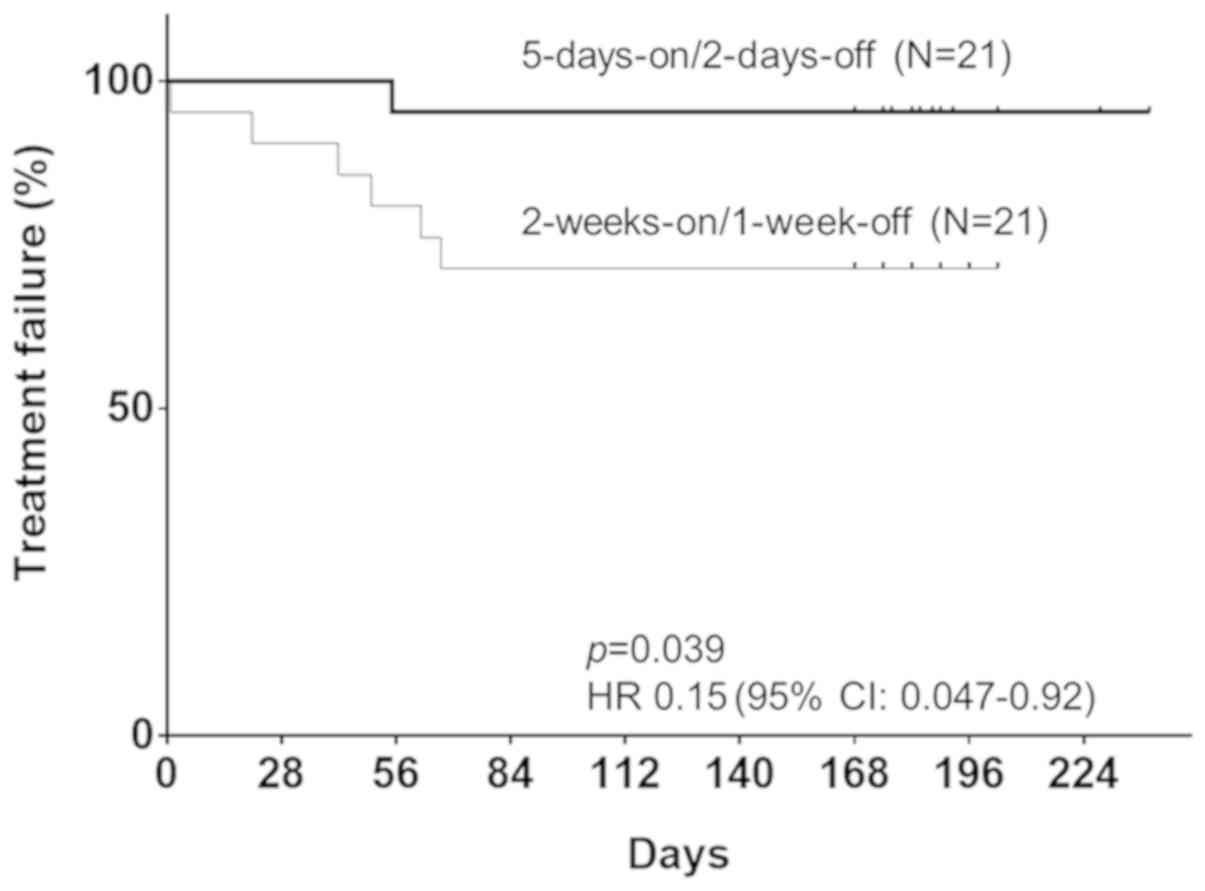
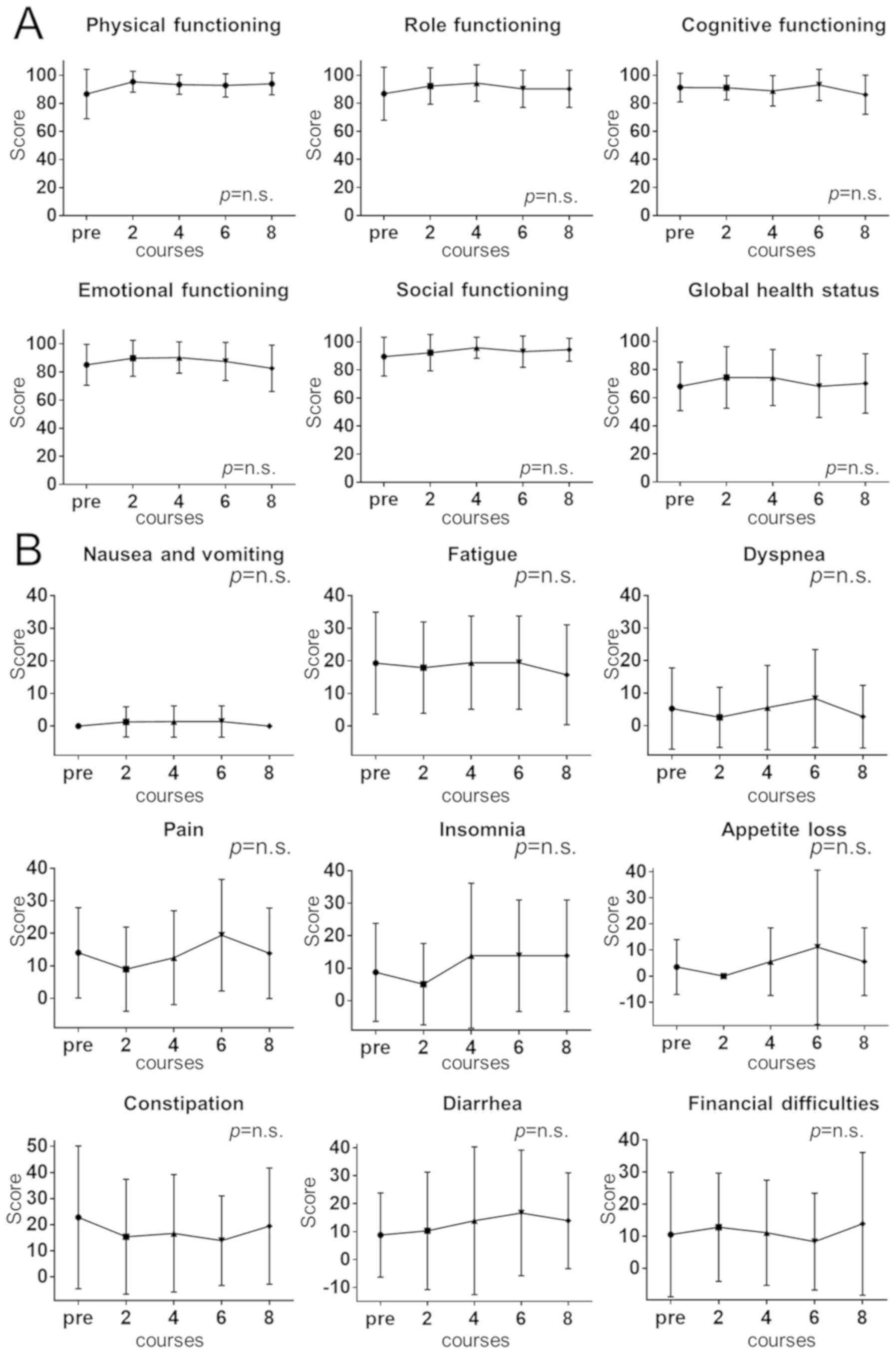
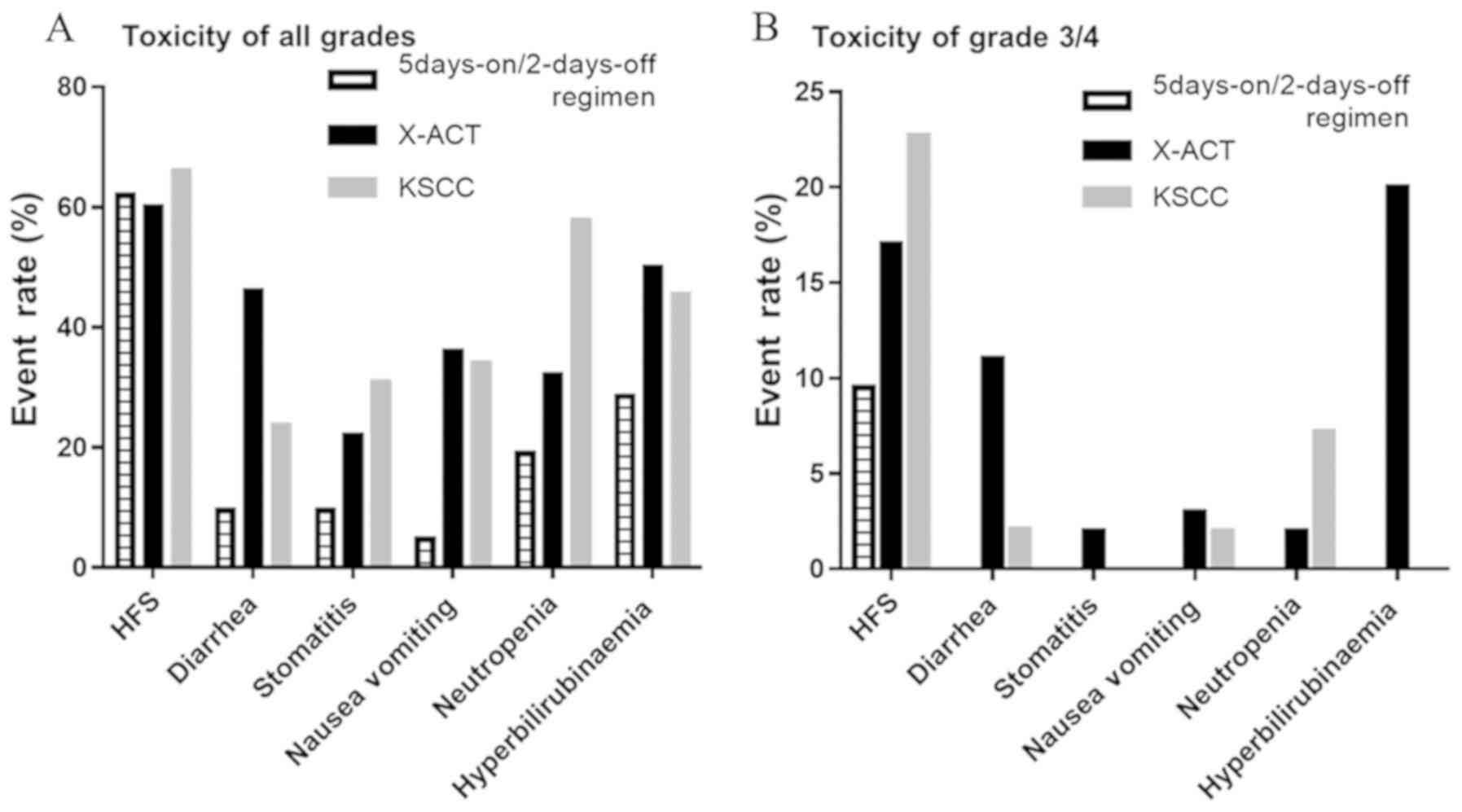
Spandidos Publications style
Mizumoto Y, Yokoyama S, Matsuda K, Iwamoto H, Mitani Y, Tamura K, Nakamura Y, Murakami D, Oka M, Kobayashi Y, Kobayashi Y, et al: Modulation of capecitabine administration to improve continuity of adjuvant chemotherapy for patients with colorectal cancer: A phase II study. Mol Clin Oncol 12: 126-133, 2020.
APA
