Introduction
Keratins comprise a large group of structural
proteins of the intermediate filament protein family and are found
in all epithelial cells. Expression of individual keratins is cell
and tissue specific and reflects type of epithelium as well as
state of differentiation of the epithelial cells. Keratins possess
viscoelastic properties and thus provide cells with mechanical
stability and help to maintain cellular integrity. At the same time
keratins act in intracellular signalling pathways during wound
healing and stress responses (1-3).
Based on gene structure, cysteine/proline content of
the carboxy-terminal region and specific expression patterns,
keratins can be classified as ‘soft’ cytokeratins expressed in
various types of epithelia or ‘hair’ (trychocytic) keratins that
are components of epithelial appendages. The biochemical properties
of both groups classify keratins as type I (acidic) or type II
(basic to neutral isoelectric point). All keratins share a
tripartite domain structure with a central self-assembling
α-helical rod domain that allows them to form heterodimeric
complexes containing a type I and II keratin in equimolar amounts.
These subunits form the basis of the intermediate filament network.
Specific keratin pairs tend to be co-expressed and serve as markers
of epithelial differentiation (4).
Mammalian hair keratins are involved in the
formation of hard keratinizing structures such as the hair-forming
compartment of the hair follicle and distinct nail compartments.
Although their prominent expression is in hair, dorsal tongue
epithelium as well as other soft human epithelia also express hair
type keratins (4-8).
Human hair keratins comprise nine type I members (K31, K32, K33a,
K33b, K34, K35, K36, K37 and K38) and six type II members (K81,
K82, K83, K84, K85 and K86). In situ hybridization and
immunohistochemical studies have shown each member to have a
specific location within the hair follicle or tongue epithelium
corresponding to the stage of cellular differentiation (2,9,10).
Human keratin 36 [K36, previously termed Ha6 and
Ka31(2)] is classified as a type I
hair keratin, which presumably partners with an unknown type II
keratin. K36 has been described as a marker of advanced stage of
differentiation in upper hair cortex, although it does not
represent the major hair keratin (9). The human KRT36 gene that encodes
K36 is located within a type-specific cluster on chromosome
17q12-q21 and belongs to the structurally unrelated group C of
acidic hair keratins (9).
The group of oral squamous cell carcinoma (OSCC)
exerts the eighth most common cancer in the world, with a 5-year
survival <60% (11). Within this
group tumours are most commonly located in the tongue (SCCOT), and
differences between these SCCOTs and SCCs in other intra-oral sites
have been shown (12,13). Worldwide there is an increasing
incidence of SCCOT, particularly among young people (<45 years)
and in many parts of the World especially women (11).
In a patient with OSCC not only the tumour but also
the surrounding tissue shows neoplastic changes, a phenomenon
called field cancerization (14).
Genetically changed fields can in cases of OSCC be found within a
distance of 7 cm from the tumour (14), which in the case of SCCOT means that
the whole tongue can be affected.
Looking at the adjacent clinically tumour-free
tongue tissue in patients with SCCOT we have identified 554 genes
to be dysregulated compared to healthy tongue (15). Among these KRT36 was shown to
be tongue-specific. KRT36 mRNA levels were significantly
lower in clinically normal tissue adjacent to SCCOT tumours and
KRT36 was one of the most progressively downregulated genes
in SCCOT tumours. High KRT36 levels were observed in normal
tongue from healthy volunteers and the intermediate levels in
clinically normal tongue from SCCOT patients (15). Therefore, K36 was speculated to
represent a potential marker of early neoplastic changes in the
tongue and a representative indicator of field cancerization
effects in OSCCs, where molecular changes can be seen in cells
distant from the malignancy (16-18).
In the present study we aimed at mapping the
expression pattern of K36 in a panel of human tissues with
particular focus on normal and malignant SCCOT tissue from the
mobile tongue. For this study, a specific K36 antibody was
produced, affinity purified and used for immunohistochemical
studies.
Materials and methods
Antibody production and
purification
Two peptide sequences were chosen for antibody
production; TPTFSTGSIKGLC from the N-terminus and CKPVIRVPSVPPV
from the C-terminal region of human K36. These peptides were chosen
based on their lack of sequence similarity with other human
keratins using BLASTP search of the human proteome. The peptides
were synthesised and individually coupled to keyhole limpet
hemocyanin using their terminal cysteine residues, and antibodies
were prepared in two rabbits using an equimolar mixture of the two
peptide conjugates in each rabbit. Sera were collected after four
immunisations and affinity purified against the individual
peptides, to provide four separate affinity-purified rabbit K36
antibodies. Peptide synthesis, coupling, immunisation and affinity
purification was performed commercially (Moravian
Biotechnology).
