Introduction
Lung cancer is the leading malignancy in terms of
both incidence and mortality according to recent global cancer
statistics and as such is a major health issue requiring attention
(1). One major risk factor of lung
cancer is smoking. However, the number of non-smoker patients with
lung cancer is reported to have increased (2), which implies the existence of other
unknown risk factors (1). Surgery is
a key treatment modality for patients with non-metastatic lung
cancer. In Japan, 20% of patients with lung cancer are diagnosed
with stage IV (metastatic) lung cancer (3), requiring systemic anticancer therapy
including molecular targeted agents.
Along with the development of molecular targeted
therapy for lung cancer, the need for repeated genetic testing of
cancer cells has increased (4). This
has further increased the need for capturing samples from cancer
tissues or cells. It is not easy to obtain tumor tissues or cell
samples from patients with lung cancer. Therefore, less invasive
methods are in demand. Blood has been targeted as a sample for
capturing circulating tumor cells (CTC) or DNA for molecular
testing in a less invasive manner (5). Moreover, some investigators reported
that CTCs are detectable in patients with non-metastatic lung
cancer, and that the detected CTCs correlate with clinical outcome
(6,7). However, it is unknown whether CTCs
detected in patients with metastatic and non-metastatic disease
have the same biological characteristics. Establishing the
biological differences of CTCs in each clinical state would
facilitate understanding of the mechanism of metastasis. Thus, we
have developed a metallic micro-cavity array filter and an
automated CTC detection system. We had previously reported that
this device can isolate significantly more CTCs from patients with
metastatic lung cancer compared to methods depending on epithelial
cell-adhesion molecule (EpCAM) (8).
In this pilot study, we tested the device for CTC detection ability
in lung cancer patients of every clinical stage, including the
non-metastatic stage.
Patients and methods
Metallic micro-cavity array (MCA)
filter and the automated detection system
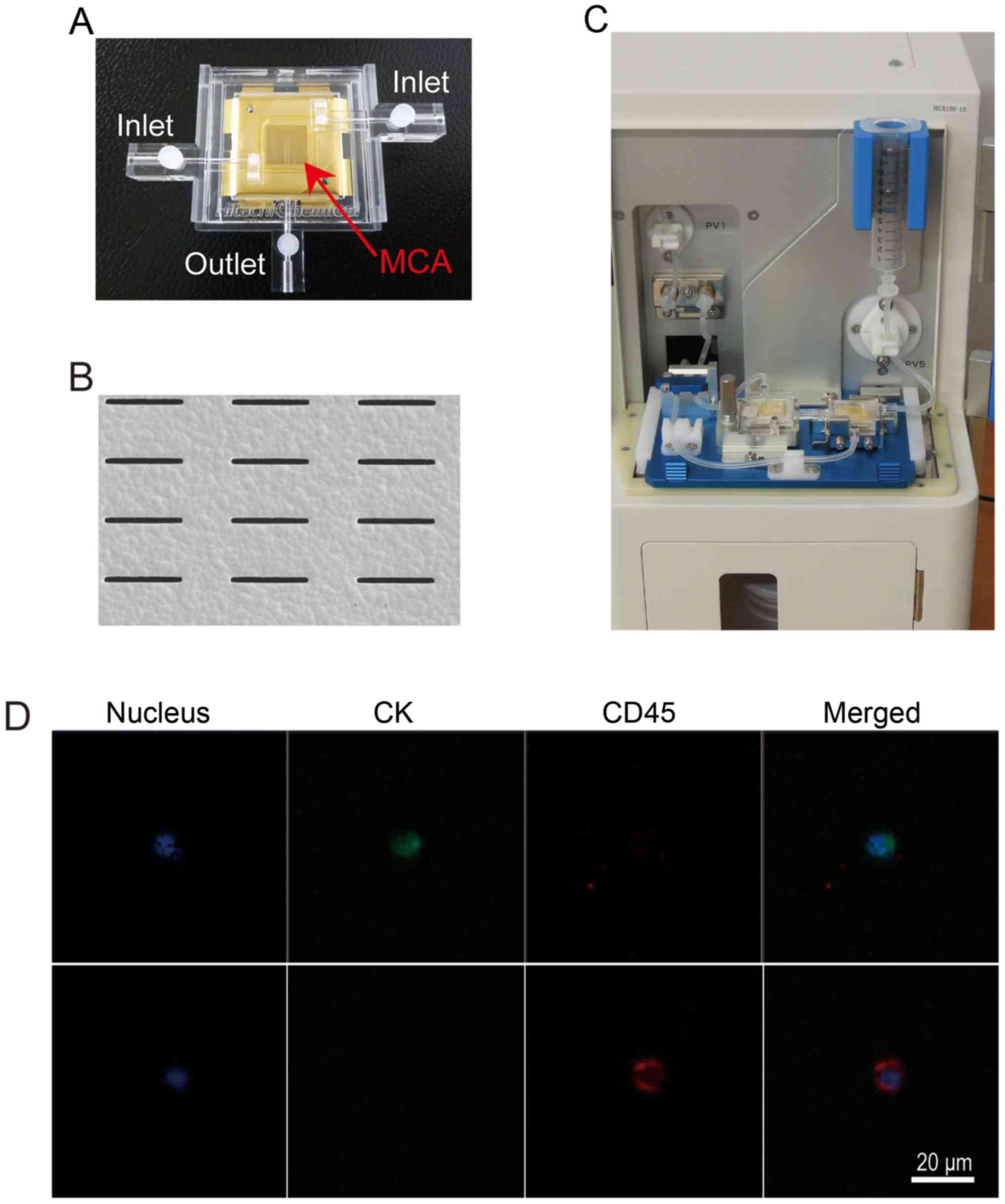
Hitachi Chemical Co., Ltd. has developed a metallic
MCA filter and an automated detection system for CTCs (Fig. 1A-C) (8-10).
A total of 3 ml of whole blood was added to the reservoir in the
system, and sample blood was passed through the MCA filter using a
