25) |
| Ditto | 2018 | CC | NS | NS | NS | NS | NS | (26) |
| van Gent | 2017 | CC | PS | 1994-2005 | 246 | 124 | 122 | (27) |
| Kuwabara | 2000 | CC | PS | 1993-1994 | 37 | 18 | 19 | (28) |
| Liang | 2010 | CC | PS | 2006-2009 | 163 | 81 | 82 | (29) |
| Liu | 2016 | CC | PS | 2011-2012 | 120 | 60 | 60 | (30) |
| Makowski | 2014 | CC | NS | 2001-2012 | 73 | 53 | 20 | (31) |
| Possover | 2000 | CC | PS | 1997-1999 | 38 | 28 | 10 | (32) |
| Querleu | 2002 | CC | RS | 1991-1996 | 95 | 47 | 48 | (33) |
| Raspagliesi | 2006 | CC | PS | NS | 110 | 51 | 59 | (34) |
| Raspagliesi | 2017 | CC | PS | 2009-2016 | 83 | 36 | 47 | (35) |
| Roh | 2015 | RCT | PS | 2003-2005 | 86 | 40 | 46 | (36) |
| Sakuragi | 2005 | CC | PS | 2000-2002 | 27 | 5 | 22 | (37) |
| Shi | 2016 | CC | RS | 2003-2013 | 108 | 42 | 64 | (38) |
| Skret-Magierlo | 2010 | CC | PS | 2007-2008 | 20 | 10 | 10 | (39) |
| van den
Tillaart | 2009 | CC | PS | 1994-1999 | 246 | 124 | 122 | (40) |
| | | | | 2001-2005 | | | | |
| Tseng | 2012 | CC | PS | 2010-2011 | 30 | 12 | 18 | (41) |
| Wu | 2010 | RCT | PS | 2007-2008 | 31 | 16 | 15 | (11) |
| Yang | 2016 | CC | PS | 2012-2015 | 76 | 38 | 38 | (42) |
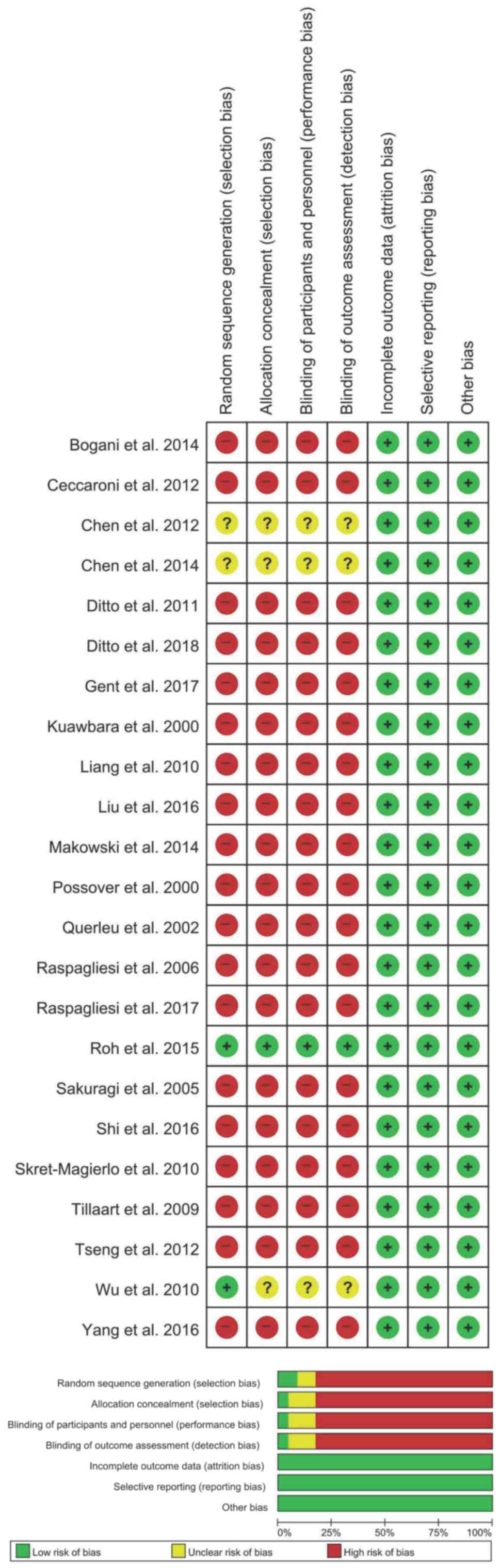
Study quality
All 23 studies included were retrospective. The risk
of bias was deemed to be high in all the studies due to the lack of
blinding of the participants or personnel and outcome assessors.
Moreover, all studies were characterized by high risk of allocation
bias. A detailed risk of bias assessment is described in Fig. 2.