Tissue specimens
A panel of morphologically normal tissue samples was
collected for analysis of K36 expression. This panel comprised
samples from different areas of the mobile tongue, excluding the
base of the tongue, skin from different anatomical locations, lip,
mamilla, nail, thymus, vaginal epithelium, cervix, appendix and
oesophagus. The tumour tissue panel included skin cancers such as
melanoma (1 case) and basal cell carcinoma (1 case), SCCOT (14
cases) and cervical squamous cancers (12 cases) (Table III). SCCOT samples were derived
from the archive at Clinical Pathology between 2012 and 2017 from
patients with primary SCCOT and sufficient material for
immunohistochemical analysis. The total number of sections tested
was 59 and out of these 28 were cancer samples. All tested samples
of normal and cancer tissue were obtained from patients undergoing
treatment in Umea and Brno. The present study was approved by the
Ethical review boards in Umea and Brno (Dnr 08-003M; 03-201).
Clinical information about the samples included are summarised in
Tables I and II.
 | Table IIIOverview of K36 antibody stained
tissues. |
Table III
Overview of K36 antibody stained
tissues.
| Tissue | No. of tested
cases | Staining
pattern | Localisation |
|---|
| Normal tissue | | | |
|
Appendix | 1 | Negative | |
|
Cervix | 3 | Negative | |
|
Hair, cross
section | 1 | Negative | |
|
Hair,
longitudinal section | 2 | Positive | Cortex |
|
Lip | 2 | Negative | |
|
Mammilla | 2 | Negative | |
|
Nail | 2 | Positive | Nail bed |
|
Oesophagus | 1 | Negative | |
|
Palmar
skin | 2 | Negative | |
|
Scalp
skin | 1 | Negative | |
|
Skin other
location | 5 | Negative | |
|
Thymus | 2 | Positive | Hassal's
corpuscles |
|
Tongue | 6 | Positive | Dorsal
epithelium/filiform papillae |
|
Vaginal
epithelium | 1 | Negative | |
| Cancer tissue | | | |
|
Basal cell
carcinoma | 1 | Negative | |
|
Cervical
squamous cancer | 12 | Negative | |
|
Melanoma | 1 | Negative | |
|
Tongue
cancer | 14 | Negative | |
 | Table ISCCOT clinical information. |
Table I
SCCOT clinical information.
| Sex | Age (years) | TNM |
|---|
| F | 54 | T1N0M0 |
| M | 57 | T2N0M0 |
| F | 74 | T1N0M0 |
| M | 64 | T1N0M0 |
| F | 87 | T3N2cM0 |
| F | 74 | T2N0M0 |
| M | 67 | T2N0M0 |
| M | 55 | T4aN2bM0 |
| F | 74 | T2N0M0 |
| F | 71 | T2N0M0 |
| M | 51 | T2N1M0 |
| F | 71 | T1N0M0 |
| F | 42 | T1N1M0 |
| M | 52 | T4aN2bM0 |
 | Table IIClinical information of the additional
samples studied. |
Table II
Clinical information of the additional
samples studied.
| Tissue | Sex | Mean age (years) | No. of cases |
|---|
| Appendix | F | 36 | 1 |
| Cervix | F | 50 | 3 |
| Hair, cross
section | M | 10 | 1 |
| Hair, longitudinal
section | F | 33 | 2 |
| Lip | F | 46 | 2 |
| Mammilla | F | 55 | 2 |
| Nail | F/M | 63/33 | 2 |
| Oesophagus | F | 55 | 1 |
| Palmar skin | F | 82 | 2 |
| Skin of the
scalp | M | 64 | 1 |
| Skin | 3F, 2M | 49/74 | 5 |
| Thymus | F/M | 35/23 | 2 |
| Tongue | F/M | 24/52 | 6 |
| Vaginal
epithelium | F | 45 | 1 |
| Basalioma | M | 42 | 1 |
| Cervical
cancer | F | 47 | 12 |
| Melanoma | F | 73 | 1 |
Immunohistochemistry
Immunohistochemical staining was performed on 4 µm
thick tissue sections and the optimal antibody concentration and
retrieval were set. The procedure included section
deparaffinization in xylene and rehydration into PBS through a
graded series of ethanol. Endogenous peroxidase activity was
quenched in 3% hydrogen peroxide in PBS for 5 min. Antigen
retrieval was performed by boiling in 1 mM EDTA pH 8.0 for 20 min.
Afterwards the sections were incubated overnight at 4̊C with
affinity purified rabbit serum CKP 39 fraction 1.4. HRP polymer
conjugated anti-rabbit (K4003 Agilent Technologies, Inc.) was used
according to the manufacturer's instructions (Envision + System;
Agilent Technologies, Inc.). Signal was visualized by
3,3'-diaminobenzidine (Liquid DAB + substrate chromogen system;
Agilent Technologies, Inc.). Nuclear counterstaining was performed
with Gill's haematoxylin. Slides were graded as positive or
negative.
Illumina HT-12 bead chip array
data
The gene expression array data come from our
previous study of SCCOT samples using Illumina HT-12 bead chip
array containing 47,231 probes (15)
that was enlarged by new samples. Here we retrieved and analysed
mRNA levels of hair-keratins in data from 14 cases of normal
control tongue from healthy volunteers, 22 SCCOT samples and 22
corresponding samples of tumour-free area of the tongue. Raw data
has been deposited at http://www.ebi.ac.uk/arrayexpress, Array Express
accession numbers E-MTAB-4678(14)
(Cases; all controls, tumour/tumour-free 11, 35, 51, 56, 58, 59,
61, 65, 73, 79, 85) and E-MTAB-5534 (Cases 20, 35, 49, 76, 98, 105,
111, 119, 124, 131, 137, 138).
