Introduction
The term subepithelial tumor (SET) is clinically
used for protuberant lesions covered by an intact mucosa (1). The distribution of SETs in the upper
gastrointestinal tract varies among different reports, with the
stomach being the organ most frequently involved (2-4).
SETs were previously referred to as submucosal tumors (SMTs). SETs
are classified into non-neoplastic and neoplastic lesions. The
majority of the lesions are asymptomatic. However, carcinoid
tumors, lymphomas, glomus tumors and gastrointestinal stromal
tumors (GISTs) may be malignant or have malignant potential
(5,6).
Recently, there have been important developments in
minimally invasive full-thickness resection for SETs of the upper
gastrointestinal tract, but there remain certain challenges.
Laparoscopy-assisted endoscopic full-thickness resection (EFTR)
techniques, such as laparoscopic-endoscopic cooperative surgery
(LECS) and non-exposed endoscopic wall-inversion surgery (NEWS),
are the most common procedures, but their applicability remains a
matter of debate. LECS is a safe procedure that allows for very
precise resection, preventing unnecessary and excessive resection
(7-11). NEWS
carries the major advantage of being highly accurate in determining
the resection line with no risk of peritoneal contamination, and
avoids exposure of the tumor into the peritoneal cavity, and it is
is feasible for SETs <3 cm in greatest diameter (8-17).
To the best of our knowledge, our group reported the first case on
NEWS of the stomach (18) and first
part of the duodenum in Thailand (19). The aim of the present study was to
further investigate the feasibility, efficacy and safety of
laparoscopy-assisted EFTR for upper gastrointestinal SETs and to
evaluate the clinical outcome.
Patients and methods
Patients
Patients with upper gastrointestinal SETs who were
referred to the Department of Surgery, Faculty of Medicine,
Thammasat University (Pathumthani, Thailand) between July 2016 and
December 2017 and were identified in our electronic documentation
system, were included in this retrospective study. The study
protocol was approved by the Human Ethics Committee of Thammasat
University (Faculty of Medicine); reference no. MTU-EC-SU-1-170/60.
All patients included in this study provided their consent to the
use of their clinical data for scientific and academic
purposes.
Inclusion and exclusion criteria
The SETs were treated according to the National
Comprehensive Cancer Network (20),
the European Society for Medical Oncology (21) and the Asian consensus guidelines for
the diagnosis and management of gastrointestinal stromal tumors
(22). The inclusion criteria for
laparoscopy-assisted EFTR were SETs ≥2 cm and SETs <2 cm with
high-risk endoscopic ultrasound characteristics, including
irregular border, cystic spaces, ulceration, echogenic foci and
heterogeneity. Patients who were not deemed suitable for
laparoscopy and endoscopic resection were excluded.
Preoperative assessment and treatment
selection
The location and local invasion of tumors were
evaluated with upper gastrointestinal endoscopy and abdominal
computed tomography. The patients were informed of multiple
treatment options and consented to undergo endoscopy and
laparoscopic surgery. The patients who had SET without evidence of
lymph node and/or distant metastasis and who underwent
laparoscopy-assisted EFTR (LECS and NEWS) were enrolled in the
present study. LECS was conducted for tumors >3 cm in diameter
on preoperative imaging, whereas NEWS was performed for tumors
<3 cm, as the tumors were removed perorally using an endoscopic
retrieval device (9,13,14).