peristaltic pump at a flow rate of 600 µl/min. Washing, fixation,
and permeabilization were performed automatically by the system.
Captured cells were stained automatically to distinguish the
nucleus (DAPI), cytokeratin (CK), and CD45 expression (fluorescent
dyes and antibodies for staining for nucleus, CK, and CD45 are
undisclosed because the device is still under development)
(Fig. 1D). The cavity size and
density are undergoing an optimization process. In Fig. 1B, a scanning electron microscope
image shows an array of micro-cavities used in this series.
Patients and methods
Patients at Hitachi general hospital who were
diagnosed with lung cancer, undergoing planned lung resection for
lung cancer, or suspected of having lung cancer, were recruited
from June 2014 to May 2015. The number of patients recruited was
planned as 40 for this pilot study. Written informed consent was
obtained from all participating patients. A 7-ml blood sample that
included backup volume for re-examination was collected in an EDTA
tube twice, namely before treatment (1st) and at approximately 3
months after the initial sample collection (2nd). Each sample was
anonymized and sent to the laboratory at Hitachi Chemical Co., Ltd.
Two operators at the Hitachi Chemical laboratory who were blinded
to the clinical information counted the CTCs according to
predetermined criteria [nucleus (+), CK (+), and CD45(-)]. Surplus
samples were discarded according to the protocol. All participating
patients were treated and followed up at Hitachi general hospital.
Their clinical data were collected from medical records. Smoking
index is an indicator of smoking history that is clinically used in
Japan, and was calculated as the number of cigarettes per day
multiplied by years smoking during his/her lifetime. We used the
7th edition of TNM for lung cancer classification.
The present study protocol was approved by The
Institutional Review Board of Ibaraki Hospital Headquarters at
Hitachi, Ltd. (approval no. 2014-64) and written informed consent
was obtained from all participants.
Statistical analysis
Continuous variables are presented as mean ± SE and
were tested using Shapiro-Wilk tests for normal distribution. If
the variable followed normal distribution, mean CTC counts in each
clinicopathological factor were compared using an independent
t-test. Otherwise, Mann-Whitney U tests were applied. For nominal
variables, χ2 test or Fisher's exact test was applied.