Mizumoto, Y., Yokoyama, S., Matsuda, K., Iwamoto, H., Mitani, Y., Tamura, K. ... Yamaue, H. (2020). Modulation of capecitabine administration to improve continuity of adjuvant chemotherapy for patients with colorectal cancer: A phase II study. Molecular and Clinical Oncology, 12, 126-133. https://doi.org/10.3892/mco.2019.1961
MLA
Mizumoto, Y., Yokoyama, S., Matsuda, K., Iwamoto, H., Mitani, Y., Tamura, K., Nakamura, Y., Murakami, D., Oka, M., Kobayashi, Y., Yamaue, H."Modulation of capecitabine administration to improve continuity of adjuvant chemotherapy for patients with colorectal cancer: A phase II study". Molecular and Clinical Oncology 12.2 (2020): 126-133.
Chicago
Mizumoto, Y., Yokoyama, S., Matsuda, K., Iwamoto, H., Mitani, Y., Tamura, K., Nakamura, Y., Murakami, D., Oka, M., Kobayashi, Y., Yamaue, H."Modulation of capecitabine administration to improve continuity of adjuvant chemotherapy for patients with colorectal cancer: A phase II study". Molecular and Clinical Oncology 12, no. 2 (2020): 126-133. https://doi.org/10.3892/mco.2019.1961