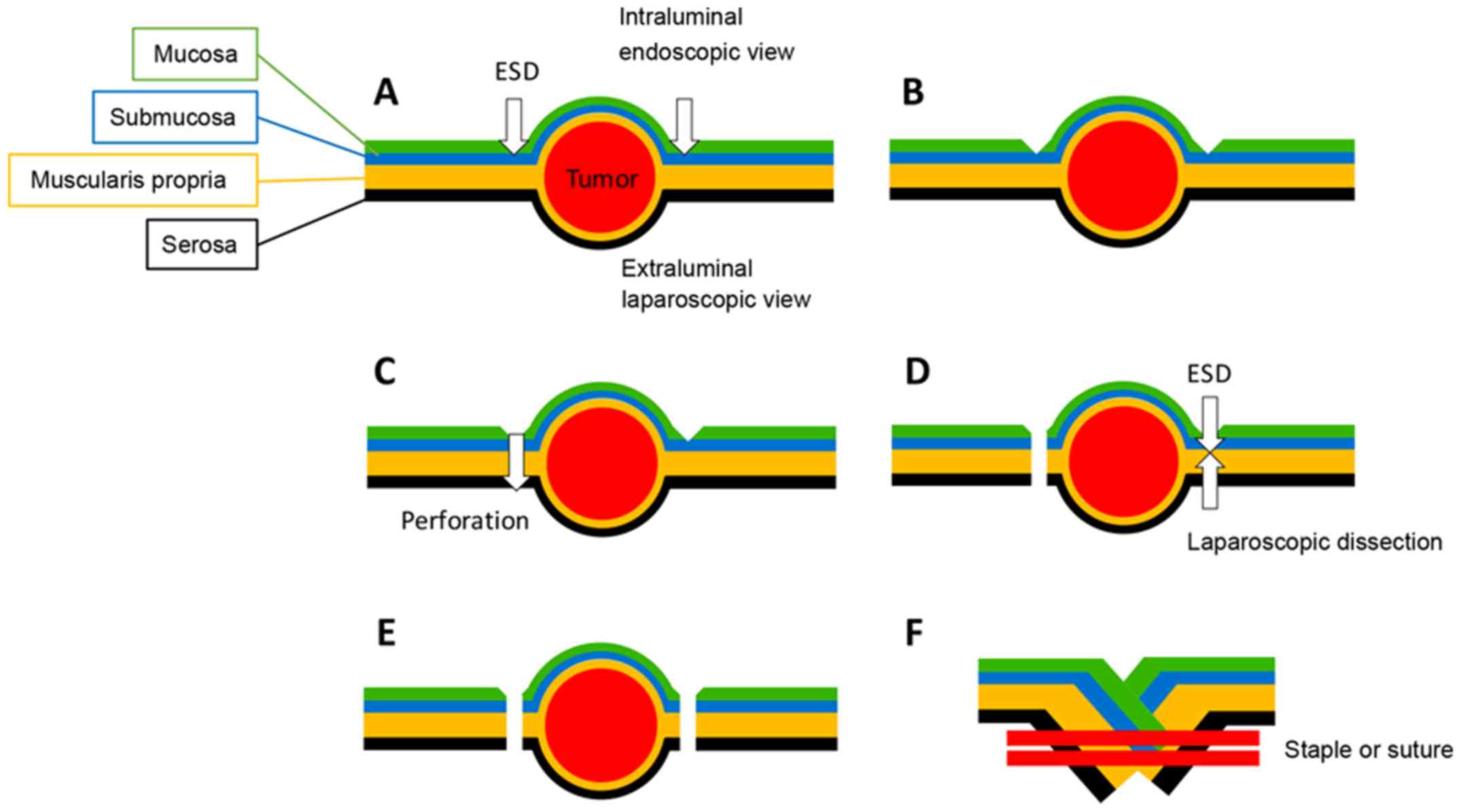
LECS
Briefly, the LECS procedure was performed as
follows: The lesion was identified and the mucosal markings created
using endoscopy. Next, the laparoscopic outer serosal markings
opposite to the previously created inner mucosal markings were
incised. The lesion was circumferentially resected by endoscopic
mucosal and submucosal dissection, followed by laparoscopic
seromuscular resection. The lesion was removed through the
abdominal incision. The resection defect was closed by
full-thickness suturing with the hand-sewn technique in the lesser
curvature close to the esophagogastric junction and the pylorus. In
other areas, it was closed using a laparoscopic linear stapling
device (Fig. 1).