For correlation analyses, we applied both Pearson's and Spearman's
rank correlation analysis between CTC counts or change in CTC
counts and clinicopathological factors. Survival time was
calculated using the Kaplan-Meier method and compared using a
log-rank test. P<0.05 was considered to indicate a statistically
significant difference. We used SPSS version 24 (SPSS, Inc.) for
all statistical analyses.
Results
Patient characteristics
Of the 40 recruited patients, two cases were
excluded as lung cancer was not histologically proven in one case,
whereas the other case was diagnosed as metastatic lung tumor and
recurrence from a prior resected lung cancer. Therefore, we
analyzed 38 cases. Their clinicopathological characteristics are
summarized in Table I. The mean age
of the patients was 69.9 years (48-82), and they included 27 males
and 11 females. Adenocarcinoma was the dominant histological type,
including 1 small cell carcinoma. Considering clinical stages, the
number of patients in stages I, II, III, and IV was 19, 6, 6 and 7,
respectively. As for the treatment modality, surgery was dominant
(26 cases). Tyrosine kinase inhibitors were used in 3 cases for
EGFR mutation and in one for ALK rearrangement.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristic | Result |
|---|
| Age | 69.9 (48-82) |
| Sex, M:F | 27:11 |
| Histology | |
|
AD | 32 |
|
SQ | 4 |
|
SM | 1 |
|
NSCLC | 1 |
| Clinical stage | |
|
IA | 15 |
|
IB | 4 |
|
IIA | 3 |
|
IIB | 3 |
|
IIIA | 4 |
|
IIIB | 2 |
|
IV | 7 |
| Treatment
modalities | |
|
Surgery | 26 |
|
Radiotherapy | 1 |
|
Chemotherapy | 7 |
|
TKI | 4 |
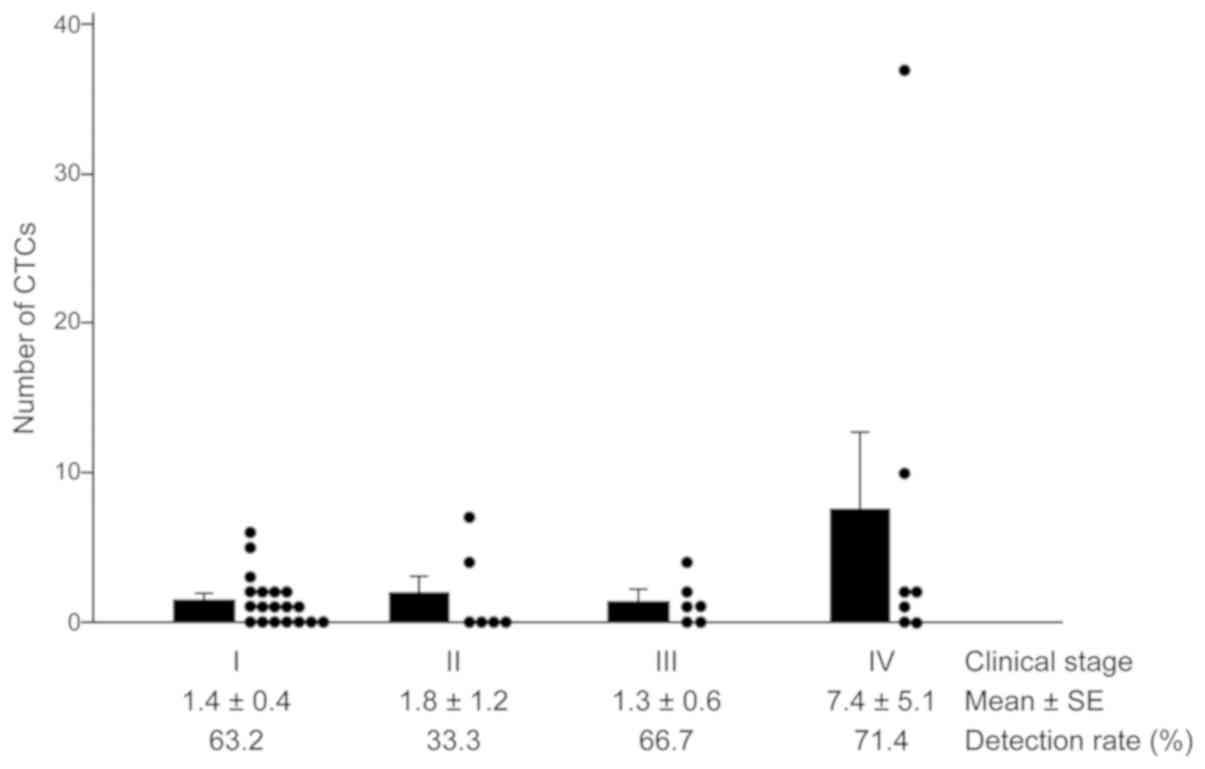
CTC counts in each clinical stage
CTC counts were 1.4±0.4, 1.8±1.2, 1.3±0.6 and
7.4±5.1 in clinical stages I, II, III, and IV, respectively.