Statistical analysis
Statistical analysis was performed on the previously
published array data (14) using IBM
SPSS 25 (IBM Corp.). Differences at P≤0.05 were considered to be
statistically significant. Changes in the level of expression of
each KRT gene within group of samples (control, tumour-free,
tumour) was analysed using ANOVA with post hoc Tukey HSD test. Data
are analysed as the mean ± standard deviation.
Expression analysis
Microarray data were analysed using application
Multiple Experiment Viewer (MeV) (19).
Results
Production of K36 antibodies
A mixture of two peptides from the human K36 protein
(conjugated to keyhole-limpet hemocyanin) was used to immunise
rabbits for antibody production. Sera were then used in dot-blots
against the individual peptides (conjugated to bovine serum
albumin) to assess antibody production. In both rabbits, a stronger
response was seen against the CKPVIRVPSVPPV peptide (C-terminal
region) than against the N-terminal peptide (Fig. S1). Sera were then individually
affinity-purified against each peptide and fractions were assessed
for immunoglobulin purity and amount by polyacrylamide gel
electrophoresis, again showing higher levels of antibodies to the
C-terminal region peptide in both rabbits (Fig. S2). These initial assessments were
performed and provided by the supplier.
Affinity-purified antibodies and non-affinity
purified sera were then tested at various concentrations on normal
human tongue samples, employing antigen retrieval (EDTA or citrate)
or no antigen retrieval. Pre-immune sera from the two rabbits were
used as controls for their respective non-purified sera and