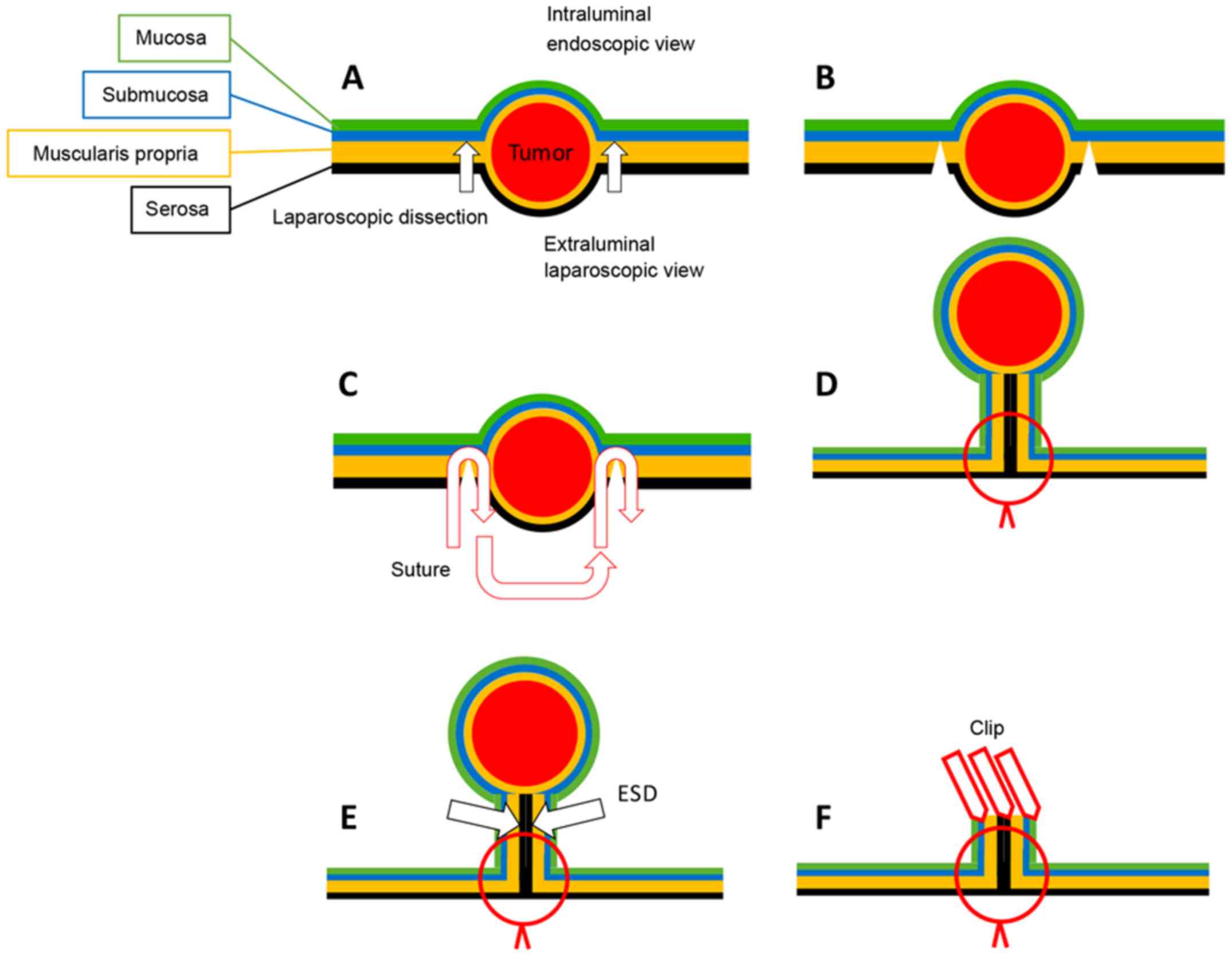
NEWS
Briefly, NEWS was performed as follows: Several
endoscopic mucosal markings were created around the subepithelial
mass, and several serosal markings were made using a laparoscopic
technique on the opposite side. The injection solution was prepared
with Glyceol and a small amount of indigo carmine dye. The solution
was endoscopically injected into the submucosal layer. A
circumferential seromuscular incision was carefully performed, and
was continuously sutured to invert the lesion into the lumen.