Detection rates (defined as CTC counts of one or more) of each
clinical stage were: 63.2% (I), 33.3% (II), 66.7% (III), and 71.4%
(IV) (Fig .2). The ratios of CTC
counts=0 in each clinical stage were: 36.8% (I), 66.7 % (II), 33.3%
(III), and 28.6% (IV). Two-group comparisons between each stage or
between each possible combination, such as stage I and other
stages, stage I/II and III/IV, stage IV and other stages, using
Mann-Whitney U tests showed no statistically significant
difference.
Comparison of CTCs and
clinicopathological factors
We compared CTC counts and clinicopathological
factors and summarized them in Table
II. Sex, histological type, smoking index, presence or absence
of interstitial pneumonitis, clinical stage, and carcinoembryonic
antigen (CEA) levels were analyzed. None of the clinicopathological
factors showed a statistically significant difference (Mann-Whitney
U test). In this study, we did not define a specific number of CTCs
as positive. If one or more CTCs were defined as positive, we could
compare CTC numbers with clinicopathological factors as means of
continuous variables (age, smoking index, WBC, lymphocyte counts,
CRP, CEA, Cyfra, and c-tumor size) and as frequency of nominal
variables (sex, presence or absence of interstitial pneumonitis,
adenocarcinoma or other than adenocarcinoma, stage I and more than
II, and stage IV and others). A significant difference was observed
only in the smoking index. The CTC-positive group showed a smaller
smoking index than the negative group (382±485 vs. 892±769,
P=0.018).
 | Table IIComparison between CTC counts and
clinical factors. |
Table II
Comparison between CTC counts and
clinical factors.
| Clinical factor | CTC counts (mean ±
SE) | P-value |
|---|
| Sex | | |
|
Male
(n=27) | 1.74±0.49 | 0.505 |
|
Female
(n=11) | 4.64±3.27 | |
| Histology | | |
|
AD
(n=32) | 2.69±1.15 | 0.422 |
|
Non-AD
(n=6) | 2.00±1.63 | |
| Smoking
indexa | | |
|
≥600
(n=17) | 2.06±0.74 | 0.685 |
|
<600
(n=20) | 3.00±1.72 | |
|
0
(n=13) | 4.23±2.76 | 0.429 |
|
Other than 0
(n=24) | 1.71±0.52 | |
| IP | | |
|
Present
(n=5) | 3.20±2.06 | 0.967 |
|
Absent
(n=33) | 2.48±1.12 | |
| Clinical stage | | |
|
I
(n=19) | 1.42±0.39 | 0.795 |
|
II or more
(n=19) | 3.74±1.95 | |
|
I and II
(n=25) | 1.52±0.41 | 0.447 |
|
III and IV
(n=13) | 4.62±2.80 | |
|
IV (7) | 7.43±5.10 | 0.299 |
| Other than IV
(n=31) | 1.48±0.34 | |
| CEA | | |
|
≥3.5
(n=21) | 3.67±1.77 | 0.622 |
|
<3.5
(n=17) | 1.24±0.34 | |
Correlation analyses between CTC
counts and clinical factors
We performed correlation analyses between changes in
CTC counts based on time and clinical factors. Changes in CEA, WBC,
lymphocyte, and CRP did not show a correlation with differences in
CTC counts in both Pearson's and Spearman's rank correlation
analyses (Table III).
