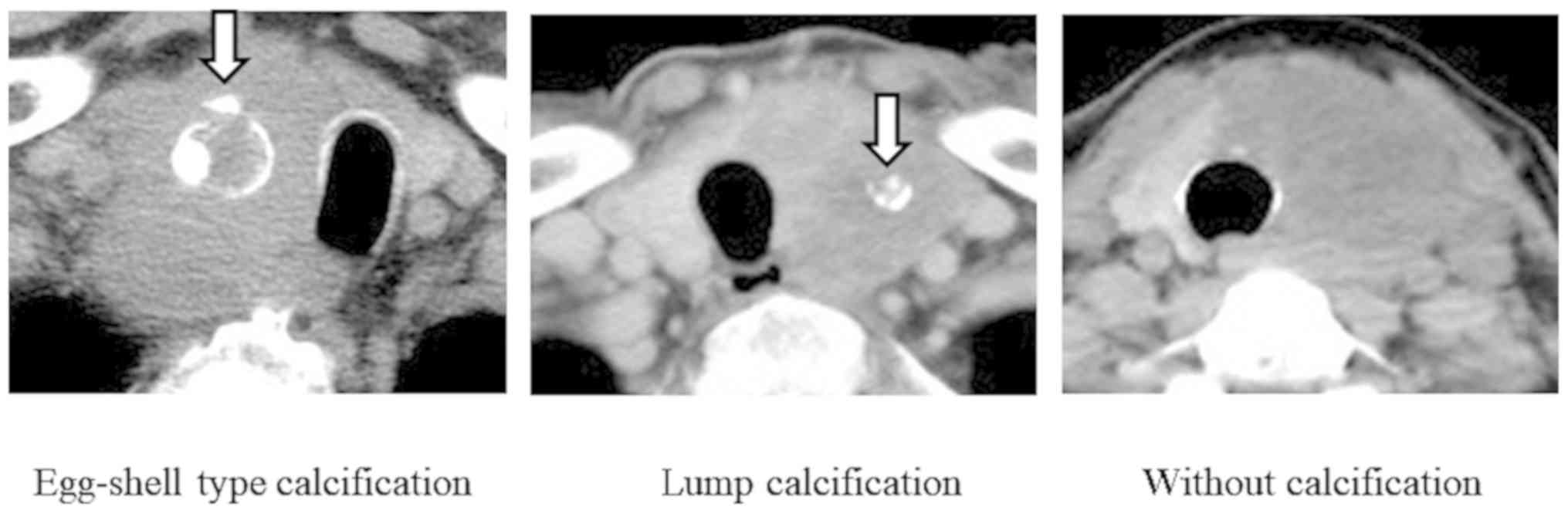
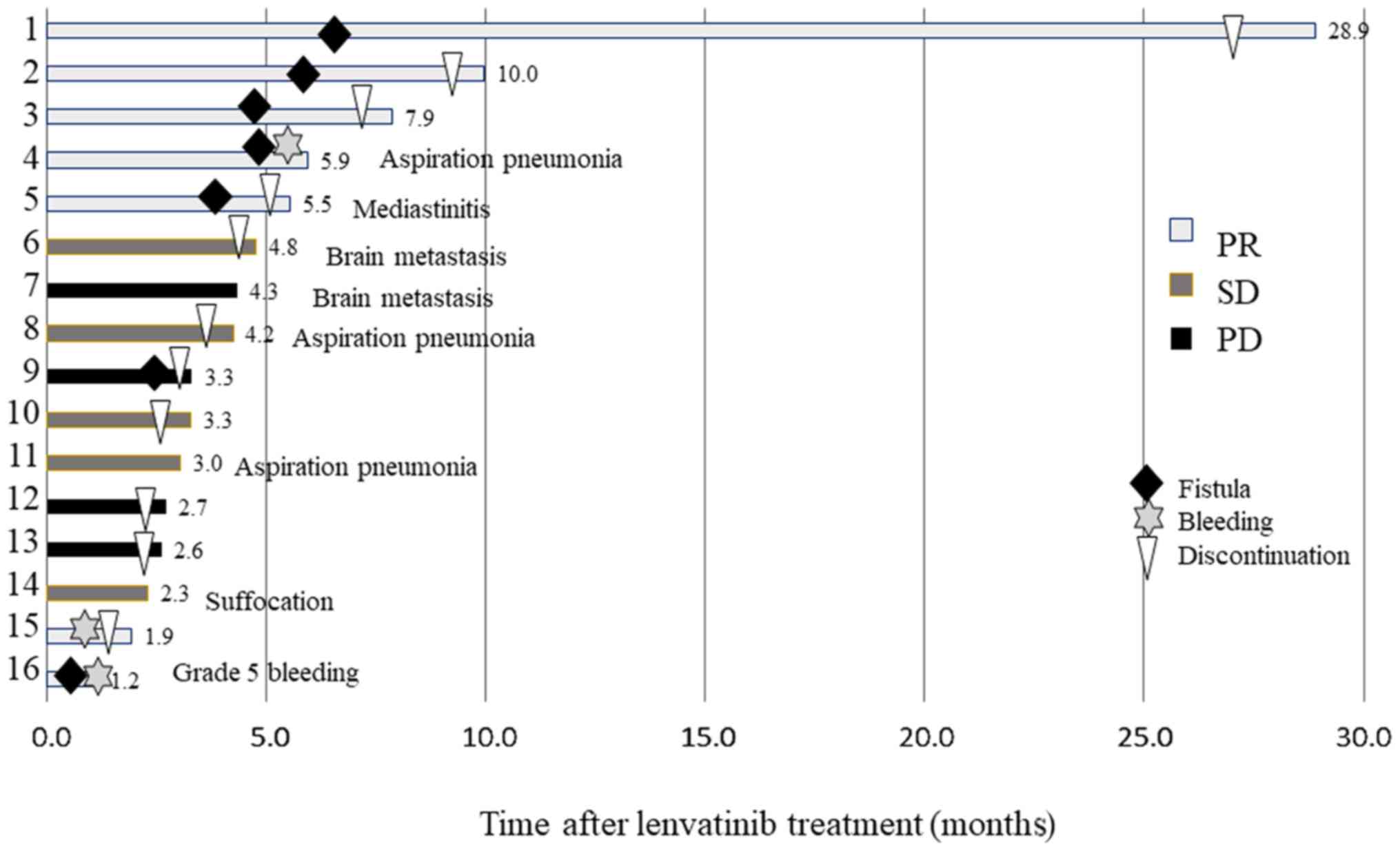
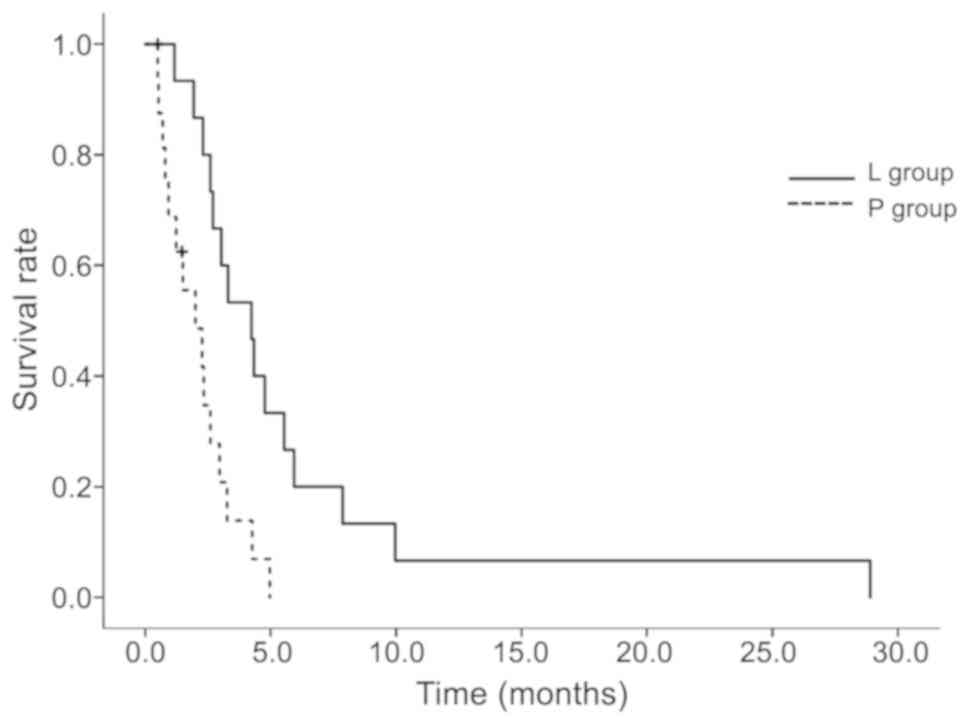
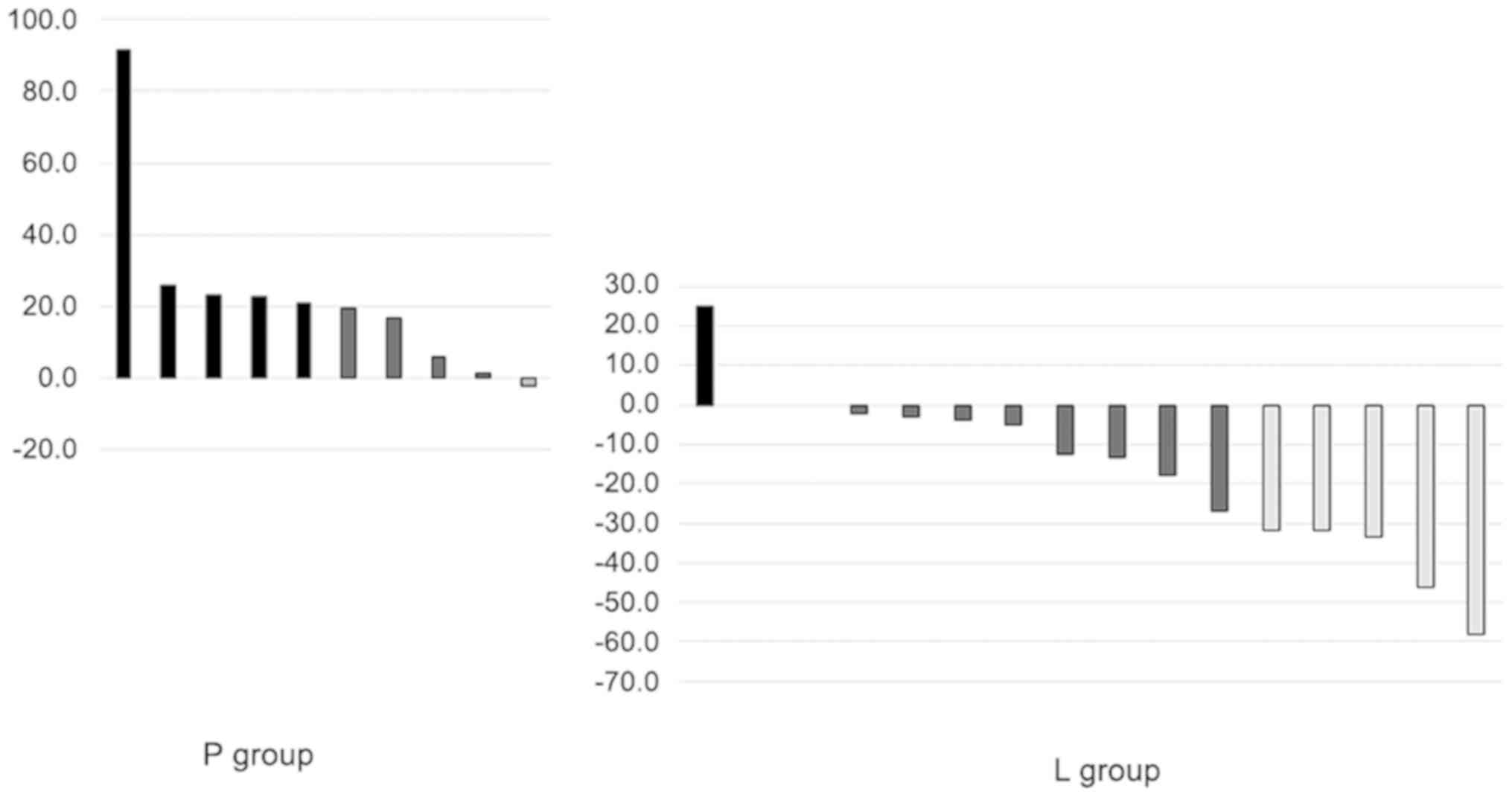
|
1
|
Oh EM, Lee KE, Kwon H, Kim EY, Bae DS and
Youn YK: Analysis of patients with anaplastic thyroid cancer
expected to have curative surgery. J Korean Surg Soc. 83:123–129.
2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shimaoka K, Schoenfeld DA, DeWys WD,
Creech RH and DeConti R: A randomized trial of doxorubicin versus
doxorubicin plus cisplatin in patients with advanced thyroid
carcinoma. Cancer. 56:2155–2160. 1985.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mooney CJ, Nagaiah G, Fu P, Wasman JK,
Cooney MM, Savvides PS, Bokar JA, Dowlati A, Wang D, Agarwala SS,
et al: A phase II trial of fosbretabulin in advanced anaplastic
thyroid carcinoma and correlation of baseline serum-soluble
intracellular adhesion molecule-1 with outcome. Thyroid.
19:233–240. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sosa JA, Elisei R, Jarzab B, Balkissoon J,
Lu SP, Bal C, Marur S, Gramza A, Yosef RB, Gitlitz B, et al:
Randomized safety and efficacy study of fosbretabulin with
paclitaxel/carboplatin against anaplastic thyroid carcinoma.