Meta-analysis results
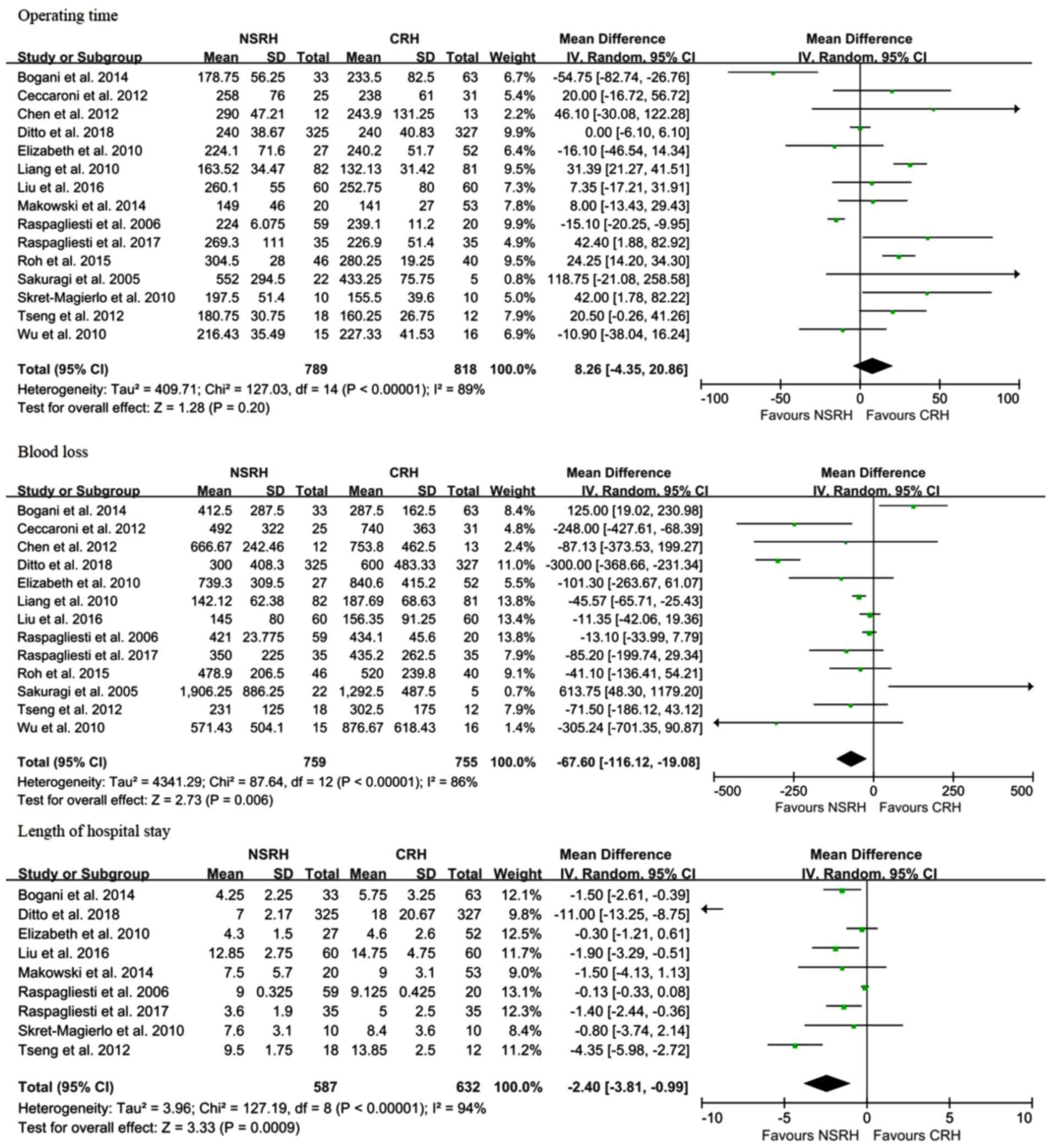
Outcomes included the following perioperative
parameters: Mean operative time, mean estimated blood loss and
length of hospital stay. Outcomes as an indicator of quality of
life were as follows: Duration of postoperative catheter (days),
urinary dysfunction, rectal dysfunction and sexual dysfunction. The
analysis of oncological outcome was performed through radicality,
PFS and OS. Radicality was measured by the resected parametrium and
vagina. The operative time (WMD, 8.45 min; 95% CI: -2.79 to 19.67;
P=0.14) did not differ statistically significantly between patients
undergoing CRH and NSRH (Fig. 3). As
regards perioperative parameters, NSRH was found to be associated
with lower intraoperative blood loss (WMD, -87.29 ml; 95% CI:
-139.91 to -34.66; P=0.001) and a shorter length of hospital stay
(WMD, -5.37 days; 95% CI: -8.08 to -2.67; P<0.0001) in
comparison with CRH.
