Introduction
Head and neck cancer affects ~686,000 new patients
annually, 375,000 of whom eventually succumb to the disease
according to the latest data from the World Health Organization
(1). These data indicate that head
and neck squamous cell carcinoma (HNSCC) is the sixth most common
type of cancer worldwide. Thus, a growing number of patients
diagnosed with head and neck cancer are confronted with not only
the risk of local and regional relapse following curative therapy,
but also the occurrence of a synchronous or metachronous secondary
carcinoma. According to GLOBOCAN, the incidence of secondary
carcinoma in different localizations in the head and neck is as
follows: Oral cavity 4.3, larynx 2.2, pharynx 2.0, and nasopharynx
1.2 per 100,000 (2,3). Mortality depends on the localization of
the primary tumor and the stage of the disease. Globally, a total
of 4.58% of all cancer patients succumb to head and neck carcinoma
(2).
Multiple primary malignancies is a term used to
describe the occurrence of two or more malignancies at different
anatomical localizations. These are defined as synchronous
malignancies if the secondary carcinoma is diagnosed within 6
months of the primary carcinoma, or as metachronous if it is
diagnosed >6 months after the primary carcinoma (4). In a retrospective study of 6,545
oncological patients in China, a secondary malignancy was
identified in 72 cases (1.1%). In the present study, the highest
incidence of secondary malignancies was noted among patients with
head and neck cancer, followed by colorectal carcinoma and lung
tumors (5). A retrospective study
from India analyzed the data of 23,260 patients and found that 41
patients (0.18%) had developed a secondary carcinoma; these
secondary malignancies were most common among patients with head
and neck cancer, followed by gynecological tumors (6). An analysis from the USA, including
2,116,163 patients, identified 170,865 (8.1%) cases with a
secondary carcinoma; in that study, the most common localizations
of a secondary malignancy were the lung (18%), colon (12%),
prostate (9%), and bladder (8%) (7).
These differences confirm a wide variation in different parts of
the world. Precise epidemiological data regarding the incidence and
distribution of synchronous and metachronous malignancies are
lacking, and the incidence in the current literature varies between
1.8 and 34.6% (8-19).
A study from the Department of Health and Human Services in USA
analyzed 58,363 patients with head and neck cancer between 1973 and
2008, and revealed that 11.7% (n=6,855) of the patients developed a
secondary tumor. Patients with laryngeal carcinoma had the highest
incidence of secondary tumors outside the head and neck area
(13.6%), whereas patients with primary oral cancer had the highest
incidence of secondary malignancies within the head and neck area
[oral cavity (58.4%), larynx (18.2%) and oropharynx (13.3%)]
(8). Metachronous carcinomas
developed more often compared with synchronous carcinomas and,
according to the literature, their frequency varied between 4.7 and
24%, whilst synchronous tumors were found in 0.3-14% of the
patients (9,11,13,15,20). Di
Martino et al categorized secondary tumors based on the time
of diagnosis. Their retrospective study investigated 1,803 patients
with primary tumors in the head and neck area, among whom 34 (1.9%)
were found to have a synchronous carcinoma and 86 (4.8%) developed
a metachronous carcinoma. Of those 86 patients, 50 developed the
metachronous tumor within 5 years after primary tumor diagnosis, 22
patients developed the metachronous tumor within 6-10 years, and 14
patients after >10 years. Whether metachronous carcinomas with
such long latency should be classified as secondary malignancies,
with an etiological and pathogenic connection to the primary
carcinoma, or as independent primary tumors, warrants further
discussion (13).
The most important risk factors for HNSCC are
alcohol and nicotine abuse, the chewing of betel nuts, insufficient
dental hygiene, and infection with human papillomavirus,
particularly the high-risk subtypes 16 and 18 (21-26).
Smoking and drinking are major contributors to field cancerization
and the occurrence of secondary malignancies (27-29).
According to Ruback et al the combination of alcohol and
tobacco abuse increases the risk of SCC up to 40-fold compared with
either alcohol or tobacco alone. It may be inferred that alcohol
serves as a dissolvent for some tobacco carcinogens and facilitates
cellular uptake (30). A study by
Pezzuto et al investigated the combined use of alcohol and
tobacco in 11,221 patients with head and neck cancer; 72% of the
patients claimed to have consumed tobacco or alcohol regularly; 4%
of those patients claimed to have consumed alcohol alone, 33%
tobacco alone, and 35% reported consuming both regularly (31). Both carcinogenic substances have a
dose-effect relationship and cause cumulative toxicity.
Carcinogenic effects may appear for even up to 10 years after the
cessation of drinking and smoking (27). Smokeless forms of tobacco, such as
snuffed or chewed tobacco, sometimes combined with betel quid or
other preparations, such as the areca nut, are particularly
consumed in India, North and South America and northern Europe.
Betel nuts contain several alkaloids (guvacin, arecaidin,
guvacoline and arecoline) that are addictive and have carcinogenic
properties; they also inhibit tumor suppressor genes and DNA-repair
mechanisms, causing uncontrolled cell proliferation within the
mucosa and, thus, increasing the risk of cancer of the oral cavity
and esophagus (28,32-35).
The development of secondary malignancies in the
head and neck area may be explained by field cancerization. This
term was coined in 1953 by Jaiswal et al, who observed
multiple areas of SCC within the oropharyngeal cavity of a patient.
Field cancerization describes the occurrence of multiple sites with
invasive growth and areas of dysplasia within the mucosa. Despite
the metacentric origin, field cancerization appears to be an
important factor for the occurrence of secondary SCCs after therapy
completion (21).