Thyroid. 24:232–240. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bisof V, Rakusic Z and Despot M: Treatment
of patients with anaplastic thyroid cancer during the last 20
years: Whether any progress has been made? Eur Arch
Otorhinolaryngol. 272:1553–1567. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Smallridge RC, Ain KB, Asa SL, Bible KC,
Brierley JD, Burman KD, Kebebew E, Lee NY, Nikiforov YE, Rosenthal
MS, et al: American thyroid association guidelines for management
of patients with anaplastic thyroid cancer. Thyroid. 22:1104–1139.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Iwasaki H, Yamazaki H, Suganuma N,
Nakayama H, Toda S and Masudo K: Role of tyrosine kinase inhibitor
thyrapy in anaplastic thyroid cancer. Int J Recent Adv
Multidisciplinary Res. 05:4270–4274. 2018.
|
|
8
|
Iwasaki H, Yamazaki H, Takasaki H,
Suganuma N, Nakayama H, Toda S and Masudo K: Lenvatinib as a novel
treatment for anaplastic thyroid cancer: A retrospective study.
Oncol Lett. 16:7271–7277. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Venkatesh YS, Ordonez NG, Schultz PN,
Hickey RC, Goepfert H and Samaan NA: Anaplastic carcinoma of the
thyroid. A clinicopathologic study of 121 cases. Cancer.
66:321–330. 1990.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kebebew E, Greenspan FS, Clark OH, Woeber
KA and McMillan A: Anaplastic thyroid carcinoma. Treatment outcome
and prognostic factors. Cancer. 103:1330–1335. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Onoda N, Sugino K, Higashiyama T, Kammori
M, Toda K, Ito K, Yoshida A, Suganuma N, Nakashima N, Suzuki S, et
al: The safety and efficacy of weekly paclitaxel administration for
anaplastic thyroid cancer patients: A nationwide prospective study.
Thyroid. 26:1293–1299. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pozdeyev N, Gay LM, Sokol ES, Hartmaier R,
Deaver KE, Davis S, French JD, Borre PV, LaBarbera DV, Tan AC, et
al: Genetic analysis of 779 advanced differentiated and anaplastic
thyroid cancers. Clin Cancer Res. 24:3059–3068. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Begum S, Rosenbaum E, Henrique R, Cohen Y,
Sidransky D and Westra WH: BRAF mutations in anaplastic thyroid
carcinoma: Implications for tumor origin, diagnosis and treatment.
Mod Pathol. 17:1359–1363. 2004.PubMed/NCBI
|
|
14
|
McIver B, Hay ID, Giuffrida DF, Dvorak CE,
Grant CS, Thompson GB, van Heerden JA and Goellner JR: Anaplastic
thyroid carcinoma: A 50-year experience at a single institution.
Surgery. 130:1028–1034. 2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pacheco-Ojeda LA, Martinez AL and Alvarez
M: Anaplastic thyroid carcinoma in ecuador: Analysis of prognostic
factors. Int Surg. 86:117–121. 2001.PubMed/NCBI
|
|
16
|
Takashima S, Morimoto S, Ikezoe J, Takai
S, Kobayashi T, Koyama H, Nishiyama K and Kozuka T: CT evaluation
of anaplastic thyroid carcinoma. AJR Am J Roentgenol.
154:1079–1085. 1990.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yamazaki H, Iwasaki H, Takasaki H,
Suganuma N, Sakai R, Masudo K, Nakayama H, Rino Y and Masuda M:
Efficacy and tolerability of initial low-dose lenvatinib to treat
differentiated thyroid cancer. Medicine (Baltimore).
98(e14774)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Subbiah V, Kreitman RJ, Wainberg ZA, Cho
JY, Schellens JHM, Soria JC, Wen PY, Zielinski C, Cabanillas ME,
Urbanowitz G, et al: Dabrafenib and trametinib treatment in
patients with locally advanced or metastatic BRAF V600-mutant
anaplastic thyroid cancer. J Clin Oncol. 36:7–13. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bastman JJ, Serracino HS, Zhu Y, Koenig
MR, Mateescu V, Sams SB, Davies KD, Raeburn CD, McIntyre RC Jr,
Haugen BR, et al: Tumor-Infiltrating T cells and the PD-1
checkpoint pathway in advanced differentiated and anaplastic
thyroid cancer. J Clin Endocrinol Metab. 101:2863–2873.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Vanden Borre P, Gunda V, McFadden DG,
Sadow PM, Varmeh S, Bernasconi M, Parangi S, et al: Combined
BRAF(V600E)-and SRC-inhibition induces apoptosis, evokes an immune
response and reduces tumor growth in an immunocompetent orthotopic
mouse model of anaplastic thyroid cancer. Oncotarget. 5:3996–4010.
2014.PubMed/NCBI View Article : Google Scholar
|