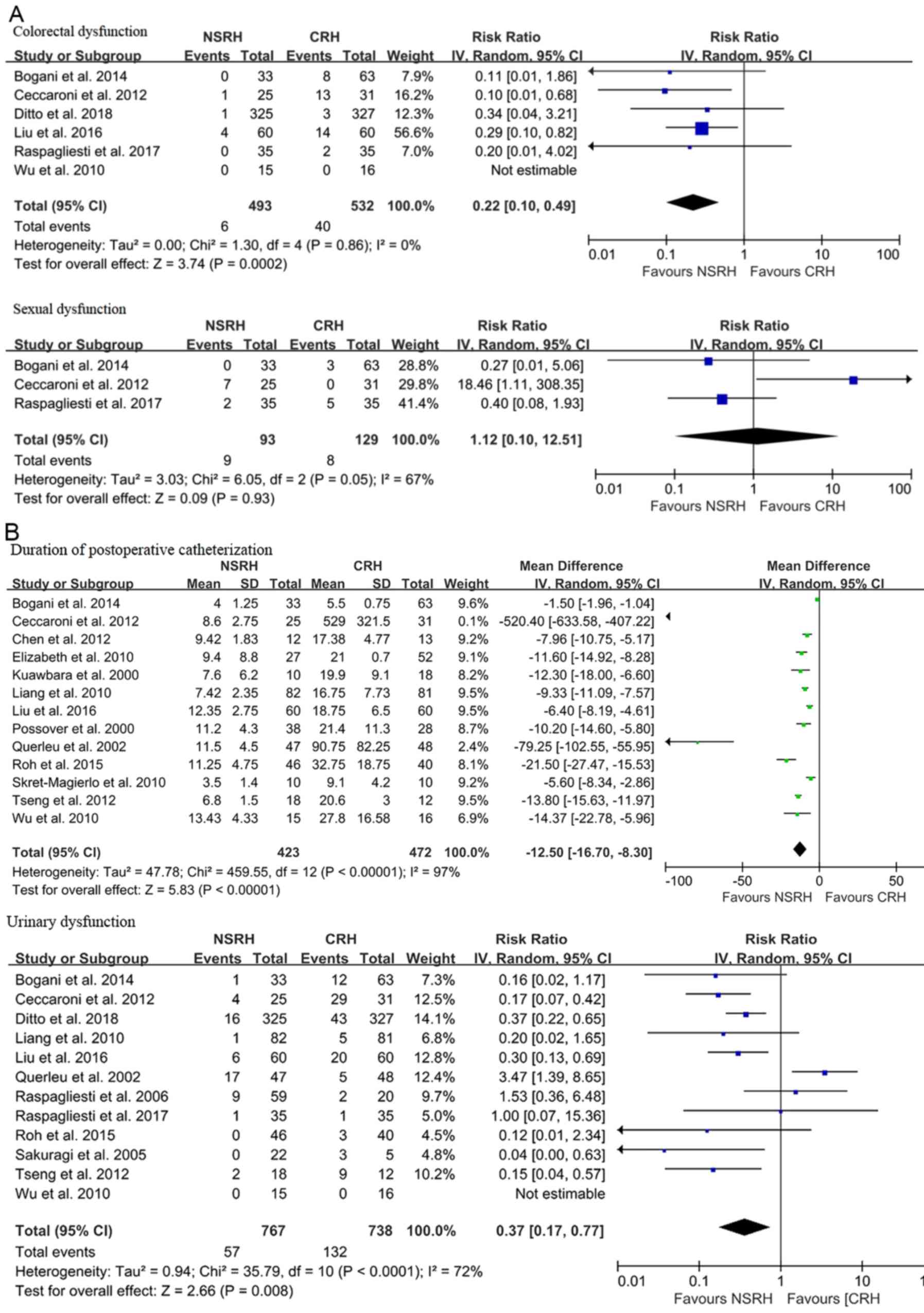
Data on pelvic floor dysfunction rates are presented
in Fig. 4. Patients undergoing NSRH
experienced lower urinary (RR=0.34; 95% CI: 0.18 to 0.63;
P=0.0007), colorectal (RR=0.24; 95% CI: 0.13 to 0.45; P<0.00001)
and sexual (RR=0.27; 95% CI; 0.08 to 0.86; P=0.03) dysfunction
rates compared with patients undergoing CRH (Fig. 4A). In particular, a shorter duration
of postoperative catheterization (WMD, -8.59 days; 95% CI: -12.17
to -5.02; P<0.00001) was observed among patients undergoing NSRH
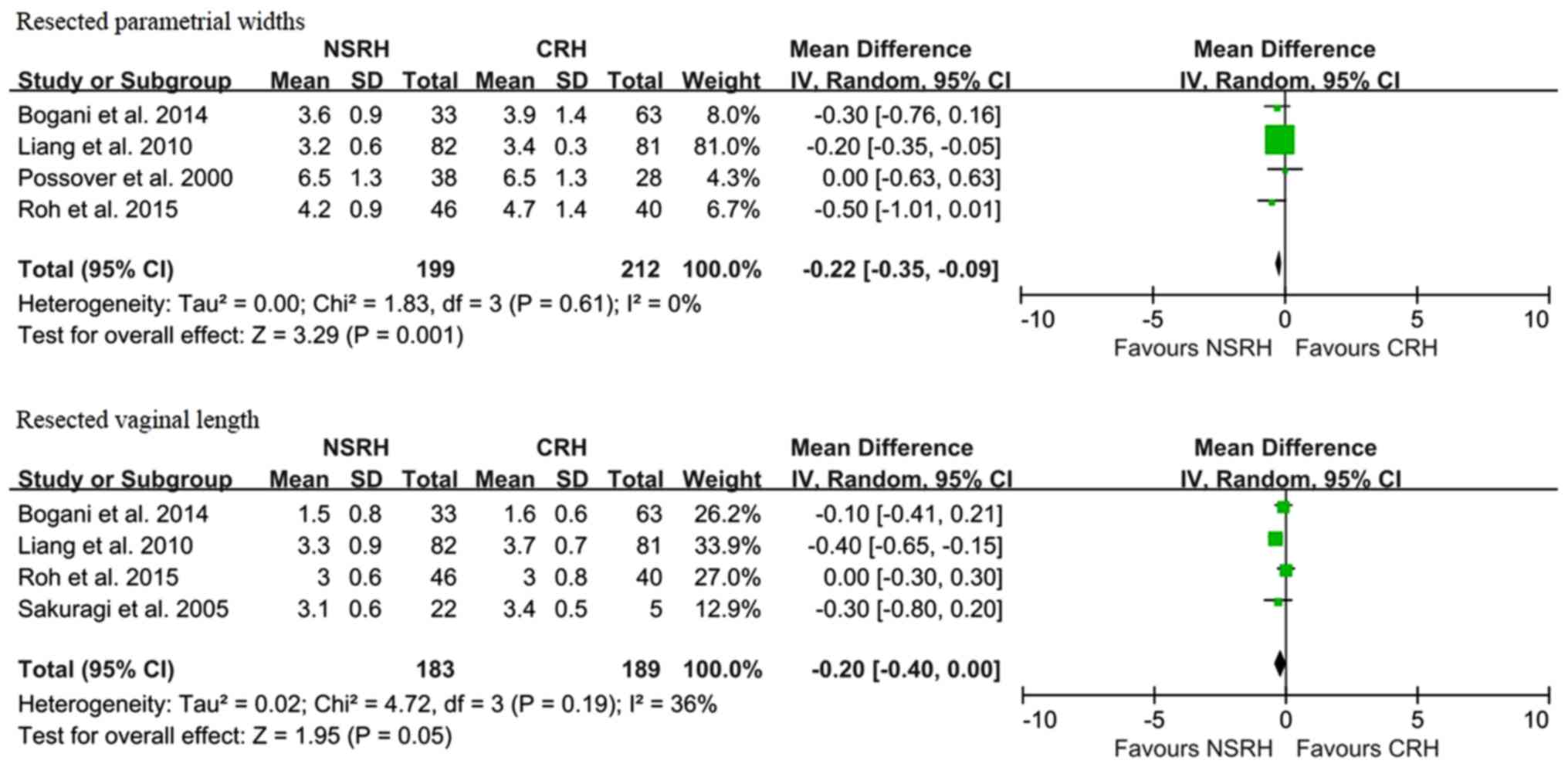
compared with patients undergoing CRH (Fig. 4B). Resected parametrial width was a
favorable factor in patients with CRH (WMD, -0.78 cm; 95% CI: -1.45
to -0.11; P=0.02). This result suggests that NSRH is inferior to
CRH in terms of radicality (Fig. 5).
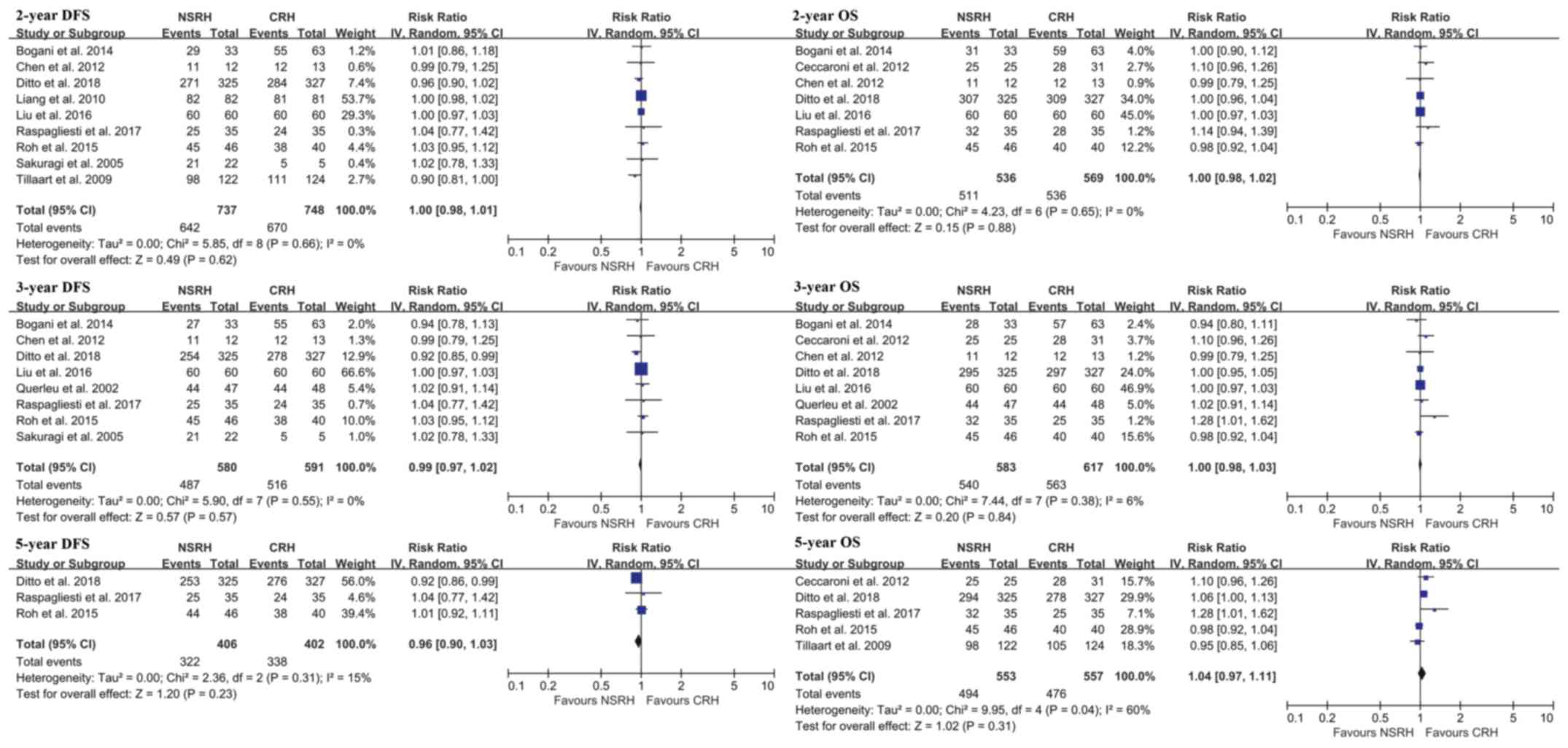
The 5-year disease free survival (RR=0.98; 95% CI: 0.90 to 1.06;
P=0.62) and 5-year OS (RR=0.97; 95% CI: 0.92 to 1.02; P=0.26) rates
were similar between groups (Fig.
6).
Discussion
The present study was a systematic review and
meta-analysis of the current evidence on the role of the
nerve-sparing approach to surgical treatment for ECC, collecting
data from studies comparing NSRH with CRH. Considering the
heterogeneity between studies, we were able to obtain valuable data
regarding pelvic dysfunction rate and oncological outcome. First,