 | Table IIICorrelation between changes in
circulating tumor cell counts and clinical factors. |
Table III
Correlation between changes in
circulating tumor cell counts and clinical factors.
| Difference in
clinical factors with time | r | P-value | rs | P-value |
|---|
| Δ CEA (ng/ml) | 0.020 | 0.905 | 0.056 | 0.743 |
| Δ WBC (µl) | -0.082 | 0.624 | -0.159 | 0.339 |
| Δ lymphocyte
(µl) | -0.051 | 0.821 | 0.089 | 0.692 |
| Δ CRP (mg/dl) | 0.102 | 0.544 | 0.139 | 0.407 |
Survival analyses related to CTC
counts and changes in CTC counts
During the follow-up period, 12 deaths were
recorded, of which 11 resulted from lung cancer and one from acute
myeloid leukemia. The median follow-up period in survivors was 36
months. The 3-year survival rate for all patients was 68% and did
not reach 50%, and therefore the median survival time was not
calculated. We divided cases into two groups according to CTC
counts or changes in CTC counts: 1st CTC=0 and >0; 1st CTC=0 or
1 and >1; 1st CTC=0 to 2 and >2; 1st CTC=0 to 3 and >3;
1st CTC=0 to 4 and >4; 1st CTC=0 to 5 and >5; CTC decreased
group and other than decreased group; and CTC increased group and
other than increased group. We compared the survival of each group
and none of the analyses showed any significant difference. Because
the cohort of this pilot study included various treatment
modalities, we further analyzed only non-surgical cases (n=12).
Analyses between 1st CTC=0 and >0; 1st CTC=0 or 1 and >1; 1st
CTC=0 to 2 and >2; 1st CTC=0 to 3 and >3; CTC decreased group
and other than decreased group; and CTC increased group and other
than increased group; 2nd CTC=0 and >0; 2nd CTC=0 or 1 and >1
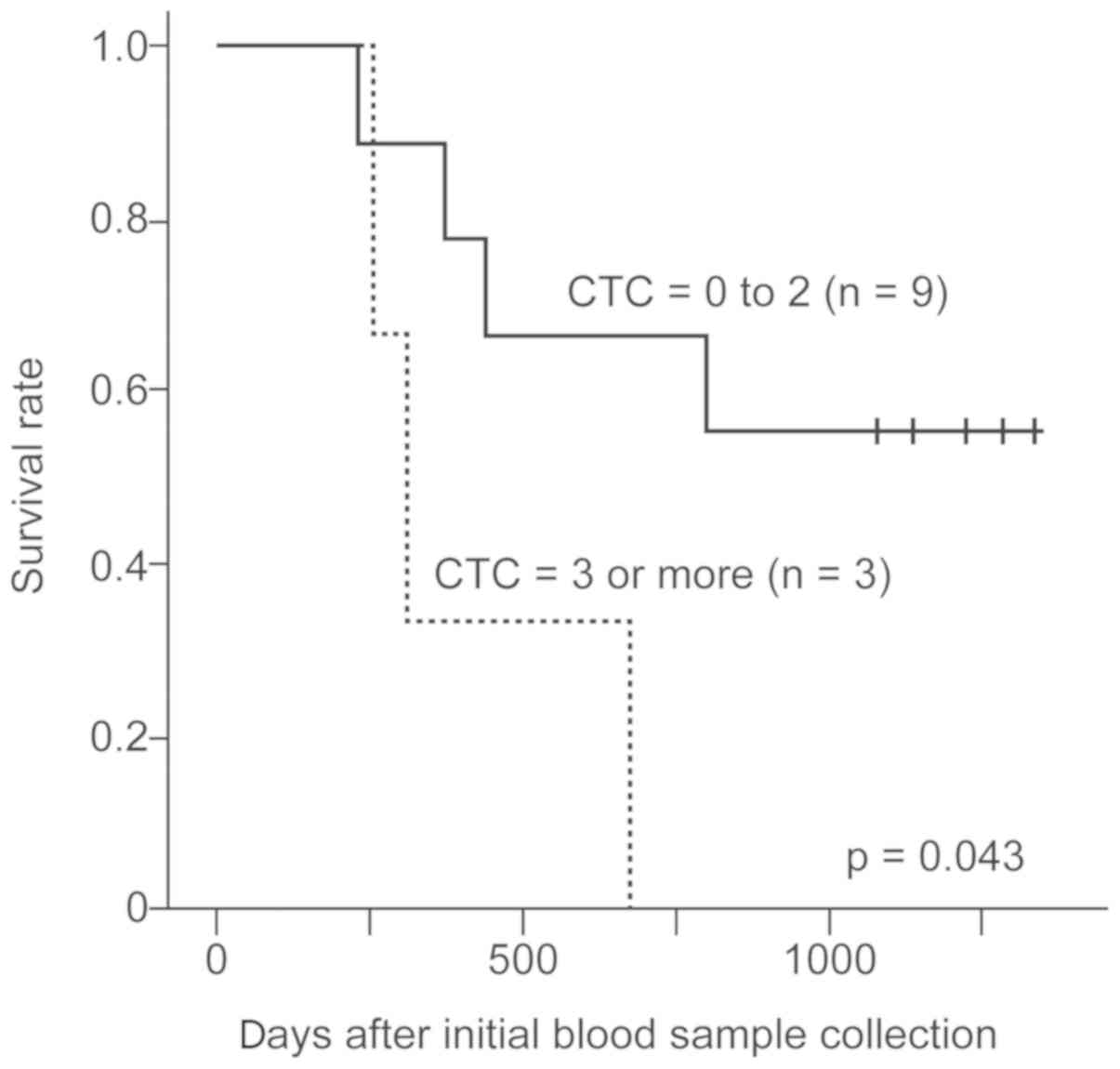
showed no significant differences. In contrast, cases of 2nd CTC
>2 showed significantly worse survival that those with 2nd CTC=0