Secondary malignancies occur frequently in patients
with head and neck carcinoma and may seriously affect the prognosis
of the patients, which warrants a close follow-up; therefore, we
herein sought to evaluate the frequency of secondary malignancies
among head and neck cancer patients in our center and identify
possible clinical indicators of their occurrence following curative
tumor therapy. The aim of the present study was to help predict the
occurrence of secondary cancer within smaller cohorts of other
certified tumor centers.
Patients and methods
Ethics statement
The present study was conducted in full accordance
with the Declaration of Helsinki and was approved by the Ethics
Committee II of the Medical Faculty of Mannheim at the University
of Heidelberg, Mannheim, Germany (file no. 2016-827R-MA).
Patients
A total of 380 patients with HNSCC who were treated
at University Hospital Mannheim between 2006 and 2015 were
enrolled. Patients for whom no complete follow-up data could be
obtained, or those who died during the follow-up period, were
excluded. Patients with secondary carcinomas other than SCC, i.e.,
sarcomas, lymphomas, etc., as well as patients receiving palliative
treatment were also excluded. All included patients underwent a
thorough clinical inspection, including a physical and endoscopic
examination of the head and neck area, as well as sonographic and
radiological imaging for staging of the disease. Bronchoscopy,
gastroscopy, pharyngoscopy and microlaryngoscopy were part of
staging for all patients in order to identify secondary
malignancies, as well as regional and distant metastases. After
staging, patients received therapy according to the primary tumor
stage. Multimodal therapy included tumor resection, neck
dissection, adjuvant chemoradiotherapy, or definitive
chemoradiotherapy when surgical therapy was refused or deemed not
suitable. All patients underwent regular follow-ups at the clinic,
including head and neck examination, ultrasonography of the neck,
microlaryngoscopy, esophagogastroscopy and bronchoscopy, with a
mean interval of 4.8 months (standard deviation, 1.8 months).
Patient data were retrieved from the internal tumor database of our
institution, patient files, medical reports, surgical reports, and
interdisciplinary tumor board protocols. Information was also
obtained on patient sex, age, TNM stage, date of diagnosis,
localization of the primary tumor, secondary carcinomas and their
localization (synchronous and metachronous), and tobacco and
alcohol consumption. The initial hypothesis was that no more than
20% of all cases would be positive for a secondary carcinoma.
Another goal of the present study was to investigate possible
clinical indicators for the occurrence of secondary carcinomas.
Statistical analysis
Statistical analysis was performed with JMP 11
software (SAS Institute, Inc.). A t-test was performed for
independent variables. P≤0.05 was considered to indicate
statistically significant differences.
Results
Patient characteristics
The majority of the 380 patients were male (n=269;
70.8%). The mean age was 63.5 years, with a standard deviation of
10.9 years (range, 24-94 years). The majority of the patients were
diagnosed with laryngeal carcinoma (n=133; 35%), followed by 128
(33.7%) patients with oropharyngeal cancer. A total of 67 patients
(17.6%) had carcinoma of the oral cavity. The distribution of tumor
localization is shown in Table
I.
 | Table IDistribution of primary tumor
localization. |
Table I
Distribution of primary tumor
localization.
| Localization | Frequency | % |
|---|
| Larynx | 133 | 35 |
| Oropharynx | 128 | 33.7 |
| Oral cavity | 67 | 17.6 |
| Hypopharynx | 52 | 13.7 |
| Total | 380 | 100.0 |
The 7th edition of TNM classification was applied
for cancer staging (36). The most
common T stage was T2 (32.6%), followed by T1 (29.5%). The most
common nodal status was N0 (46.8%), followed by N2 (41.1%). The
majority of the patients had no distant metastases (M0; 91.8%). The
distribution of TNM stage of the primary tumors is shown in
Table II.
 | Table IIDistribution of TNM stage of primary
tumors within the cohort. |
Table II
Distribution of TNM stage of primary
tumors within the cohort.
| A, T stage | | |
|---|
| Stage | Frequency | % |
|---|
| Carcinoma in
situ | 7 | 1.8 |
| T1 | 112 | 29.5 |
| T2 | 124 | 32.6 |
| T3 | 76 | 20 |
| T4 | 61 | 16.1 |
| Total | 380 | 100.0 |
| B, N stage | | |
| Stage | Frequency | % |
| N0 | 178 | 46.8 |
| N1 | 32 | 8.4 |
| N2 | 156 | 41.1 |
| N3 | 10 | 2.6 |
| Nx | 4 | 1.1 |
| Total | 380 | 100.0 |
| C, M stage | | |
| Stage | Frequency | % |
| M0 | 349 | 91.8 |
| M1 | 12 | 3.2 |
| Mx | 19 | 5 |
| Total | 380 | 100.0 |
Localization of secondary
carcinomas
Between 2011 and 2015, a total of 39 patients
(10.3%) developed a secondary carcinoma. Secondary carcinomas
exhibited a similar distribution pattern to that of the primary
tumors. The most common localizations of the secondary malignancies
were the oropharynx (41%), larynx (25.6%) and oral cavity (20.6%).