During suturing, a sponge was cut to approximately the size of the
lesion and was inserted between the serosal layer of the inverted
lesion and the continuous serosal suture line. The lesion was
removed by careful endoscopic mucosal dissection. The resected
lesion and sponge were removed perorally. Finally, the mucosal
edges were closed with several endoscopic clips (Fig. 2).
Statistical analysis
The patient characteristics, surgical outcomes,
postoperative courses, results of the histopathological examination
and short-term outcomes were analyzed in LECS and NEWS. Data are
expressed as mean ± standard error of the mean. Statistical
analysis was performed using the χ2 test and Fisher's
test for categorical data and the Mann-Whitney U test for
continuous data. All data were analyzed with SPSS 22.0 (IBM Corp.).
P<0.005 was considered to indicate statistically significant
differences.
Results
Patient characteristics
A total of 16 SET patients without evidence of lymph
node and distant metastasis, who consented to undergo
laparoscopy-assisted EFTR (LECS and NEWS) after being informed on
all treatment options, were included in this study (Table I). A total of 10 patients in the LECS
group and 6 patients in the NEWS group, with no significant
differences by age and BMI, were selected. All patients in the LECS
group received surgery on the stomach. SETs were treated by gastric
NEWS in 5 patients and duodenal NEWS in one. The mean tumor size in
the LECS group was larger compared with that in the NEWS group
(LECS, 5.6±1.9 cm; NEWS, 2.1±0.5 cm; P<0.001); 6 tumors in the
LECS group had ulceration, with potential risk of tumor seeding
into the abdominal cavity.
 | Table ICharacteristics of patients and tumors
treated by laparoscopy-assisted endoscopic full-thickness
resection. |
Table I
Characteristics of patients and tumors
treated by laparoscopy-assisted endoscopic full-thickness
resection.
| | Procedure, n | |
|---|
| Characteristics | LECS (n=10) | NEWS (n=6) | P-value |
|---|
| Age, mean ± SD,
years | 68.3±14.7 | 52.0±19.0 | 0.106 |
| Sex, male/female | 4/6 | 2/4 | 0.807 |
| BMI,
kg/m2 | 23.8±3.8 | 28.9±11.6 | 0.339 |
| Location, n | | | |
|
Stomach | 10 | 5 | |
|
Upper
third | 6 | 1 | |
|
Middle
third | 3 | 1 | |
|
Lower
third | 1 | 3 | |
|
Duodenum | 0 | 1 | |
| Position, n | | | |
|
Stomach | 10 | 5 | |
|
Anterior
wall | 1 | 1 | |
|
Greater
curvature | 4 | 1 | |
|
Posterior
wall | 1 | 3 | |
|
Lesser
curvature | 4 | 0 | |
|
1st part of
the duodenum, anterior wall | 0 | 1 | |
| Tumor size, mean ±
SD, cm | 5.6±1.9 | 2.1±0.5 | <0.001 |
| Tumors with
ulceration, n | 6 | 0 | |
The duration of the surgery did not differ
significantly between the two groups (LECS, 211.1±36.6 min; NEWS,
207.5±30.7 min; P=0.836), with R0 resection in both. The
intraoperative blood loss was higher in the LECS group compared
with that in the NEWS group (LECS, 23.0±13.5 ml; NEWS, 1.5±0.8 ml;
P<0.001). The mean resected specimen area/tumor area ratio did
not differ significantly between the two groups. On the first
postoperative day, all the patients were stable; however, the white
blood cell count (WBC), the mean first postoperative day
WBC/preoperative WBC ratio and the level of C-reactive protein
(CRP) were higher in the LECS group compared with those in the NEWS
group (first postoperative day WBC: LECS, 10.1±1.0x103
µl; NEWS, 6.6±2.5x103/l, P=0.018; 1st postoperative day
WBC/preoperative WBC: LECS, 174.9±31.6%; NEWS, 107.8±5.6%,
P<0.001; and CRP: LECS, 84.9±18.4 mg/l; NEWS, 24.1±8.9 mg/l,
P<0.001). The final pathological diagnosis of the SETs was
gastrointestinal stromal tumor (n=9) and leiomyoma (n=1) in the
LECS group, and gastrointestinal stromal tumor (n=3), schwannoma
(n=1), pancreatic ectopia (n=1) and neuroendocrine tumor (n=1) in
the NEWS group. The postoperative hospitalization was shorter in
the NEWS group compared with that in the LECS group (LECS, 6.2±0.4
days; NEWS, 5.3±0.8 days; P<0.048). Both patients undergoing
LECS and those undergoing NEWS were in a good overall condition,
without adverse events, rehospitalization or tumor recurrence. The
mean follow-up period for patients in the LECS group was 333.2 days
and in the NEWS group 345.7 days (range, 1-537 days) (Tables II and III).