affinity-purified reagents. From this initial screening, serum from
rabbit 39 affinity-purified against the CKPVIRVPSVPPV peptide was
chosen for further analysis, using antigen retrieval in 1 mM EDTA
boiling for 20 min. Serum from rabbit 33 purified against the same
peptide showed similar staining characteristics but required a
lower dilution, whilst both sera purified against the TPTFSTGSIKGLC
peptide showed similar staining but required high concentrations
with attendant background staining. The non-immune sera did not
show any staining when used at similar or higher
concentrations.
Keratin 36 expression in normal tongue
and SCCOT
Having established optimal conditions, we analysed
K36 in the set of selected tissues using immunohistochemistry with
affinity purified serum (designated 39-CKP). In view of our finding
of altered KRT36 mRNA levels in SCCOT, we focused on
staining K36 in normal and malignant human tongue epithelium.
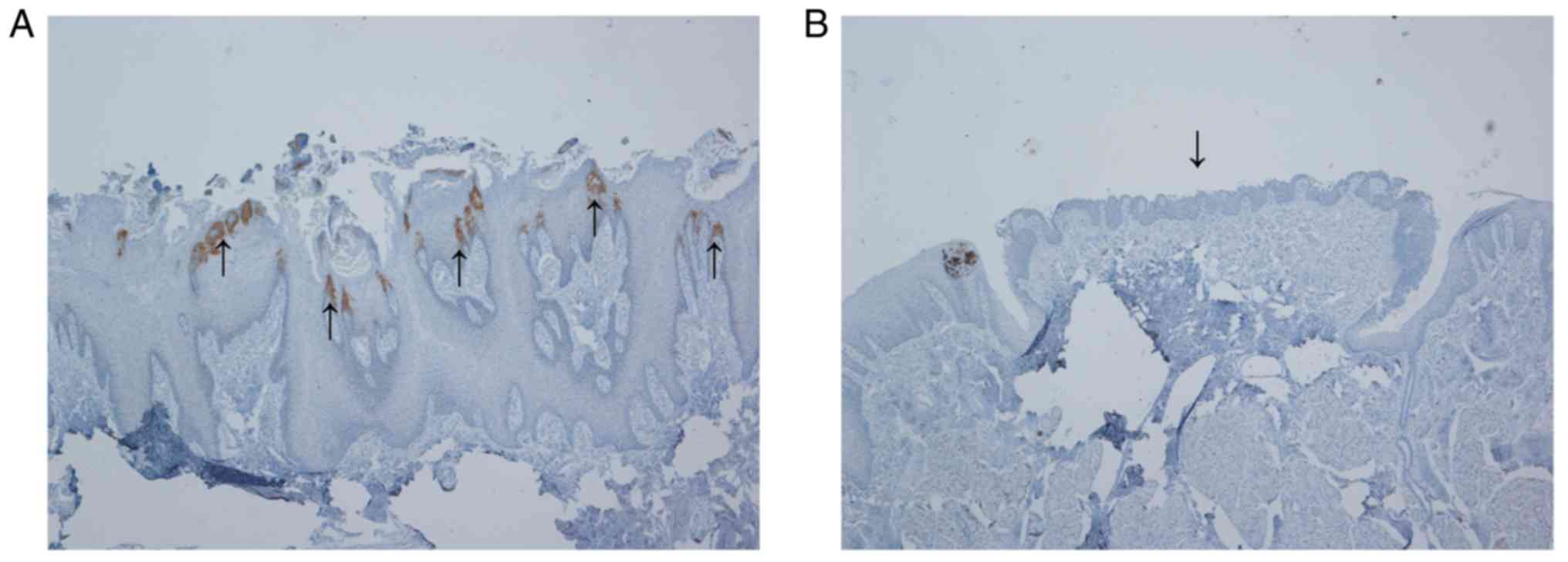
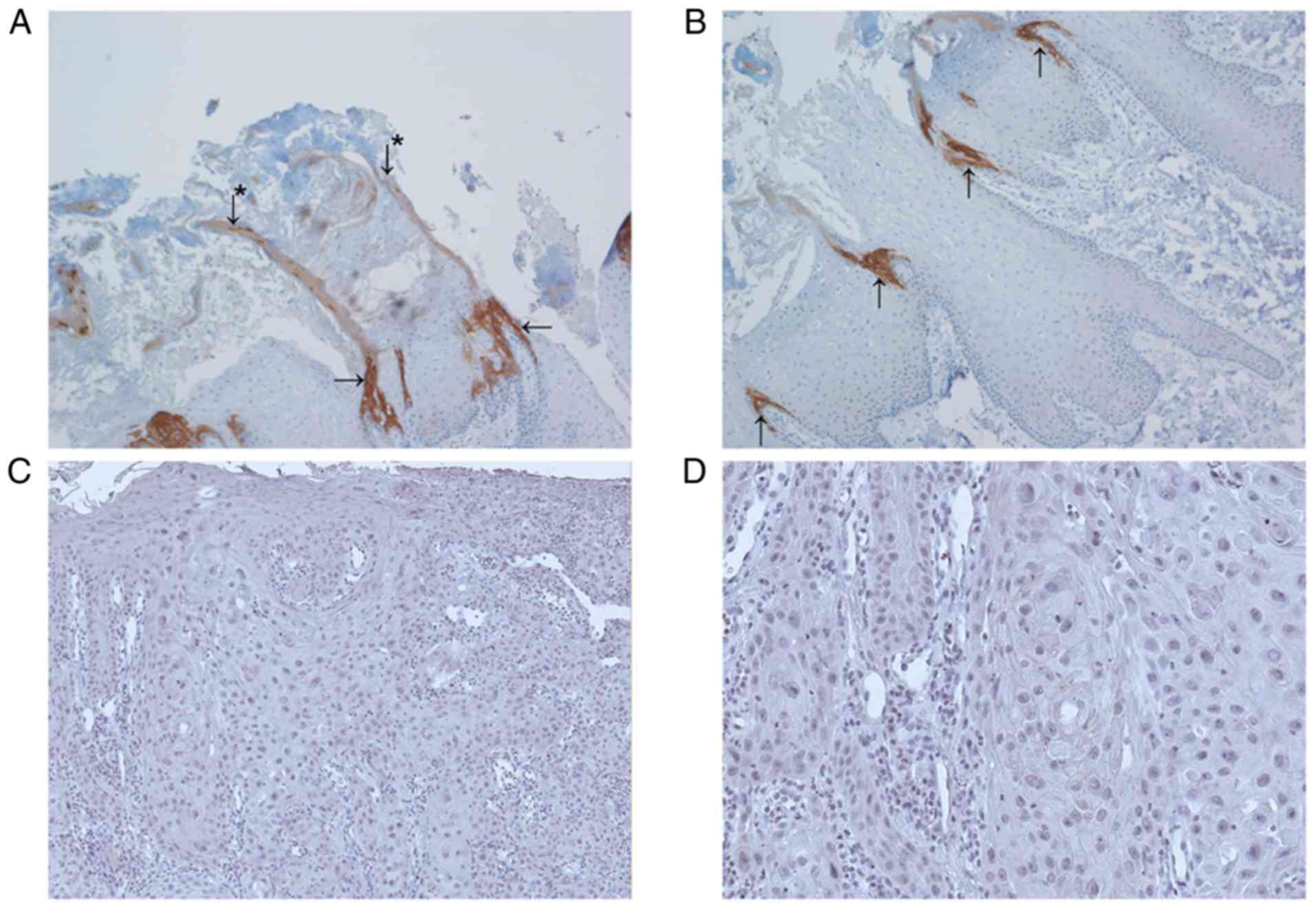
No scoring of percentage K36 expressing cells or
intensity in expression was performed. Tongue epithelium is a
morphologically variable tissue with diverse structures. K36
immunostaining was localised solely to filiform papillae and not
detected in other structures (Fig.
1). Within the filiform papilla, K36 was present in the
cytoplasm of epithelial cells organised in arcs at the periphery.
These cell clusters with elongated shape are called secondary
filiform papillae and give rise to cornified spines (6). Other compartments of filiform papillae
such as primary structure and interpapillary epithelium were
K36-negative (Fig. 2).