The localizations of the secondary carcinomas diagnosed in the
patients of the present study are summarized in Table III.
 | Table IIIDistribution of secondary carcinoma
localization. |
Table III
Distribution of secondary carcinoma
localization.
| Localization | Frequency | % |
|---|
| Oropharynx | 16 | 41 |
| Larynx | 10 | 25.6 |
| Oral cavity | 8 | 20.6 |
| Hypopharynx | 5 | 12.8 |
| Total | 39 | 100.0 |
Synchronous and metachronous secondary
cancers
Secondary malignancies were differentiated into
synchronous and metachronous. The time of diagnosis of the
secondary carcinoma was used to distinguish between the two patient
cohorts. A total of 22 (5.8%) of the patients developed a
synchronous tumor (diagnosed within the first 6 months after the
primary tumor), whilst 17 patients (4.5%) developed a metachronous
tumor (occurring between 6 months and 5 years after the primary
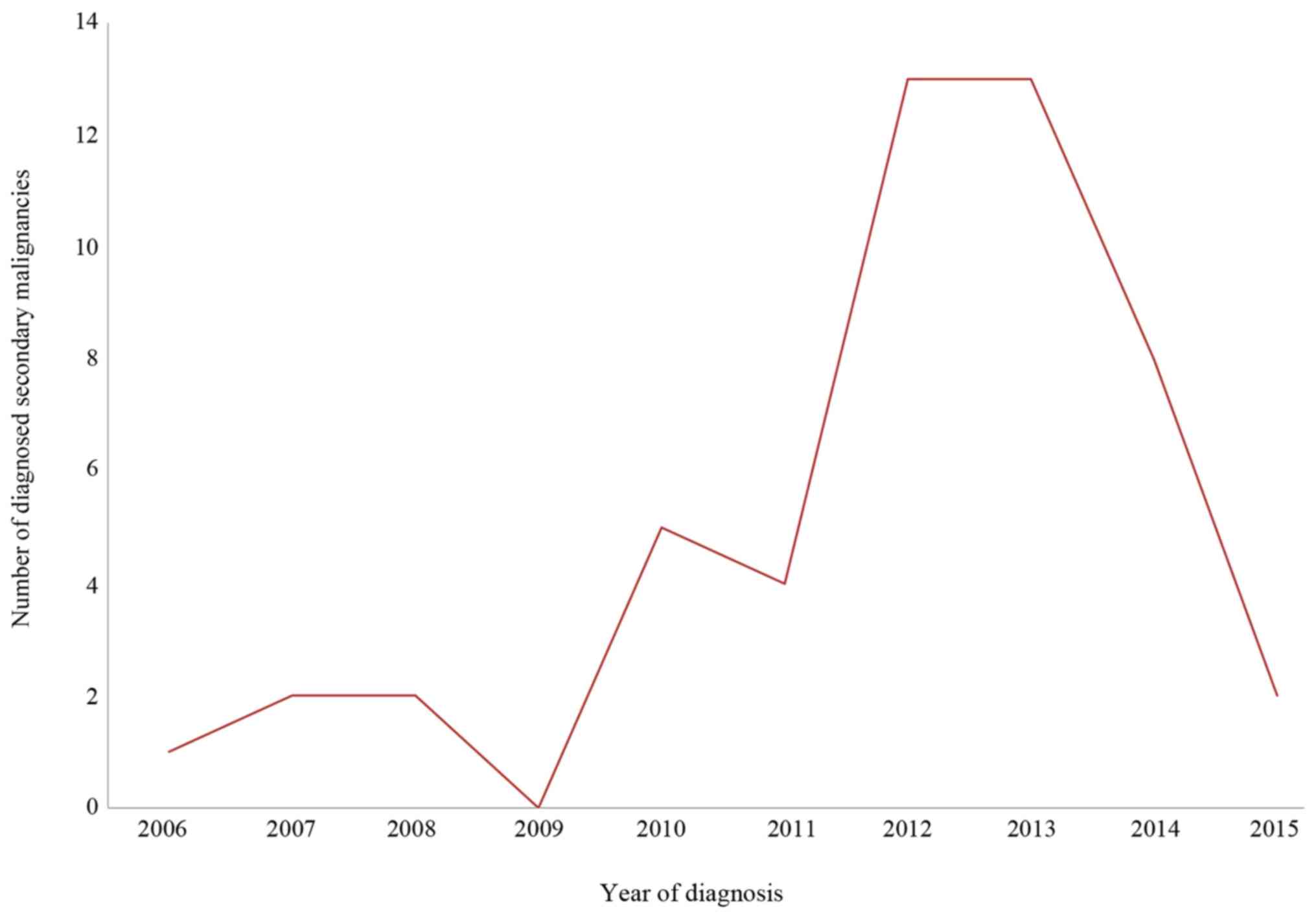
tumor). Figure 1 displays the number
of diagnosed secondary malignancies (synchronous and metachronous)
within the collective between 2006 and 2015. Whilst there was an
increase in diagnosed secondary malignancies from 2006 to 2012, the
number reached a steady state between 2012 and 2013 and decreased
between 2014 and 2015.
Associations between
clinicopathological characteristics and secondary cancer
development
A Chi-square test and a Fisher's exact test were
performed to analyze the associations between different primary
tumor localizations and the development of secondary carcinomas. A
P-value of 0.1 confirmed There was no significant association
between the localization of the primary tumor and the development
of a secondary carcinoma (P=0.1), although the majority of
secondary carcinomas affected patients with primary oropharyngeal
(n=16; 4.2% of all patients; 41% of all secondary carcinomas) and
laryngeal carcinomas (n=13; 3.4% of all patients; 25.6% of all
secondary carcinomas).
Whether patient sex was a relevant factor for the
occurrence of secondary carcinomas was investigated. No significant
correlation between sex and secondary malignancies was observed
(P=0.51), although more men (31; 8.2% of all patients) rather than
women (8; 2.1% of all patients) developed a secondary carcinoma.