to 2 (P=0.043, Fig. 3).
Discussion
In this study, we tried to assess the ability to
detect CTC at each clinical stage of lung cancer using the metallic
micro-cavity array filter developed by Hitachi Chemical. Although
CTC counts at each clinical stage did not show any significant
differences, our data show that CTCs were detected in patients with
non-metastatic lung cancer, which indicates the possible utility of
this device in early stage lung cancer. CTC studies targeting
non-metastatic lung cancer are limited and the CTC detection rate
(positive rate) varies (6,7,11-13).
As we did not confirm whether the cells captured on our device are
cancer cells using other methods such as morphological examination
under white light in all cases, we did not define the number of
CTCs as positive in this study. If we could define one or more CTCs
as positive, the positive rate in clinical stage I would be 63.2%,
which is comparable to that reported for a size-based device
(7,12). Regarding the specificity of captured
cells, we washed the filter and stained the captured cells with
Diff-quick stain kit (Sysmex) in some cases. The morphology of the
captured CK-positive cells was confirmed as CTCs, and that of
double positive (CK and CD45 positive) cells was determined as
hematologic cells (data not shown). Recently, an earlier version of
this device was tested in patients with metastatic lung cancer
(8). In that investigation,
non-detection rates (CTC=0) in metastatic non-small cell lung
cancer and small cell lung cancer were 23 and 0%, respectively. In
our series, 2 among 7 cases of metastatic non-small cell lung
cancer did not show any CTCs. This non-detection rate (28.6%) might
be comparable but requires further large-scale assessment. One
patient with small cell lung cancer who did not show CTCs in our
series was at clinical stage I.