We also analysed the distribution of K36-positive
filiform papillae on the dorsal front tongue epithelium and found
that all filiform papillae recognized in the anterior two-thirds of
the mucosa were K36-positive.
To see whether K36 is dysregulated in cancer tissue,
we also included samples of SCCOT, but K36 was not detected in any
of these samples (Table III and
Fig. 2).
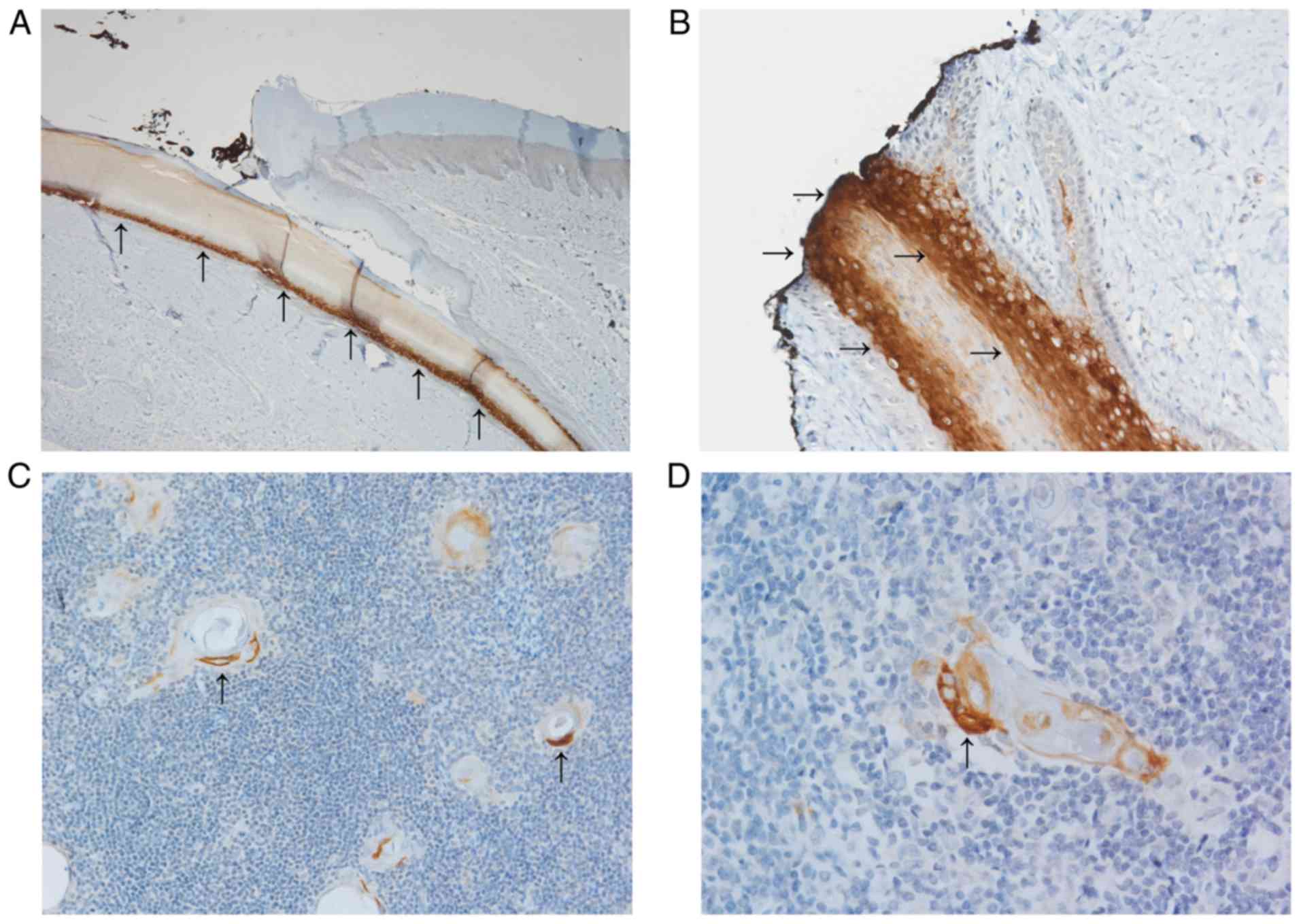
Keratin 36 in various normal
epithelial tissues and corresponding cancer tissue
We also used a panel of normal epithelial tissues
chosen according to RNA sequencing data available in the Human
protein atlas open access database (19). Based on these data and the
literature, we compiled a set of human normal and cancer tissues
that express KRT36 mRNA but that were not yet evaluated at
protein level. The panel of normal tissue samples included several
positive cases, namely nail bed, Hassal's corpuscles of thymus and
hair cortex (Fig. 3). Squamous
epithelia from different anatomical locations, lip, mammilla,
cervix, oesophagus and vagina were K36-negative and non-squamous
epithelia in these tissues and appendix were also negative. Tumour
samples of melanoma, basal cell carcinoma and cervical carcinoma
were also negative for K36. An overview of staining results is
provided in Table III.