The distribution of secondary malignancies amongst men and women is
summarized in Table IV.
 | Table IVDistribution of secondary carcinoma
amongst male and female patients. |
Table IV
Distribution of secondary carcinoma
amongst male and female patients.
| Secondary
carcinoma | Male | Female | Total |
|---|
| No, n (%) | 269 (70.8) | 72 (18.9) | 341 (89.7) |
| Yes, n (%) | 31 (8.2) | 8 (2.1) | 39 (10.3) |
| Total | 300 (78.9) | 80 (21.1) | 380 (100.0) |
The youngest patient in our study population with a
secondary carcinoma was 50 years old, while the oldest was 87 years
old. A t-test was performed in order to evaluate patient age as a
possible risk factor for the development of secondary carcinomas. A
P-value of 0.27 (95% CI: -1.07, 3.7) could not prove this
hypothesis, which suggests that the development of secondary
malignancies is not significantly associated with patient age.
A total of 42% of all secondary carcinomas had a T1
primary tumor and 26% had a T2 primary tumor (P=0.39). A total of
48% of the patients had a lymph node status of N0, and another 48%
had N2a, b or c disease (P=0.39). Of all secondary tumors, 98% had
no distant metastasis at the time of diagnosis (P=0.22).
Chi-squared tests proved that the TNM status of the primary tumors
did not represent a risk factor for the development of a secondary
carcinoma.
A total of 138 patients reported regular consumption
of carcinogenic substances, such as tobacco products, or alcoholic
beverages; a total of 17.4% (70 patients) reported tobacco use
alone, 5.1% (20 patients) reported alcohol use alone, and 12.2% (48
patients) reported use of both alcohol and tobacco. Two of the
patients (0.5%) reported other forms of drug consumption. No
information regarding the consumption of carcinogenic substances
was available for the remaining 242 patients. A Chi-square test was
performed to evaluate the association between consumption of
carcinogenic substances and the development of secondary
malignancies. Due to incomplete data regarding the substance abuse
profile of 242 patients, no reliable general conclusion can be
drawn from this statistical analysis. Although no significant
association could be confirmed (P=0.89), a larger cohort with
greater data availability should be investigated. A summary of the
aforementioned statistical results is presented in Table V.
 | Table VAssociation between the development
of secondary carcinoma and different cofactors. |
Table V
Association between the development
of secondary carcinoma and different cofactors.
| Cofactors | P-value |
|---|
| Primary tumor
location | 0.1 |
| Sex | 0.51 |
| Age | 0.27 |
| T stage of primary
tumor | 0.39 |
| N stage of primary
tumor | 0.39 |
| M stage of primary
tumor | 0.22 |
| Continued exposure
to carcinogenic substances | 0.89 |
Discussion
The present study was designed to investigate
indicators for secondary carcinomas in patients with head and neck
cancer. The data of 380 patients with head and neck cancer were
assessed to retrospectively analyze the incidence and frequency of
synchronous and metachronous secondary carcinomas.
Patients with head and neck cancer are prone to
develop multiple primary tumors (5,6). A
possible explanation for this phenomenon is the concept of field
cancerization, coined by Jaiswal et al in 1953(21). The reported incidence of secondary
malignancies ranges between 1.8 and 34.6% (8-19).
We were able to confirm our hypothesis that secondary head and neck
malignancies occur in <20% of the patients in the present study.
Of the 380 patients, 39 developed a secondary malignancy (10.3%).
Increasing numbers of secondary carcinomas were detected between
2006 (n=1) and 2012 (n=13). After 2012, the number of secondary
carcinomas stabilized and then declined again in 2014. During this
period, our hospital established a certified head and neck cancer
center, which may explain the growing number of diagnosed secondary
carcinomas due to structured follow-ups, followed by a decline as a
result of multi-disciplinary therapy. Furthermore, the
establishment of withdrawal programs may have had a positive impact
on the incidence of secondary malignancies (37-39).
The pattern of anatomical distribution for secondary carcinomas was
similar to that for primary tumors. The most common localization
was the oropharynx (32%) followed by the larynx (20%) and oral
cavity (16%). The most common localization of secondary
malignancies varies in the current literature, and includes the
lung, larynx and oropharynx (2,13,11).
However, different follow-up standards were used. In the present
study, all patients underwent a chest X-ray and a full endoscopic
evaluation. Wax et al compared a computed tomography (CT)
scan of the chest with simple X-ray and bronchoscopy. The CT scan
exhibited the highest sensitivity at 87% (40). Another study reported that
early-stage secondary malignancies can barely be detected on CT
scans, so this examination should only be used in advanced-stage
secondary carcinomas (stage III and IV) (41). Bronchoscopy is an invasive procedure,
yet it allows biopsies and is associated with no exposure to
radiation (14). In our cohort, only
a small number (n=2; 5%) of secondary carcinomas were successfully
diagnosed by bronchoscopy. According to current evidence,
metachronous carcinomas are found more often compared with
synchronous carcinomas (20).
Depending on the study, the incidence varies between 4.7 and 24%
for metachronous tumors, whereas synchronous secondary carcinomas
are reported in 0.3-14% of the patients (9,11,13,15,20).
In the present study, 6.2% of the cohort were found to have a
synchronous tumor and 4.1% were found to have a metachronous tumor.