our findings supported the results of studies reporting that NSRH
was associated with a shorter duration of postoperative
catheterization compared with CRH. These findings indicated that
bladder function recovered faster and the incidence of bladder
dysfunction was lower compared with that of CRH when using the NSRH
approach. Second, postoperative flatulence, constipation and fecal
incontinence are the main manifestations of anorectal dysfunction,
and the results are more favorable for NSRH compared with CRH. The
negative effect of CRH on bowel function was also reported in other
studies (43). Similarly, the
nerve-sparing approach was associated with a lower rate of sexual
dysfunction. There was no difference in operative time between the
two groups. However, estimated blood loss and length of hospital
stay were favorable for NSRH. In a meta-analysis with a
non-randomized study, NSRH was reported to be associated with a
longer operative time compared with CRH (44). Unlike our results, estimated blood
loss and length of hospital stay were similar between the two
groups in this non-randomized study.
The results of present systematic review and
meta-analysis suggest that NSRH is associated with fewer
complications and faster recovery of pelvic function compared with
CRH. Therefore, the radicality and oncological outcome were
compared between the two groups. Radicality was analyzed by
resected parametrium width and vaginal length, and NSRH exhibited
lower radicality compared with CRH. Liang et al investigated
the safety of 163 ECC patients and observed a statistically
significant reduction in the length of the resected parametrium and
vagina in the NSRH group (29). The
lower radicality per se may also be a concern. However,
other studies have reported favorable results of less radical
surgery in combination with neoadjuvant chemotherapy (45). There was no significant difference in
the 5-year PFS and OS between NSRH and CRH. The reason for the less
radical approach not affecting the oncological outcome may be
explained by previous studies (12,36,39).
Chen et al (12) analyzed the
cardinal ligament tissue specimens of 12 and 13 patients undergoing
NSRH and CRH, respectively, and found that, compared with CRH,
fewer pelvic nerves were removed in NSRH during cardinal ligament
dissection. In addition, they confirmed that the same number of
blood and lymphatic vessels were eliminated with both approaches.
The metastasis of cervical cancer occurs mainly through the blood
vessels and the lymphatic system, whereas metastasis through the
nerves is extremely rare, with only one such case reported to date
(46). However, even this single
case was one with advanced cervical cancer, rather than ECC. These
results may clarify why oncological outcome did not differ between
the two groups, and why the pelvic dysfunction rate was lower in
the NSRH group.