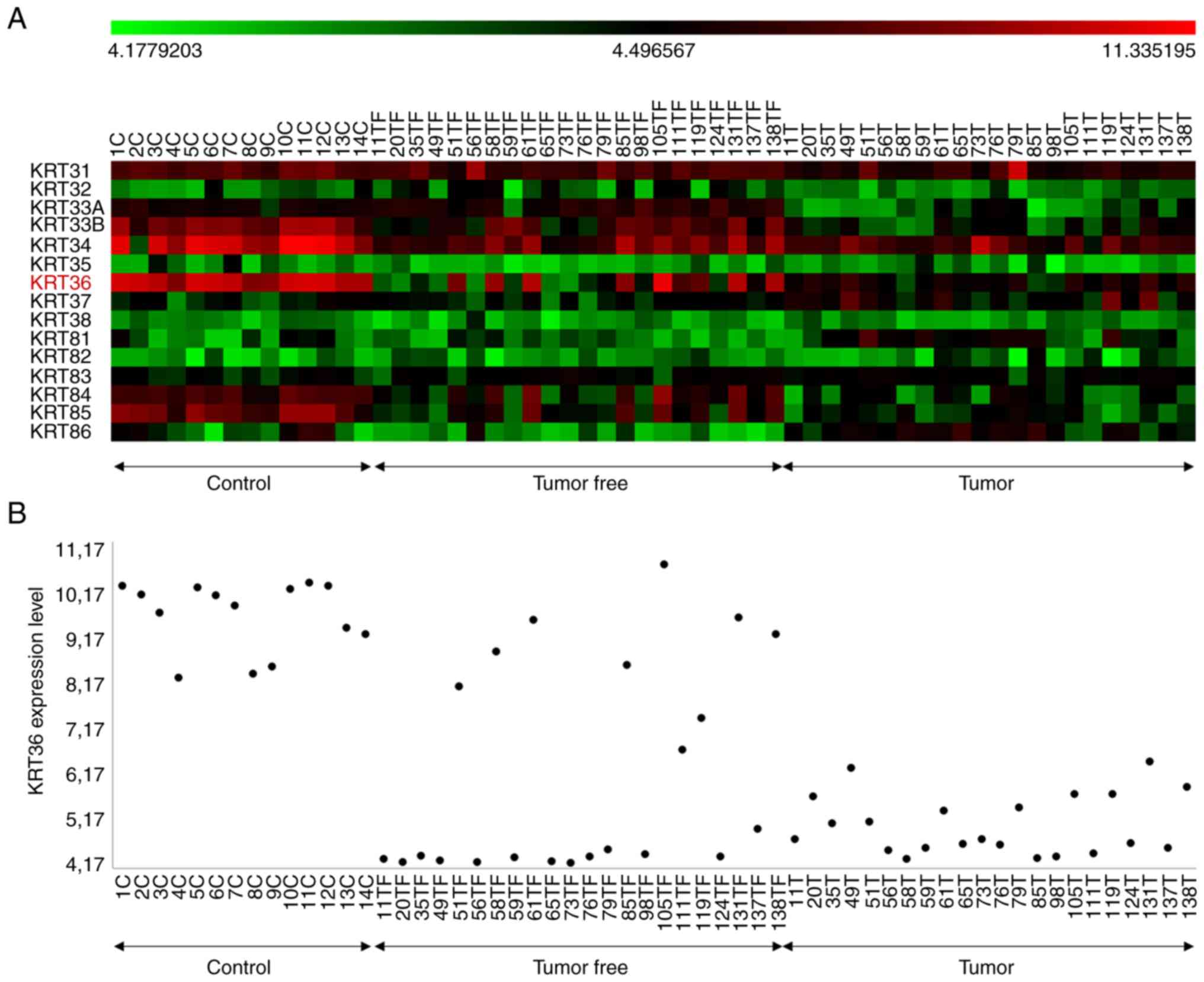
Hair keratin RNA expression in tongue
tumour, tumour-free and control samples
We analysed mRNA data from tongue tumours,
tumour-free and healthy controls (14) concerning levels of individual hair
type keratins (KRT31-KRT38 and KRT81-KRT86) in each
group of samples (Fig. 4).
Statistical analysis revealed significant downregulation of
KRT31 (P=0.003), KRT33A (P=0.000), KRT33B
(P=0.000), KRT34 (P=0.000), KRT36 (P=0.000),
KRT84 (P=0.000) and KRT85 (P=0.000) in tumour samples
compared to healthy controls. Of these, KRT84 mRNA showed
the highest correlation with KRT36 mRNA levels. Levels of
KRT37 (P=0.000) and KRT81 (P=0.000) in contrast were
significantly upregulated in cancer (ANOVA/Tukey HSD test)
(Table SI and Fig. S3).