The long interval of 6 months and 5 years leaves room for detection
bias. Not all patients comply to the full scale of the follow-up
program, whereas some patients relocate and cannot be evaluated to
the full extent. Although the cohort of the present study cannot
match a multicentered international cohort, the aim of this study
was to evaluate the occurrence of secondary carcinomas within the
confined environment of a single treatment center and, thus, help
predict numbers and occurrences within smaller cohorts. The present
study further aimed to evaluate the quality of data collected
during the establishment of a certified tumor center.
As regards demographic patient characteristics, the
mean age of the cohort was 64 years, and 78.9% of the patients were
men, which is consistent with other studies (8,42).
Malignancies in the head and neck area were more common among
patients aged 60-70 years and significantly more frequent in men
(n=31; 79.5% of all secondary carcinomas) rather than women (n=8;
20.5% of all secondary carcinomas). A t-test revealed that the
association between age and secondary malignancy development in our
study was not statistically significant (P=0.27, 95% CI: -1.08,
3.74). The sex predilection may be explained by the higher
consumption of noxious substances by men. Similar studies report
men to be more prone to the development of secondary malignancies
(11,20). The majority of patients in our cohort
were diagnosed with a primary T2 (31.98%) or T1 tumor (28.93%),
which confirms the findings reported by Ruback et al
(30). A similar distribution of
nodal and metastatic status was found in a cohort formed by the
University of Tokyo (9). Patients
are more likely to recognize lesions and abnormalities in the upper
airway at an early stage. However, non-specific symptoms, such as
hoarseness, which may be initially attributed to a common cold, may
delay primary diagnosis. Regardless, 60% of all laryngeal
malignancies are diagnosed at an earlier stage compared with
subglottic or supraglottic malignancies (28). Tumors of the oral cavity may also be
detected at an early stage. Of the 112 cases, 88.4% had a tumor
size of T1 or T2(12). A
retrospective study of 52 patients with secondary carcinomas in the
head and neck area conducted by Saito et al also found the
index tumor size to be T1 or T2 in 62% of the cases (9). However, TNM classification was not
found to be a significant indicator for the occurrence of secondary
carcinomas in the present study.
A secondary tumor most often developed among
patients with primary oropharyngeal carcinoma (16 cases; 4.2% of
all patients; 41% of all secondary carcinomas), although
oropharyngeal tumors were not the most common primary tumors.
Patients with laryngeal cancer ranked second regarding the
occurrence of secondary carcinomas (13 cases; 3.4% of all patients;
33% of secondary carcinomas). The lowest incidence of secondary
carcinomas was observed for primary oral cavity and hypopharyngeal
cancers (Table III). Dequanter
et al reported the larynx, hypopharynx and oropharynx (in
descending order) as the most common sites of secondary carcinoma
development (43); Patrucco et
al confirmed these results (11). In a large study with 58,363
carcinomas of the head and neck area, the majority of secondary
carcinomas were diagnosed in patients with a primary carcinoma of
the oral cavity (967 cases), larynx (353 cases), oropharynx (281
cases) and hypopharynx (76 cases) (8). Thus, there is an inconsistency in the
current literature regarding the association between the
localization of the primary tumor and secondary carcinomas
(44,45). We were unable to identify a
significant association between the localization of the primary
carcinoma and the development of secondary carcinomas in the
present study (P=0.09).
The combined abuse of tobacco and alcohol increases
the risk of SCC (30). A
retrospective study by Ruback et al reported consumption of
tobacco and alcohol by 1,351 patients with malignancies in the head
and neck area. A total of 747 patients (75.15%) reported regularly
consuming tobacco, 579 (58.25%) alcohol, and 547 (54%) both
(30). Another publication by Leon
et al categorized 88.9% as smokers and 87.7% as regular
alcohol consumers (n=4,298) (44).
In the present study, 142 of the 380 patients reported regular
alcohol and/or tobacco consumption. Tobacco use was more frequent
(18.27% of patients), whereas 48 patients (12.18%) reported regular
use of both alcohol and tobacco. In comparison to the available
evidence, the number of patients consuming noxious substances in
our data is low and was only obtained from 142 patients due to
insufficient patient history, or patients not admitting to
substance abuse. However, our results support the fact that
nicotine and alcohol have carcinogenic properties. A total of 18.3%
of patients without and 18% with secondary carcinomas were regular
smokers. Regular smokers and drinkers developed secondary
carcinomas more often compared with those who did not consume both
substances regularly. Our data were not sufficient to establish a
statistically significant effect of smoking and drinking on the
development of secondary carcinomas. A study from the Head and Neck
Center in St. Pau investigated 3,631 patients and confirmed that
continued smoking and/or alcohol consumption after primary therapy
significantly increased the risk for secondary tumors (P<0.001)
(45). It is a matter for discussion
whether invasive or mutilating procedures, such as laryngectomy,
may trigger changes in drinking or smoking habits, since the
majority of our patients with secondary carcinomas had T1 or T2
primary tumors. Eichler et al investigated 359 patients
after laryngectomy; 68.5% of the patients quit smoking completely,
10.6% continued smoking, 6.1% had not been smokers before, whereas
no data could be obtained for 14.8% of the cases. Regarding alcohol
consumption, 28.1% of the patients continued alcohol consumption,
whilst 70.5% tried to reduce consumption (42). Alcohol appears to have a higher
addictive factor than nicotine; however, a more aggressive surgical
approach does not appear to significantly affect the patient's
behavior for either noxious substance.
Infection with high-risk types of human
papillomavirus (HPV) is a well-known risk factor for the
development of multiple carcinomas (8-10,30).