 | Table IIOutcome of laparoscopic-assisted
endoscopic full-thickness resection for upper gastrointestinal
subepithelial tumors. |
Table II
Outcome of laparoscopic-assisted
endoscopic full-thickness resection for upper gastrointestinal
subepithelial tumors.
| | Procedure | |
|---|
| Variables | LECS (n=10) | NEWS (n=6) | P-value |
|---|
| Operative duration,
mean ± SD, min | 211.1±36.6 | 207.5±30.7 | 0.836 |
| Blood loss, ml | 23.0±13.5 | 1.5±0.8 | <0.001 |
| R0 resection, n
(%) | 10 (100.0) | 6 (100.0) | |
| Area of tumor
(cm2) | 25.8±14.3 | 3.6±1.5 | <0.001 |
| Area of resection
specimen (cm2) | 40.7±20.2 | 5.9±1.7 | <0.001 |
| Specimen area/tumor
area (%) | 165.6±43.9 | 171.6±32.6 | 0.756 |
| Postoperative
hospitalization, mean ± SD, days | 6.2±0.4 | 5.3±0.8 | 0.048 |
| Body temperature and
laboratory data on 1st postoperative day | | | |
| Body temperature
(̊C) | 37.0±0.2 | 37.1±0.3 | 0.469 |
| Preoperative WBC,
mean ± SD, x103/µl | 5.9±1.2 | 6.1±2.3 | 0.851 |
| Postoperative day 1
WBC, mean ± SD, x103/µl | 10.1±1.0 | 6.6±2.5 | 0.018 |
| Postoperative day 1
WBC/preoperative WBC (%) | 174.9±31.6 | 107.8±5.6 | <0.001 |
| CRP, mean ± SD,
mg/l | 84.9±18.4 | 24.1±8.9 | <0.001 |
| Adverse events, n
(%) | 0 (0.0) | 0 (0.0) | |
| Pathological
diagnosis, n (%) | | | |
|
GIST | 9 (90.0) | 3 (50.0) | |
|
Schwannoma | 0 (0.0) | 1 (16.7) | |
|
Leiomyoma | 1 (10.0) | 0 (0.0) | |
|
Pancreatic
ectopia | 0 (0.0) | 1 (16.7) | |
|
Neuroendocrine
tumor | 0 (0.0) | 1 (16.7) | |
| Recurrence, n
(%) | 0 (0.0) | 0 (0.0) | |
| Survival, n
(%) | 10 (100.0) | 6 (100.0) | |
| Mean follow-up,
days | 333.2±167.4 | 345.7±132.4 | 0.793 |
 | Table IIIDetails of 16 patients with upper
gastrointestinal subepithelial tumors treated by
laparoscopic-assisted endoscopic full-thickness resection. |
Table III
Details of 16 patients with upper
gastrointestinal subepithelial tumors treated by
laparoscopic-assisted endoscopic full-thickness resection.