However, only 4 RCTs were included in the evaluated
studies, whereas the majority were case-control and comparative
cohort studies. There was also heterogeneity between studies, and
there was no level A evidence on this issue. However, the studies
were well-conducted and the data extracted were sufficient to
understand the impact of the nerve-sparing approach compared with
CRH. Recently, Chinese study groups conducted larger studies
(28,29,47), and
a total of 172 patients (82, 60 and 30 patients, respectively)
underwent NSRH. Lower pelvic dysfunction rate and improved safety
were confirmed with the nerve-sparing approach in these
studies.
There were certain limitations to the present study:
i) As mentioned earlier, the first limitation of this meta-analysis
was the considerable heterogeneity among the studies. There are
inherent biases in the various-design papers included in the
present study. Therefore, this must be taken into consideration
when interpreting the results. The risk of bias of the included
studies was systematically assessed, as seen in Fig. 6. ii) There were several omitted data
across different studies. Therefore, the results should be
interpreted with caution. iii) The mean number of patients included
in the reviewed studies was only 50 per group, which is relatively
small. iv) RR rather than hazard ratio was used to assess survival
outcomes. RR only measures the number of events and takes no
account of when they occur; thus, it is suitable for measuring
dichotomous outcomes, but less appropriate for analyzing
time-to-event outcomes (48). When
the total number of events reported for each study is used to
calculate RR, the result is an estimate that is difficult to
interpret. Although interpretation may be difficult, RRs can be
calculated at specific time points, making estimates comparable and
easier to interpret, at least at those time points.
In conclusion, the data collected in this systematic
review and meta-analysis demonstrated that the nerve-sparing
approach guarantees minimized risk of surgical-related pelvic
dysfunction, while achieving a similar oncological outcome as CRH,
supporting the preferred use of NSRH over CRH as a treatment for
ECC patients. However, further RCTs should be conducted to
establish the superiority and safety of NSRH in ECC.
Acknowledgements
Not applicable.
Funding
The present study was supported by Dong-A
University.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SHL wrote the manuscript and analyzed the data. JWB,
MH, YJC, JWP and SRO analyzed the data. SJK, SYC, JHY and YL
collected the data. All authors have read and approved the final
version of this manuscript for publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rob L, Halaska M and Robova H:
Nerve-sparing and individually tailored surgery for cervical
cancer. Lancet Oncol. 11:292–301. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Long Y, Yao DS, Pan XW and Ou TY: Clinical
efficacy and safety of nerve-sparing radical hysterectomy for
cervical cancer: A systematic review and meta-analysis. PLoS One.
9(e94116)2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ditto A, Martinelli F, Borreani C,
Kusamura S, Hanozet F, Brunelli C, Rossi G, Solima E, Fontanelli R,
Zanaboni F, et al: Quality of life and sexual, bladder, and
intestinal dysfunctions after class III nerve-sparing and class II
radical hysterectomies: A questionnaire-based study. Int J Gynecol
Cancer. 19:953–957. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kim HS, Choi CH, Lim MC, Chang SJ, Kim YB,
Kim MA, Kim TJ, Park SY, Kim BG, Song YS, et al: Safe criteria for
less radical trachelectomy in patients with early-stage cervical
cancer: A multicenter clinicopathologic study. Ann Surg Oncol.
19:1973–1979. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Maas CP, Trimbos JB, DeRuiter MC, Van De
Velde CJ and Kenter G: Nerve sparing radical hysterectomy: Latest
developments and historical perspective. Crit Rev Oncol.
48:271–279. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Querleu D and Morrow CP: Classification of
radical hysterectomy. Lancet Oncol. 9:297–303. 2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Höckel M, Konerding MA and Heussel CP:
Liposuction-assisted nerve-sparing extended radical hysterectomy:
Oncologic rationale, surgical anatomy, and feasibility study. Am J
Obstet Gynecol. 178:971–976. 1998.PubMed/NCBI View Article : Google Scholar
|
|
10
|
van Gent MD, Romijn LM, van Santen KE,
Trimbos JB and de Kroon CD: Nerve-sparing radical hysterectomy
versus conventional radical hysterectomy in early-stage cervical
cancer. A systematic review and meta-analysis of survival and
quality of life. Maturitas. 94:30–38. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wu J, Liu X, Hua K, Hu C, Chen X and Lu X:
Effect of nerve-sparing radical hysterectomy on bladder function
recovery and quality of life in patients with cervical carcinoma.