We did not identify any clinicopathological factors
associated with the number of CTCs other than smoking index.
However, our results on the association between CTC counts and
smoking history are inconsistent with the data presented by
Dandachi et al (6). We
speculate that our prospectively recruited series was
adenocarcinoma-dominant (84% of all cases), which would confound
any correlation with the smoking index. As low-dose CT screening,
which is effective in finding a small peripheral ground glass
nodules representing a lepidic-growth type adenocarcinoma, is being
carried out since 1998 in Hitachi city (14), the histological type in our hospital
might have a tendency to be adenocarcinoma-dominant.
Although 1st CTC counts and changes in CTC counts
did not show any significant contribution to survival in all 38
cases and 12 non-surgical cases, cases with 2nd CTC count >2 in
non-surgical cases showed significantly worse survival than those
with 2nd CTC=0 to 2 (Fig. 3).
Because the cohort of this study included various treatment
modalities, we further selected only non-surgical cases for
survival analyses. Whereas these results might indicate the
possibility that CTC counts using this device after non-surgical
treatment would be of prognostic value, the clinical implication
and utility of CTC counts captured by this device require further
investigation with a larger number of patients.
The goal of CTC detection would then be to recognize
and evaluate the clinical utility of captured CTCs. For advanced
metastatic lung cancer, a possible application in genetic analyses
could facilitate precision medicine (4,15).
Additionally, if the cutoff number of CTC counts for postoperative
recurrence were available, patients undergoing lung resection for
lung cancer could avoid radiation exposure upon follow-up
examination. In both settings, single-cell analysis would not be
necessarily required. Our development concept of this device was
for simple capture of CTCs without sample preparation that would
combine clinical convenience with high throughput, and not for
single-cell manipulation. The development of techniques and methods
and evaluation of the utility of captured cells remain future
challenges. We developed this filter and device step by step that
included experiments using whole human blood spiked with cultured
cancer cells and measurements of CTC in healthy controls (8-10).
Because this prospective study was carried out according to a
protocol determined in advance, we could not recruit further cases
for more data nor add data from healthy controls. In a future
study, concurrent acquisition of healthy control samples and a
sufficiently large sample size should be considered.
In conclusion, this pilot study shows that the
metallic micro-cavity array filter developed by Hitachi Chemical
captured CTCs in patients with lung cancer even in early clinical
stages.
Acknowledgements
The authors would like to thank Ms. Masayo Okawa
(Division of Clinical Trial Management, Pharmacy Department,
Hitachi General Hospital) for helping with the informed consent
process, Ms Fumiko Kikuchi (Clinical Laboratory Center, Hitachi
General Hospital) for taking blood samples, and Mr Atsushi Yanagida
(Diagnostic Pathology Department, Hitachi General Hospital) for
blood sample management.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HI wrote the manuscript. TN designed the present
study. HI, TN, YY, KS, KK and SK treated and cared for the
patients. HK, TO, KE, TM, SN and SY developed the micro-cavity
array filter and the device. TN and YS comprehensively supervised
the present study. HI, TN, YY, KS, KK, SK, HK, TO, KE, TM, SN, SY
and YS interpreted the data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study protocol was approved by The
Institutional Review Board of Ibaraki Hospital Headquarters at
Hitachi, Ltd. (approval no. 2014-64) and written informed consent
was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The filter and device were developed by Hitachi
Chemical Co., Ltd. The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cufari ME, Proli C, De Sousa P,
Raubenheimer H, Al Sahaf M, Chavan H, Shedden L, Niwaz Z, Leung M,
Nicholson AG, et al: Increasing frequency of non-smoking lung
cancer: Presentation of patients with early disease to a tertiary
institution in the UK. Eur J Cancer. 84:55–59. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sawabata N, Asamura H, Goya T, Mori M,
Nakanishi Y, Eguchi K, Koshiishi Y, Okumura M, Miyaoka E and Fujii
Y: Japanese Joint Committee for Lung Cancer Registry: Japanese Lung
Cancer Registry Study: First prospective enrollment of a large
number of surgical and nonsurgical cases in 2002. J Thorac Oncol.