| Patient number | Age, years | Sex | Site | Location | Tumor size, mm | Ulceration | Procedure Type | Procedure time,
min | Pathological
diagnosis | R0 resection | Adverse events | Recurrence | Survival |
|---|
| 1 | 61 | Female | Stomach | Upper body,
posterior wall | 2.2 | No | NEWS | 219 | GIST | Yes | No | No | Alive |
| 2 | 44 | Female | Stomach | Cardia | 3.5 | No | LECS | 246 | GIST | Yes | No | No | Alive |
| 3 | 84 | Female | Stomach | Middle body, lesser
curvature | 7 | Yes | LECS | 260 | GIST | Yes | No | No | Alive |
| 4 | 67 | Male | Stomach | Middle body,
posterior wall | 3.5 | No | LECS | 186 | GIST | Yes | No | No | Alive |
| 5 | 85 | Male | Stomach | Fundus | 8 | Yes | LECS | 186 | GIST | Yes | No | No | Alive |
| 6 | 79 | Female | Stomach | Antrum, greater
curvature | 6.5 | Yes | LECS | 171 | GIST | Yes | No | No | Alive |
| 7 | 75 | Female | Stomach | Middle body,
posterior wall | 3 | No | NEWS | 192 | GIST | Yes | No | No | Alive |
| 8 | 18 | Male | Stomach | Antrum, greater
curvature | 2 | No | NEWS | 209 | Pancreatic
ectopia | Yes | No | No | Alive |
| 9 | 63 | Female | Stomach | Fundus | 5.5 | No | LECS | 218 | GIST | Yes | No | No | Alive |
| 10 | 75 | Male | Stomach | Middle body,
anterior wall | 4.5 | Yes | LECS | 188 | GIST | Yes | No | No | Alive |
| 11 | 51 | Female | Duodenum | 1st part | 1.3 | No | NEWS | 261 | Neuroendocrine
tumor | Yes | No | No | Alive |
| 12 | 80 | Male | Stomach | Cardia | 9 | Yes | LECS | 268 | GIST | Yes | No | No | Alive |
| 13 | 50 | Female | Stomach | Antrum, posterior
wall | 2.1 | No | NEWS | 178 | Schwannoma | Yes | No | No | Alive |
| 14 | 57 | Male | Stomach | Antrum, anterior
wall | 2.2 | No | NEWS | 185 | GIST | Yes | No | No | Alive |
| 15 | 49 | Female | Stomach | Fundus | 4.5 | Yes | LECS | 170 | GIST | Yes | No | No | Alive |
| 16 | 57 | Female | Stomach | Cardia | 4.3 | No | LECS | 218 | Leiomyoma | Yes | No | No | Alive |
Discussion
Several studies on the R0 resection of SETs without
evidence of lymph node and distant metastasis using the endoscopic
and laparoscopic approaches to reduce morbidity report these
methods as challenging and under development. The advantages of
performing intraluminal and intraperitoneal procedures during the
same operation are minimal invasion and precise resection at the
tumor margin. Hiki et al (7)
first reported LECS as a safe minimally invasive procedure that
maintained the patients' quality of life by resecting a lesion with
minimal margins and preserving gastric function. NEWS is a novel
technique developed and published by Goto et al (17), which includes a minimally invasive
procedure that removes the tumor perorally with full-thickness
resection of the gastric wall, thereby avoiding the risk of
intraperitoneal seeding. In our institute, LECS was performed for
upper SETs >3 cm in diameter, and NEWS was employed for SETs
<3 cm due to the peroral removal, as previously reported
(9,13,14).