Int J Gynecol Cancer. 20:905–909. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen C, Li W, Li F, Liu P, Zhou J, Lu L,
Su G, Li X, Guo Y and Huang L: Classical and nerve-sparing radical
hysterectomy: An evaluation of the nerve trauma in cardinal
ligament. Gynecol Oncol. 125:245–251. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kim HS, Kim K, Ryoo SB, Seo JH, Kim SY,
Park JW, Kim MA, Hong KS, Jeong CW, Song YS, et al: Conventional
versus nerve-sparing radical surgery for cervical cancer: A
meta-analysis. J Gynecol Oncol. 26:100–110. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bogani G, Rossetti DO, Ditto A, Signorelli
M, Martinelli F, Mosca L, Scaffa C, Leone Roberti Maggiore U,
Chiappa V, Sabatucci I, et al: Nerve sparing approach improves
outcomes of patients undergoing minimally invasive radical
hysterectomy: A systematic review and meta-analysis. J Minim
Invasive Gynecol. 25:402–410. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xue Z, Zhu X and Teng Y: Comparison of
nerve-sparing radical hysterectomy and radical hysterectomy: A
systematic review and meta-analysis. Cell Physiol Biochem.
38:1841–1850. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li B, Zhang R, Wu LY, Zhang GY, Li X and
Yu GZ: A prospective study on nerve-sparing radical hysterectomy in
patients with cervical cancer. Zhonghua Fu Chan Ke Za Zhi.
43:606–610. 2008.(In Chinese). PubMed/NCBI View Article : Google Scholar
|
|
17
|
Review Manager (RevMan) [Computer
program]. Version 5.3. Copenhagen: Then Nordic Cochrane Centre, The
Cochrane Collaboration, 2014.
|
|
18
|
Gagnier J: The Cochrane risk of bias tool.
2011; Available at: http://bmg.cochrane.org.
Accessed July 12, 2016.
|
|
19
|
Guyatt GH, Oxman AD, Vist GE, Kunz R,
Falck-Ytter Y, Alonso-Coello P, Schünemann HJ and GRADE Working
Group: GRADE: An emerging consensus on rating quality of evidence
and strength of recommendations. BMJ. 336:924–926. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Moher D, Shamseer L, Clarke M, Ghersi D,
Liberati A, Petticrew M, Shekelle P, Stewart LA and PRISMA-P Group:
Preferred reporting items for systematic review and meta-analysis
protocols (PRISMA-P) 2015 statement. Syst Rev. 4(1)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bogani G, Cromi A, Uccella S, Serati M,
Casarin J, Pinelli C, Nardelli F and Ghezzi F: Nerve-sparing versus
conventional laparoscopic radical hysterectomy: A minimum 12
months' follow-up study. Int J Gynecol Cancer. 24:787–793.
2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ceccaroni M, Roviglione G, Spagnolo E,
Casadio P, Clarizia R, Peiretti M, Bruni F, Peters I and Aletti G:
Pelvic dysfunctions and quality of life after nerve-sparing radical
hysterectomy: A multicenter comparative study. Anticancer Res.
32:581–588. 2012.PubMed/NCBI
|
|
23
|
Chen C, Li W, Li F, Liu P, Zhou J, Lu L,
Su G, Li X, Guo Y and Huang L: Classical and nerve-sparing radical
hysterectomy: an evaluation of the nerve trauma in cardinal
ligament. Gynecol Oncol1. 25:245–251. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen C, Huang L, Liu P, Su G, Li W, Lu L,
Wang L, Li X, Duan H, Zou C and Hatch K: Neurovascular quantitative
study of the uterosacral ligament related to nerve-sparing radical
hysterectomy. Eur J Obstet Gynecol Reprod Biol. 172:74–79.
2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ditto A, Martinelli F, Mattana F, Reato C,
Solima E, Carcangiu M, Haeusler E, Mariani L and Raspagliesi F:
Class III nerve-sparing radical hysterectomy versus standard class
III radical hysterectomy: An observational study. Ann Surg Oncol.
18:3469–3478. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ditto A, Bogani G, Leone Roberti Maggiore
U, Martinelli F, Chiappa V, Lopez C, Perotto S, Lorusso D and
Raspagliesi F: Oncologic effectiveness of nerve-sparing radical
hysterectomy in cervical cancer. J Gynecol Oncol.
29(e41)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
van Gent MDJM, Rademaker M, van der Veer
JCB, van Poelgeest MIE, Gaarenstroom KN, Putter H, Trimbos JBMZ and
de Kroon CD: Long-term oncological outcome after conventional
radical hysterectomy versus 2 nerve-sparing modalities for early
stage cervical cancer. Int J Gynecol Cancer. 27:1729–1736.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kuwabara Y, Suzuki M, Hashimoto M, Furugen
Y, Yoshida K and Mitsuhashi N: New method to prevent bladder
dysfunction after radical hysterectomy for uterine cervical cancer.
J Obstet Gynaecol Res. 26:1–8. 2000.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liang Z, Chen Y, Xu H, Li Y and Wang D:
Laparoscopic nerve-sparing radical hysterectomy with fascia space
dissection technique for cervical cancer: Description of technique
and outcomes. Gynecol Oncol. 119:202–207. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu Z, Li X, Tao Y, Li W, Yang Y, Yao Y
and Zhu T: Clinical efficacy and safety of laparoscopic
nerve-sparing radical hysterectomy for locally advanced cervical
cancer. Int J Surg. 25:54–58. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Makowski M, Nowak M, Szpakowski M,
Wladzinski J, Serwach-Nowinska A, Janas L and Wilczyński JR:
Classical radical hysterectomy and nerve-sparing radical
hysterectomy in the treatment of cervical cancer. Prz Menopauzalny.