In the present cohort, no data could be acquired regarding the HPV
status or cumulative dose of radiation for the primary tumors.
However, several studies suggest that infection with high-risk HPV
types 16 and 18 increases the risk of development of secondary
carcinomas. A study by Li et al from 2018 stated that
HPV-infected patients are generally immunosuppressed and, thus, may
be predisposed to the development of secondary cancers (46). However, a current meta-analysis by
Götz et al, investigating the diagnosis of HPV infection,
reported a widely varying number of infections with HPV amongst
cohorts, and concluded that previously published studies regarding
this subject should be read critically and may not represent a
basis for therapeutic decisions (47). Further immunosuppression in patients
with HNSCC occurs during chemoradiation (48). Al-Taei et al observed an
overall decreased T-cell response and accumulation of
immunosuppressive effects in oropharyngeal cancer patients
following chemoradiation (49). As
regards chemoradiation alone, a study by Berrington de Gonzalez
et al demonstrated a significantly increased risk of
developing secondary carcinomas in patients who received a
radiation dose of >5 Gy (50).
Since HNSCC patients regularly receive radiation with a cumulative
dose of 40-60 Gy, these findings should be investigated further.
Overall, the combination of virus- and treatment-induced
immunosuppression may increase the risk of multiple carcinomas
(51).
The primary limitation of this study was its
retrospective design. The data on the consumption and abuse of
noxious substances was particularly inconsistent and, thus, must be
interpreted with caution. Another issue was recall bias: Treatment
histories, medications and use of noxious substances, could not be
fully recalled by the patients and physicians. When comparing the
cohort of the present study to those of similar studies, it was
found to be a representative sample. However, increasing the cohort
size may help achieve statistically significant results in the case
of smaller differences between groups. Further studies should
collect data over a longer period of time, producing more
consistent and detailed information. In conclusion, the study
cohort is comparable to those of other studies, and appears to be a
representative sample of head and neck cancer patients. The
incidence of secondary carcinoma in this study was 10.3%. The
localization pattern of secondary carcinomas was similar to that of
primary tumor sites. The majority of secondary carcinomas were
found in the oropharynx (32%), followed by the larynx (20%) and the
oral cavity (16%). These results are in line with the concept of
field cancerization, suggesting that secondary carcinomas may
develop near the primary tumor site. However, the results of the
present study were not statistically significant. Synchronous
tumors occurred slightly more often compared with metachronous
tumors (6.2 vs. 4.1%, respectively). Age and sex did not
significantly affect the occurrence or development of secondary
carcinomas. Furthermore, no significant association was identified
between the TNM status of the primary cancer and that of the
secondary carcinoma. Regarding the consumption of noxious
substances, a clear trend was observed in that persistent
consumption of alcohol and tobacco after the primary diagnosis
promoted the development of secondary carcinomas. Yet, due to the
inconsistencies in the data collected from 252 patients in the
cohort, the statistical significance of this association was not
proven (P=0.89). Overall, avoiding the continued exposure to
noxious substances, as well as early diagnosis and consistent
treatment in a certified head and neck cancer center, appear to be
key factors for decreasing the incidence of secondary carcinomas.
Furthermore, the role of infection with high-risk HPV types, as
well as the impact of chemoradiation, should be investigated
further, as they appear to play a key role in the development of
secondary malignancies.
Acknowledgements
The authors would like to thank Mrs. Sylvia Büttner,
of the Institute for Medical Statistics, Biomathematics and
Information Processing in Mannheim, for creating the statistical
breakdown.
Funding
No funding was received.
Availability of data and materials
Data were obtained from the oncological database for
head and neck cancer at the ENT Tumor Center Mannheim.
Authors' contributions
VN analyzed and interpreted the data retrieved from
the oncological database and was a major contributor to literature
research. BKu interpreted the data and wrote the manuscript. BKr,
NR and CA were major contributors in correcting and proofreading
the final manuscript. CA and NR helped with acquiring the data.
Further, NR and CA have given final approval of the version to be
published. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee II of the Medical Faculty of Mannheim at the University
of Heidelberg, Mannheim, Germany (file no. 2016-827R-MA).
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
International Agency for Research on
Cancer (IARC): Cancer GLOBOCAN 2012: Cancer Tomorrow. http://globocan.iarc.fr/Pages/summary_table_pop_sel.aspx.
|
|
3
|
Larizadeh MH, Damghani MA and Shabani M:
Epidemiological characteristics of head and neck cancers in
southeast of iran. Iran J Cancer Prev. 7:80–86. 2014.PubMed/NCBI
|
|
4
|
Xu LL and Gu KS: Clinical retrospective
analysis of cases with multiple primary malignant neoplasms. Genet
Mol Res. 13:9271–9284. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jiao F, Yao LJ, Zhou J, Hu H and Wang LW:
Clinical features of multiple primary malignancies: A retrospective
analysis of 72 Chinese patients. Asian Pac J Cancer Prev.