The mean specimen area/tumor area ratio did not
differ significantly between the two groups, reflecting the
avoidance of excessive, unnecessary resection and precise cutting
of the lesion. The operative duration of both techniques was also
not significantly different, but the intraoperative blood loss was
higher in the LECS group compared with that in the NEWS group,
which was attributed to the tumor size and area of resection. Both
techniques are effective and minimally invasive, and achieved R0
resection without recurrence, a short length of hospital stay and
lack of adverse events. However, the LECS group had higher WBC
compared with the NEWS group on the 1st postoperative day, as well
as higher mean 1st postoperative day WBC/preoperative WBC ratio and
CRP levels, reflecting the inflammatory process. The procedure of
LECS includes dissecting the lesion and removing the resected
specimen via the abdominal incision. The process of LECS also
involves a step of transmural communication, meaning that the
intraperitoneal cavity may be exposed to the gastrointestinal
fluid. The cautious and delicate handling of the tissues during
surgery is crucial for minimizing the contamination risk of the
LECS procedure. The NEWS technique involves resecting a non-exposed
tumor and removing it via the oral route, which prevents activation
of the inflammatory process by peritoneal contamination. The
patients in the NEWS group had lower levels of inflammatory markers
and shorter postoperative hospitalization. The limitation of this
study lies with its inability to draw definitive conclusions on the
advantages of each technique in terms of patient characteristics,
surgical outcomes and postoperative course, due to the limited
number of cases in the LECS and NEWS groups. The aim of the present
study was to report our early experience with laparoscopy-assisted
EFTR in Thailand, and the results were in accordance with the first
reports of this technique in previous studies (11,14,23).
In conclusion, the present study successfully
demonstrated that laparoscopy-assisted EFTR by LECS and NEWS may be
a feasible and safe minimally invasive treatment option for upper
gastrointestinal SETs. NEWS is the non-exposed technique, which is
preferred if the lesion is sized <3 cm. This study describes
early findings and its main limitation is the small patient sample.
Further studies are required to verify that LECS and NEWS can be
introduced as the standard treatment for small gastric and duodenal
tumors in Thailand.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
PM conceived and designed the present study,
performed the experiments, and analyzed and interpreted the data.
PM and PC collected the data and performed the experiments. All the
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Human Ethics
Committee of Thammasat University (Faculty of Medicine)
(Pathumthani, Thailand); reference no. MTU-EC-SU-1-170/60. All the
patients in this study provided consent to the use of their
clinical data for scientific and academic purposes.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wiech T, Walch A and Werner M:
Histopathological classification of nonneoplastic and neoplastic
gastrointestinal submucosal lesions. Endoscopy. 37:630–634.
2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rösch T, Lorenz R, Dancygier H, von
Wickert A and Classen M: Endosonographic diagnosis of submucosal
upper gastrointestinal tract tumors. Scand J Gastroenterol. 27:1–8.
1992.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Polkowski M: Endoscopic ultrasound and
endoscopic ultrasound-guided fine-needle biopsy for the diagnosis
of malignant submucosal tumors. Endoscopy. 37:635–645.
2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kawamoto K, Yamada Y, Utsunomiya T,
Okamura H, Mizuguchi M, Motooka M, Hirata N, Watanabe H, Sakai K,
Kitagawa S, et al: Gastrointestinal submucosal tumors: Evaluation
with endoscopic US. Radiology. 205:733–740. 1997.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cho JW: Korean ESD Study Group: Current
guidelines in the management of upper gastrointestinal
subepithelial tumors. Clin Endosc. 49:235–240. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nishida T, Goto O, Raut CP and Yahagi N:
Diagnostic and treatment strategy for small gastrointestinal
stromal tumors. Cancer. 122:3110–3118. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi
T, Nunobe S, Tokunaga M, Miki A, Ohyama S and Seto Y: Laparoscopic
and endoscopic cooperative surgery for gastrointestinal stromal
tumor dissection. Surg Endosc. 22:1729–1735. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Matsuda T, Hiki N, Nunobe S, Aikou S,
Hirasawa T, Yamamoto Y, Kumagai K, Ohashi M, Sano T and Yamaguchi
T: Feasibility of laparoscopic and endoscopic cooperative surgery
for gastric submucosal tumors (with video). Gastrointest Endosc.
84:47–52. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hiki N, Nunobe S, Matsuda T, Hirasawa T,
Yamamoto Y and Yamaguchi T: Laparoscopic endoscopic cooperative
surgery. Dig Endosc. 27:197–204. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Matsuda T, Nunobe S, Ohashi M and Hiki N:
Laparoscopic endoscopic cooperative surgery (LECS) for the upper
gastrointestinal tract. Transl Gastroenterol Hepatol.