Discussion
Hair type keratins are expressed in hard keratinized
structures such as hair and nails. Surprisingly, using a
pan-specific antibody against hair keratins, it has been recognized
that some hair keratins also occur in soft tissues such as mouse
and human tongue (4). Specifically
for K36, there is no record on protein expression up to now and
mRNA data suggest it to be present in skin, thymus, oesophagus,
spleen, vagina, testis (20), tongue
epithelium and hair cortex (4). Here
we investigated expression of the K36 protein using newly developed
antibodies and found expression in thymus, tongue and hair only. In
addition, we found K36 in nail beds. Most of these K36 expressing
tissues are located in mechanically stressed regions of the human
body. It has been stated that keratin filaments generally function
as a major component of the epithelial cytoskeleton important for
mechanical integrity of tissues (1)
and thus we suggest that K36 may help to protect against mechanical
stress. This is particularly in keeping with K36 as a component of
filiform papillae which serve to provide a roughened surface of the
dorsal tongue and also are important for providing traction during
mastication. These structures therefore undergo extensive
mechanical stress and must be firm as well as flexible.
Previously it has been described that Hassal's
corpuscles of thymus display a very complex pattern of cytokeratins
(CKs) with specific localisation within the structure. Hassal's
corpuscles originate in medullary epithelial cells and resemble
immature keratinocytes (21). Here
K36 was mostly located in the intermediate to outer layers of
cellular swirls, where CKs of simple epithelia such as CK7, 8, 18,
10, and CK4 and 17 from transitional epithelium are known to be
located (21).
Our original data on mRNA expression profiling,
showed that KRT36 mRNA was absent or levels very low in
SCCOT, high in normal healthy tongue and intermediate in
tumour-adjacent tongue. Here we focused on protein levels of K36 to
see if this protein could be used as a diagnostic and/or prognostic
marker in SCCOT. In accordance with our RNA data, we confirmed lack
of K36 expression in all tumour samples. Even if the group of
SCCOTs analysed, 14 cases, is limited, it is evident that
KRT36 is downregulated in all tumours, whereas normal tongue
tissue contains K36-positive cells. From the other tissue types
selected from available RNA data, but not yet analysed at protein
level, we also saw lack of K36 in both normal and tumourous
cervical tissue. Also the single cases of basal cell carcinoma and
melanoma included were devoid of K36.
It is unclear whether K36 downregulation is
associated primarily with the tumour originating from a
K36-negative population, or whether it comes with tumour
progression or tumour dedifferentiation. As mechano-stimulation is
rather unexplored in tumour biology, it can only be speculated that
K36 downregulation in SCCOT can contribute to tumour progression by
changing the mechanical phenotype of cancer cells.
In conclusion, using a novel affinity-purified
polyclonal antibody we have identified K36 in several normal human
tissues including filiform papillae of dorsal tongue epithelium,
nail bed and Hassal's corpuscles, whereas other stratifying and
non-stratifying epithelia were negative. In contrast, epithelial
tumours like SCCOT and other squamous cell cancers were
consistently negative for K36.
Supplementary Material
Dot blot tests of sera against
individual peptides. (A) A weak response was exhibited against the
TPTFSTGSIKGLC peptide, while (B) a strong response was visible
against the CKPVIRVPSVPPV peptide. Serum dilutions ranged from
1:100 to 1:1,600.
Affinity-purified sera test. Coomassie
staining of affinity purified IgG revealed (A) lower levels of
antibodies targeting the TPTFSTGSIKGLC peptide compared with the
(B) CKPVIRVPSVPPV peptide in rabbits 33 and 39.
Expression analysis. The chart
represents the differences in mean expression values between the
control, tumour-free and tumour groups for each KRT gene. Analysis
was made on previously published microarray data derived from 8
SCCOTs, 10 matched SCCOT/clinically tumour-free tongue samples, two
clinically tumour-free tongue samples in SCCOT patients and 14
samples from healthy normal tongues (14). KRT, keratin; C, control;
TF, tumor.free; T, tumor; SCCOT, squamous cell carcinoma of the
oral tongue.
Hair keratin RNA expression in tongue
tumour, tumour free and control samples.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Cancer
Research Foundation in Northern Sweden, the Swedish Cancer Society
Contract number 18 05 42, Västerbotten County Council, Umeå
University, European Regional Development Fund: Project ENOCH
(grant no. CZ.02.1.01/0.0/0.0/16_019/0000868) and Ministry of
Health in the Czech Republic (grant no. MMCI 00209805).
Availability of data and materials
The datasets used and/or analysed are available from
the corresponding author on reasonable request.
Authors' contributions
PJC, BV, VB and KN designed the experiments,
analysed the results and wrote the manuscript. VB, VH, LB, PF, NS
performed the experiments, analysed the results and wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the local Ethical
Review Boards of Umea and Brno (approval nos. Dnr 08-003M and
03-201).
Patient consent for publication
Not applicable.
Competing interests
BV is associated with Moravian Biotechnology, the
company that produced and supplied the K36 polyclonal antibody. The
company did not provide financial support for the present study and
had no influence on the design, execution or analysis of the
experiments. The other authors declare that they have no competing
interests.