15:331–334. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bagri PK, Singh D, Singhal MK, Singh G,
Mathur G, Jakhar SL, Beniwal S, Sharma N, Kumar HS, Sharma A and
Bardia MR: Double primary malignancies: A clinical and pathological
analysis report from a regional cancer institute in India. Iran J
Cancer Prev. 7:66–72. 2014.PubMed/NCBI
|
|
7
|
Donin N, Filson C, Drakaki A, Tan HJ
Castillo A, Kwan L, Litwin M and Chamie K: Risk of second primary
malignancies among cancer survivors in the United States, 1992
through 2008. Cancer. 122:3075–3086. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Birkeland AC, Rosko AJ, Chinn SB, Prince
ME, Sun GH and Spector ME: Prevalence and outcomes of head and neck
versus non-head and neck second primary malignancies in head and
neck squamous cell carcinoma: An analysis of the surveillance,
epidemiology, and end results database. ORL J Otorhinolaryngol
Relat Spec. 78:61–69. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Saito Y, Ebihara Y, Ushiku T, Omura G,
Kobayashi K, Ando M, Sakamoto T, Fukayama M, Yamasoba T and Asakage
T: Negative human papillomavirus status and excessive alcohol
consumption are significant risk factors for second primary
malignancies in Japanese patients with oropharyngeal carcinoma.
Japan J Clin Oncol. 44:564–569. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rennemo E, Zätterström U, Evensen J and
Boysen M: Reduced risk of head and neck second primary tumors after
radiotherapy. Radiother Oncol. 93:559–562. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Patrucco MS and Aramendi MV: Prognostic
impact of second primary tumors in head and neck cancer. Eur Arch
Otorhinolaryngol. 273:1871–1877. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Koo K, Harris R, Wiesenfeld D and Iseli
TA: A role for panendoscopy? Second primary tumour in early stage
squamous cell carcinoma of the oral tongue. J Laryngol Otol.
129(Suppl 1): S27–S31. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Di Martino E, Sellhaus B, Hausmann R,
Minkenberg R, Lohmann M and Esthofen MW: Survival in second primary
malignancies of patients with head and neck cancer. J Laryngol
Otol. 116:831–838. 2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kesting MR, Robitzky L, Al-Benna S,
Steinstraesser L, Baurecht H, Wolff KD, Hölzle F, Nieberler M,
Mücke T and Loeffelbein DJ: Bronchoscopy screening in primary oral
squamous cell carcinoma: A 10-year experience. Br J Oral Maxillofac
Surg. 47:279–283. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Schwartz LH, Ozsahin M, Zhang GN, Touboul
E, De Vataire F, Andolenko P, Lacau-Saint-Guily J, Laugier A and
Schlienger M: Synchronous and metachronous head and neck
carcinomas. Cancer. 74:1933–1938. 1994.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Friedrich RE: Primary and second primary
cancer in 649 patients with malignancies of the maxillofacial
region. Anticancer Res. 27:1805–1818. 2007.PubMed/NCBI
|
|
17
|
Panosetti E, Luboinski B, Mamelle G and
Richard JM: Multiple synchronous and metachronous cancers of the
upper aerodigestive tract: A nine-year study. Laryngoscope.
99:1267–1273. 1989.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Haughey BH, Gates GA, Arfken CL and Harvey
J: Meta-analysis of second malignant tumors in head and neck
cancer: The case for an endoscopic screening protocol. Ann Otol
Rhinol Laryngol. 101:105–112. 1992.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chuang SC, Scelo G, Tonita JM, Tamaro S,
Jonasson JG, Kliewer EV, Hemminki K, Weiderpass E, Pukkala E,
Tracey E, et al: Risk of second primary cancer among patients with
head and neck cancers: A pooled analysis of 13 cancer registries.
Int J Cancer. 123:2390–2396. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tiwana MS, Hay J, Wu J, Wong F, Cheung W
and Olson RA: Incidence of second metachronous head and neck
cancers: Population-based outcomes over 25 years. Laryngoscope.
124:2287–2291. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jaiswal G, Jaiswal S, Kumar R and Sharma
A: Field cancerization: Concept and clinical implications in head
and neck squamous cell carcinoma. J Exp Ther Oncol. 10:209–214.
2013.PubMed/NCBI
|
|
22
|
Angadi PV, Savitha JK, Rao SS and
Sivaranjini Y: Oral field cancerization: Current evidence and
future perspectives. Oral Maxillofac Surg. 16:171–180.
2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fortuna G and Mignogna MD: Oral field
cancerization. CMAJ. 183(1622)2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kreimer AR, Clifford GM, Boyle P and
Franceschi S: Human papillomavirus types in head and neck squamous
cell carcinomas worldwide: A systematic review. Cancer Epidemiol
Biomarkers Prev. 14:467–475. 2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Franceschi S, Muñoz N, Bosch XF, Snijders
PJ and Walboomers JM: Human papillomavirus and cancers of the upper
aerodigestive tract: A review of epidemiological and experimental
evidence. Cancer Epidemiol Biomarkers Prev. 5:567–575.
1996.PubMed/NCBI
|
|
26
|
Kaatsch P, Spix C, Katalinic A, Hentschel
S, Luttman S, Stegmaier C, Caspritz S, Christ M, Ernst A, Folkerts
J, et al: Cancer in Germany 2011/2012. Robert Koch-Institute (Hrsg)
and the society of the epidemiological Cancer-registry in Germany
eV (Hrsg) Berlin, 2015. 2015; 10th Issue. doi:
10.17886/rkipubl-2015-004.
|
|
27
|
Chi AC, Day TA and Neville BW: Oral cavity
and oropharyngeal squamous cell carcinoma-an update. CA Cancer J
Clin. 65:401–421. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Belcher R, Hayes K, Fedewa S and Chen AY:
Current treatment of head and neck squamous cell cancer. J Surg
Oncol. 110:551–574. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Steward BW and Wild CP (eds): World Cancer
Report 2014. International Agency for Research on Cancer, Lyon,
France. http://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-2014.
|
|
30
|
Ruback MJ, Galbiatti AL, Arantes LM,
Marucci GH, Russo A, Ruiz-Cintra MT, Raposo LS, Maniglia JV,
Pavarino ÉC and Goloni-Bertollo EM: Clinical and epidemiological
characteristics of patients in the head and neck surgery department
of a university hospital. Sao Paulo Med J. 130:307–313.