2(40)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shoji Y, Takeuchi H, Goto O, Tokizawa K,
Nakamura R, Takahashi T, Wada N, Kawakubo H, Yahagi N and Kitagawa
Y: Optimal minimally invasive surgical procedure for gastric
submucosal tumors. Gastric Cancer. 21:508–515. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mitsui T, Nimi K, Yamashita H, Goto O,
Aikou S, Hatao F, Wada I, Shimizu N, Fujishiro M, Koike K and Seto
Y: Non-exposed endoscopic wall-inversion surgery as a novel partial
gastrectomy technique. Gastric Cancer. 17:594–599. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Maehata T, Goto O, Takeuchi H, Kitagawa Y
and Yahagi N: Cutting edge of endoscopic full-thickness resection
for gastric tumor. World J Gastrointest Endosc. 7:1208–1215.
2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Goto O, Takeuchi H, Sasaki M, Kawakubo H,
Akimoto T, Fujimoto A, Ochiai Y, Maehata T, Nishizawa T, Kitagawa Y
and Yahagi N: Laparoscopy-assisted endoscopic full-thickness
resection of gastric subepithelial tumors using a nonexposure
technique. Endoscopy. 48:1010–1015. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Goto O, Takeuchi H, Kawakubo H, Matsuda S,
Kato F, Sasaki M, Fujimoto A, Ochiai Y, Horii J, Uraoka T, et al:
Feasibility of non-exposed endoscopic wall-inversion surgery with
sentinel node basin dissection as a new surgical method for early
gastric cancer: A porcine survival study. Gastric Cancer.
18:440–445. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Goto O, Takeuchi H, Kawakubo H, Sasaki M,
Matsuda T, Matsuda S, Kigasawa Y, Kadota Y, Fujimoto A, Ochiai Y,
et al: First case of non-exposed endoscopic wall-inversion surgery
with sentinel node basin dissection for early gastric cancer.
Gastric Cancer. 18:434–439. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Goto O, Mitsui T, Fujishiro M, Wada I,
Shimizu N, Seto Y and Koike K: New method of endoscopic
full-thickness resection: A pilot study of non-exposed endoscopic
wall-inversion surgery in an ex vivo porcine model. Gastric Cancer.
14:183–187. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mahawongkajit P, Techagumpuch A and
Suthiwartnarueput W: Non-exposed endoscopic wall-inversion surgery
for a gastrointestinal stromal tumor of the stomach: A case report.
Oncol Lett. 14:4746–4750. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mahawongkajit P, Techakumpuch A and
Chanswangphuvana P: Non-exposed endoscopic wall-inversion surgery
for submucosal tumor of the duodenum: Novel case report. Dig
Endosc. 29:818–819. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
National Comprehensive Cancer Network:
NCCN clinical practice guidelines in oncology: Soft tissue sarcoma,
version 1[Internet]. Fort Washington, PA: National Comprehensive
Cancer Network, 2018 (cited 2018 Jan 5). Available from: http://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf.
|
|
21
|
ESMO/European Sarcoma Network Working
Group: Gastrointestinal stromal tumours: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol 25
(Suppl 3): iii21-iii26, 2014.
|
|
22
|
Koo DH, Ryu MH, Kim KM, Yang HK, Sawaki A,
Hirota S, Zheng J, Zhang B, Tzen CY, Yeh CN, et al: Asian consensus
guidelines for the diagnosis and management of gastrointestinal
stromal tumor. Cancer Res Treat. 48:1155–1166. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mitsui T, Yamashita H, Aikou S, Niimi K,
Fujishiro M and Seto Y: Non-exposed endoscopic wall-inversion
surgery for gastrointestinal stromal tumor. Transl Gastroenterol
Hepatol. 3(17)2018.PubMed/NCBI View Article : Google Scholar
|