5:1369–1375. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rosell R, Bivona TG and Karachaliou N:
Genetics and biomarkers in personalisation of lung cancer
treatment. Lancet. 382:720–731. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lindeman NI, Cagle PT, Aisner DL, Arcila
ME, Beasley MB, Bernicker EH, Colasacco C, Dacic S, Hirsch FR, Kerr
K, et al: Updated molecular testing guideline for the selection of
lung cancer patients for treatment with targeted tyrosine kinase
inhibitors: Guideline from the college of American pathologists,
the international association for the study of lung cancer, and the
association for molecular pathology. J Thorac Oncol. 13:323–358.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dandachi N, Tiran V, Lindenmann J, Brcic
L, Fink-Neuboeck N, Kashofer K, Absenger G, Bezan A, Cote RJ, Datar
R and Balic M: Frequency and clinical impact of preoperative
circulating tumor cells in resectable non-metastatic lung
adenocarcinomas. Lung Cancer. 113:152–157. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hofman V, Ilie MI, Long E, Selva E,
Bonnetaud C, Molina T, Vénissac N, Mouroux J, Vielh P and Hofman P:
Detection of circulating tumor cells as a prognostic factor in
patients undergoing radical surgery for non-small-cell lung
carcinoma: Comparison of the efficacy of the CellSearch Assay™ and
the isolation by size of epithelial tumor cell method. Int J
Cancer. 129:1651–1660. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hosokawa M, Kenmotsu H, Koh Y, Yoshino T,
Yoshikawa T, Naito T, Takahashi T, Murakami H, Nakamura Y, Tsuya A,
et al: Size-based isolation of circulating tumor cells in lung
cancer patients using a microcavity array system. PLoS One.
8(e67466)2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Negishi R, Hosokawa M, Nakamura S, Kanbara
H, Kanetomo M, Kikuhara Y, Tanaka T, Matsunaga T and Yoshino T:
Development of the automated circulating tumor cell recovery system
with microcavity array. Biosens Bioelectron. 67:438–442.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hosokawa M, Yoshikawa T, Negishi R,
Yoshino T, Koh Y, Kenmotsu H, Naito T, Takahashi T, Yamamoto N,
Kikuhara Y, et al: Microcavity array system for size-based
enrichment of circulating tumor cells from the blood of patients
with small-cell lung cancer. Anal Chem. 85:5692–5698.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Qian C, Wu S, Chen H, Zhang X, Jing R,
Shen L, Wang X, Ju S, Jia C and Cong H: Clinical significance of
circulating tumor cells from lung cancer patients using
microfluidic chip. Clin Exp Med. 18:191–202. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bayarri-Lara C, Ortega FG, Cueto Ladrón de
Guevara A, Puche JL, Ruiz Zafra J, de Miguel-Pérez D, Ramos AS,
Giraldo-Ospina CF, Navajas Gómez JA, Delgado-Rodriguez M, et al:
Circulating tumor cells identify early recurrence in patients with
non-small cell lung cancer undergoing radical resection. PLoS One.
11(e0148659)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Okumura Y, Tanaka F, Yoneda K, Hashimoto
M, Takuwa T, Kondo N and Hasegawa S: Circulating tumor cells in
pulmonary venous blood of primary lung cancer patients. Ann Thorac
Surg. 87:1669–1675. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nawa T, Nakagawa T, Mizoue T, Kusano S,
Chonan T, Hayashihara K, Suito T and Endo K: A decrease in lung
cancer mortality following the introduction of low-dose chest CT
screening in Hitachi, Japan. Lung Cancer. 78:225–228.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Maheswaran S, Sequist LV, Nagrath S, Ulkus
L, Brannigan B, Collura CV, Inserra E, Diederichs S, Iafrate AJ,
Bell DW, et al: Detection of mutations in EGFR in circulating
lung-cancer cells. N Engl J Med. 359:366–377. 2008.PubMed/NCBI View Article : Google Scholar
|