References
|
1
|
Ramms L, Fabris G, Windoffer R, Schwarz N,
Springer R, Zhou C, Lazar J, Stiefel S, Hersch N, Schnakenberg U,
et al: Keratins as the main component for the mechanical integrity
of keratinocytes. Proc Natl Acad Sci USA. 110:18513–18518.
2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Schweizer J, Bowden PE, Coulombe PA,
Langbein L, Lane EB, Magin TM, Maltais L, Omary MB, Parry DA,
Rogers MA and Wright MW: New consensus nomenclature for mammalian
keratins. J Cell Biol. 174:169–174. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Toivola DM, Strnad P, Habtezion A and
Omary MB: Intermediate filaments take the heat as stress proteins.
Trends Cell Biol. 20:79–91. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dhouailly D, Xu C, Manabe M, Schermer A
and Sun TT: Expression of hair-related keratins in a soft
epithelium: Subpopulations of human and mouse dorsal tongue
keratinocytes express keratin markers for hair-, skin- and
esophageal-types of differentiation. Exp Cell Res. 181:141–158.
1989.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Heid HW, Moll I and Franke WW: Patterns of
expression of trichocytic and epithelial cytokeratins in mammalian
tissues II. Concomitant and mutually exclusive synthesis of
trichocytic and epithelial cytokeratins in diverse human and bovine
tissues (hair follicle, nail bed and matrix, lingual papilla,
thymic reticulum). Differentiation. 37:215–230. 1988.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Manabe M, Lim HW, Winzer M and Loomis CA:
Architectural organization of filiform papillae in normal and black
hairy tongue epithelium: Dissection of differentiation pathways in
a complex human epithelium according to their patterns of keratin
expression. Arch Dermatol. 135:177–181. 1999.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rogers MA, Winter H, Langbein L, Bleiler R
and Schweizer J: The human type I keratin gene family:
Characterization of new hair follicle specific members and
evaluation of the chromosome 17q21.2 gene domain. Differentiation.
72:527–540. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tobiasch E, Winter H and Schweizer J:
Structural features and sites of expression of a new murine 65 and
48 kD hair-related keratin pair, associated with a special type of
parakeratotic epithelial differentiation. Differentiation.
50:163–178. 1992.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Langbein L, Rogers MA, Winter H, Praetzel
S, Beckhaus U, Rackwitz HR and Schweizer J: The catalog of human
hair keratins I. Expression of the nine type I members in the hair
follicle. J Biol Chem. 274:19874–19884. 1999.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Langbein L, Rogers MA, Winter H, Praetzel
S and Schweizer J: The catalog of human hair keratins II.
Expression of the six type II members in the hair follicle and the
combined catalog of human type I and II keratins. J Biol Chem.
276:35123–35132. 2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ng JH, Iyer NG, Tan MH and Edgren G:
Changing epidemiology of oral squamous cell carcinoma of the
tongue: A global study. Head Neck. 39:297–304. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Boldrup L, Coates PJ, Laurell G and
Nylander K: Differences in p63 expression in SCCHN tumours of
different sub-sites within the oral cavity. Oral Oncol. 47:861–865.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Boldrup L, Coates PJ, Wahlgren M, Laurell
G and Nylander K: Sub-site based alterations in miR-21, miR-125b,
and miR-203 in squamous cell carcinoma of the oral cavity and
correlation to important target proteins. J Carcinog.
11(18)2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Braakhuis BJ, Tabor MP, Kummer JA, Leemans
CR and Brakenhoff RH: A genetic explanation of Slaughter's concept
of field cancerization: Evidence and clinical implications. Cancer
Res. 63:1727–1730. 2003.PubMed/NCBI
|
|
15
|
Boldrup L, Gu X, Coates PJ, Norberg-Spaak
L, Fahraeus R, Laurell G, Wilms T and Nylander K: Gene expression
changes in tumor free tongue tissue adjacent to tongue squamous
cell carcinoma. Oncotarget. 8:19389–19402. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Boldrup L, Coates PJ, Laurell G, Wilms T,
Fahraeus R and Nylander K: Downregulation of miRNA-424: A sign of
field cancerisation in clinically normal tongue adjacent to
squamous cell carcinoma. Br J Cancer. 112:1760–1765.
2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Leemans CR, Braakhuis BJ and Brakenhoff
RH: The molecular biology of head and neck cancer. Nat Rev Cancer.
11:9–22. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Lochhead P, Chan AT, Nishihara R, Fuchs
CS, Beck AH, Giovannucci E and Ogino S: Etiologic field effect:
Reappraisal of the field effect concept in cancer predisposition
and progression. Mod Pathol. 28:14–29. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Saeed AI, Sharov V, White J, Li J, Liang
W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, et
al: TM4: A free, open-source system for microarray data management
and analysis. Biotechniques. 34:374–378. 2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ponten F, Jirstrom K and Uhlen M: The
human protein Atlas - A tool for pathology. J Pathol. 216:387–393.
2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shezen E, Okon E, Ben-Hur H and Abramsky
O: Cytokeratin expression in human thymus: Immunohistochemical
mapping. Cell Tissue Res. 279:221–231. 1995.PubMed/NCBI View Article : Google Scholar
|