2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pezzuto F, Buonaguro L, Caponigro F, Ionna
F, Starita N, Annunziata C, Buonaguro FM and Tornesello ML: Update
on head and neck cancer: Current knowledge on epidemiology, risk
factors, molecular features and novel therapies. Oncology.
89:125–136. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jain V, Garg A, Parascandola M, Chaturvedi
P, Khariwala SS and Stepanov I: Analysis of alkaloids in areca
nut-containing products by liquid chromatography-tandem mass
spectrometry. J Agric Food Chem. 65:1977–1983. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Merchant A, Husain SS, Hosain M, Fikree
FF, Pitiphat W, Siddiqui AR, Hayder SJ, Haider SM, Ikram M, Chuang
SK and Saeed SA: Paan without tobacco: An independent risk factor
for oral cancer. Int J Cancer. 86:128–131. 2000.PubMed/NCBI View Article : Google Scholar
|
|
34
|
World Health Organization: World Cancer
Report 2003. Stewart BW and Kleihues P (eds). IARC Press, Lyon,
2003.
|
|
35
|
Adel M, Liao CT, Lee LY, Hsueh C, Lin CY,
Fan KH, Wang HM, Ng SH, Lin CH, Tsao CK, et al: Incidence and
outcomes of patients with oral cavity squamous cell carcinoma and
fourth primary tumors: A long-term follow-up study in a betel quid
chewing endemic area. Medicine (Baltimore).
95(e2950)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474.
2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
McCarter K, Martínez Ú, Britton B, Baker
A, Bonevski B, Carter G, Beck A, Wratten C, Guillaumier A, Halpin
SA and Wolfenden L: Smoking cessation care among patients with head
and neck cancer: A systematic review. BMJ Open.
6(e012296)2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Dresler CM, León ME, Straif K, Baan R and
Secretan B: Reversal of risk upon quitting smoking. Lancet.
368:348–349. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Marron M, Boffetta P, Zhang ZF, Zaridze D,
Wunsch-Filho V, Winn DM, Wei Q, Talamini R, Szeszenia-Dabrowska N,
Sturgis EM, et al: Cessation of alcohol drinking, tobacco smoking
and the reversal of head and neck cancer risk. Int J Epidemiol.
39:182–196. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wax MK, Myers LL, Gabalski EC, Husain S,
Gona JM and Nabi H: Positron emission tomography in the evaluation
of synchronous lung lesions in patients with untreated head and
neck cancer. Arch Otolaryngol Head Neck Surg. 128:703–707.
2002.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Glynn F, Brennan S and O'Leary G: CT
staging and surveillance of the thorax in patients with newly
diagnosed and recurrent squamous cell carcinoma of the head and
neck: Is it necessary? Eur Arch Otorhinolaryngol. 263:943–945.
2006.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Eichler M, Keszte J, Meyer A, Danker H,
Guntinas-Lichius O, Oeken J, Pabst F and Singer S: Tobacco and
alcohol consumption after total laryngectomy and survival: A German
multicenter prospective cohort study. Head Neck. 38:1324–1329.
2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Dequanter D, Shahla M, Lardinois I,
Gilbert O, Hanquet O, Tragas G, Van Meerhæghe A and Lothaire P:
Second primary lung malignancy in head and neck cancer patients.
Eur Ann Otorhinolaryngol Head Neck Dis. 128:11–13. 2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
León X, Martínez V, López M, García J,
Venegas Mdel P, Esteller E and Quer M: Second, third, and fourth
head and neck tumors. A progressive decrease in survival. Head
Neck. 34:1716–1719. 2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
León X, Martínez V, López M, Lorenzo JG
and Quer M: Risk of third and fourth tumors in patients with head
and neck cancer. Head Neck. 32:1467–1472. 2010.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Li D, Yegya-Raman N, Kim S, Ganesan S,
Sayan M, August D, Spencer K, Hathout L, Maloney-Patel N, Malhotra
U, et al: Multiple primary malignancies in patients with anal
squamous cell carcinoma. J Gastrointest Oncol. 9:853–857.
2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Götz C, Bischof C, Wolff KD and Kolk A:
Detection of HPV infection in head and neck cancers: Promise and
pitfalls in the last ten years: A meta-analysis. Mol Clin Oncol.
10:17–28. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Gao P, Gong L and Wang X: Induction
chemotherapy in patients with resectable laryngeal cancer: A
meta-analysis. Mol Clin Oncol. 9:155–162. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Al-Taei S, Banner R, Powell N, Evans M,
Palaniappan N, Tabi Z and Man S: Decreased HPV-specific T cell
responses and accumulation of immunosuppressive influences in
oropharyngeal cancer patients following radical therapy. Cancer
Immunol Immunother. 62:1821–1830. 2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Berrington de Gonzalez A, Curtis RE, Kry
SF, Gilbert E, Lamart S, Berg CD, Stovall M and Ron E: Proportion
of second cancers attributable to radiotherapy treatment in adults:
A cohort study in the US SEER cancer registries. Lancet Oncol.
12:353–360. 2011.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Morales-Sánchez A and Fuentes-Pananá EM:
Human viruses and cancer. Viruses. 6:4047–4079. 2014.PubMed/NCBI View Article : Google Scholar
|