13:180–185. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Possover M, Stober S, Plaul K and
Schneider A: Identification and preservation of the motoric
innervation of the bladder in radical hysterectomy type III.
Gynecol Oncol. 79:154–157. 2000.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Querleu D, Narducci F, Poulard V, Lacaze
S, Occelli B, Leblanc E and Cosson M: Modified radical vaginal
hysterectomy with or without laparoscopic nerve-sparing dissection:
A comparative study. Gynecol Oncol. 85:154–158. 2002.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Raspagliesi F, Ditto A, Fontanelli R,
Zanaboni F, Solima E, Spatti G, Hanozet F, Vecchione F, Rossi G and
Kusamura S: Type II versus Type III nerve-sparing radical
hysterectomy: Comparison of lower urinary tract dysfunctions.
Gynecol Oncol. 102:256–262. 2006.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Raspagliesi F, Bogani G, Spinillo A, Ditto
A, Bogliolo S, Casarin J, Leone Roberti Maggiore U, Martinelli F,
Signorelli M, Gardella B, et al: Introducing nerve-sparing approach
during minimally invasive radical hysterectomy for locally-advanced
cervical cancer: A multi-institutional experience. Eur J Surg
Oncol. 43:2150–2156. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Roh J, Lee DO, Suh DH, Lim MC, Seo SS,
Chung J, Lee S and Park SY: Efficacy and oncologic safety of
nerve-sparing radical hysterectomy for cervical cancer: A
randomized controlled trial. J Gynecol Oncol. 26:90–99.
2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sakuragi N, Todo Y, Kudo M, Yamamoto R and
Sato T: A systematic nerve-sparing radical hysterectomy technique
in invasive cervical cancer for preserving postsurgical bladder
function. Int J Gynecol Cancer. 15:389–397. 2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Shi R, Wei W and Jiang P: Laparoscopic
nerve-sparing radical hysterectomy for cervical carcinoma: Emphasis
on nerve content in removed cardinal ligaments. Int J Gynecol
Cancer. 26:192–198. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Skret-Magierlo J, Narog M, Kruczek A,
Kluza R, Kluz T, Magon T, Skret A and Wicherek L: Radical
hysterectomy during the transition period from traditional to
nerve-sparing technique. Gynecol Oncol. 116:502–505.
2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
van den Tillaart SA, Kenter GG, Peters AA,
Dekker FW, Gaarenstroom KN, Fleuren GJ and Trimbos JB:
Nerve-sparing radical hysterectomy: Local recurrence rate,
feasibility, and safety in cervical cancer patients stage IA to
IIA. Int J Gynecol Cancer. 19:39–45. 2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tseng CJ, Shen HP, Lin YH, Lee CY and
Wei-Cheng Chiu W: A prospective study of nerve-sparing radical
hysterectomy for uterine cervical carcinoma in Taiwan. Taiwan J
Obstet Gynecol. 51:55–59. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yang Y, Qin T, Zhang W, Wu Q, Yang A and
Xu F: Laparoscopic nerve-sparing radical hysterectomy for bulky
cervical cancer (≥6 cm) after neoadjuvant chemotherapy: A
multicenter prospective cohort study. Int J Surg. 34:35–40.
2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Barnes W, Waggoner S, Delgado G, Maher K,
Potkul R, Barter J and Benjamin S: Manometric characterization of
rectal dysfunction following radical hysterectomy. Gynecol Oncol.
42:116–119. 1991.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Long Y, Yao D, Gao K and Xie X:
Preliminary study on clinical effect of nerve sparing radical
hysterectomy for cervical cancer. Chin Clin Oncol. 15:1083–1090.
2010.
|
|
45
|
Angioli R, Plotti F, Aloisi A, Scaletta G,
Capriglione S, Luvero D, Fiore L, Terranova C, Montera R and Panici
PB: A randomized controlled trial comparing four versus six courses
of adjuvant platinum-based chemotherapy in locally advanced
cervical cancer patients previously treated with neo-adjuvant
chemotherapy plus radical surgery. Gynecol Oncol. 139:433–438.
2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Li L, Wu Y, Hu L, Xu H, He H and Hu D:
Metastatic nerve root tumor: A case report and literature review.
Mol Clin Oncol. 4:1039–1040. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chen L, Zhang WN, Zhang SM, Yang ZH and
Zhang P: Effect of laparoscopic nerve-sparing radical hysterectomy
on bladder function, intestinal function recovery and quality of
sexual life in patients with cervical carcinoma. Asian Pac J Cancer
Prev. 15:10971–10975. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Tierney JF, Stewart LA, Ghersi D, Burdett
S and Sydes MR: Practical methods for incorporating summary
time-to-event data into meta-analysis. Trials. 8(16)2007.PubMed/NCBI View Article : Google Scholar
|