Introduction
Experimental unilateral transection of the optic
nerve causes acute retinal glial activation on the contralateral
side (1-3).
These experimental observations led us to consider whether
bilateral glial activation processes are occurring at the level of
ganglial retinal activation in the human visual pathway. In our
experience, glial cells are activated when retinal cells are
damaged. In the retina, these include microglia, Muller cells, and
astrocytes, while in retinal ganglion cells, it appears that the
microglia are primarily activated.
Our recent work focused on traumatic optic
neuropathy (TON) (4); however, we
also studied damage to the contralateral visual pathways. This case
report on optic nerve sheath meningioma is presented mainly as
confirmation of our previous focus on TON and also to eliminate the
possibility of damage to the contralateral pathway after a
contusion. Optic nerve sheath meningioma (ONSM) is a rare benign
tumour of the central nervous system. Although the growth of these
lesions is slow and progressive, their location is critical.
Because of their location, ONSM lesions directly influence the
frontal visual pathway and can lead to severe vision problems. The
patient in this case was a 43-year-old man who had received a
transcranial intervention. As far as we are aware, there are no
references in the literature regarding damage in contralateral
visual pathways caused by ONSM.
Case report
A 43-year-old man was diagnosed with right-sided
ONSM 12 years prior to this report. At the time of diagnosis, there
was an attempted to extirpate the tumour via a frontal transcranial
approach. The tumour was inaccessible from this approach, and the
surgery was ended. The patient was seen at the first author's
workplace in April 2018. The patient was found to be completely
blind in his right eye. According to the patient, the blindness had
been present since the operation. Repeated MRIs showed a tumour of
stationary size; therefore, further surgery was not indicated. In
addition to standard ophthalmic examinations, we also examined the
visual field of the patient using automated perimetry (Medmont
M700; Medmont International Pty Ltd.). Retinal nerve fibre layers
(RNFL) and the ganglion cell complex (GCC) were examined using
spectral domain optical coherence tomography (SD-OCT) with a
RTVue-100. The pattern electroretinogram (PERG) ant the pattern
visual evoked potential (PVEP; Roland Consult Stasche & Finger
GmbH) were obtained while using standard ISCEV methods. The
potential for exophthalmos was assessed using a Hertel
exophthalmometer, intraocular pressure (IOP) was assessed using an
Ocular Response Analyzer, and colored perception was assessed using
the Ishihara test for color blindness. The ophthalmologic
examination was always performed by the same physician.
Additionally, structural and functional MRI images
of the brain were obtained. MRI examinations were carried out using
a Philips Achieva 3T TX MR system (Philips Healthcare) with a
magnetic field strength of 3 Tesla. The functional MRI (fMRI) used
blood oxygen level-dependent (BOLD) contrast. A standard 32-channel
head coil was used, and each measurement was performed with a
gradient-echo echo-planar imaging sequence (TR/TE=3,000/30 ms,
spatial resolution of 2x2x2 mm3). Optical stimulation
was performed using a black and white checkerboard pattern
alternated with its negative image at a frequency of 2 Hz. The size
of the black and white checkerboard was 25.8x16.2 degrees.
Measurements consisted of a sequence of five 30 sec active phase
periods and five resting periods of the same length (for each of 10
dynamic scans). During the resting phase, the subject was
instructed to maintain view fixation on a static crosshair
projected in the centre of the visual field. In total, each
measurement included 100 dynamic scans and took 5 min. Each eye was
examined in a separate fMRI measurement sequence (LE, RE).
Evaluation of the fMRI data was performed using the
SPM 12 software package. Data pre-processing was composed of
realignment (motion correction), slice timing (time shift between
slice acquisitions), normalization to standard MNI-152 space, and
spatial smoothing (FWHM=3x3x3 mm3). A general linear
model was used to analyse the data, and the significance threshold
was set at P=0.05 with family-wise error (FWE) correction applied
to the final t-maps.
Structural magnetic resonance imaging was performed
in the standard planes using the sequences as follows: T1-weighted
mDIXON, TR of 500 ms, TE of 47 ms, 10 ml Gd-DTPA IV; T2-weighted
mDIXON in coronal orientation, slice thickness of 2.5 mm, TR of
3,000 ms, TE of 56 ms; T2-weighted in transversal orientation,
slice thickness of 4 mm, TR of 3,000 ms, TE of 56 ms, flip angle of
57˚; FLAIR with slice thickness of 4 mm, TR of 11,000 ms, TE of 125
ms; VenBold, TR of 15 ms, TE of 50 ms, spatial resolution of 1x1x1
mm3; DWI with slice thickness of 4 mm, TR of 3,443 ms,
TE of 76 ms, b-factor 0-800 s/mm2, 30 slices.
Structural and functional MRI examinations were
carried out in June 2018.
Results
Exophthalmos was detected on the right side (up to 2
mm more than the left eye) with 5˚ of divergence and free mobility.
Anisocoria was present, and the relative afferent pupillary defect
(RAPD) was positive. Except for this pupilar disorder and simple
atrophy of the optic nerve, the ocular findings were normal. On the
left side, the findings were normal; the pupil reacted only to a
direct light impulse. Vision was with NLP and normal in the right
and left eye, respectively. The intraocular pressure (IOP) was
19/16 mm Hg. Colour perception in the left eye remained normal
(intact). The visual field on the left side exhibited no decrease
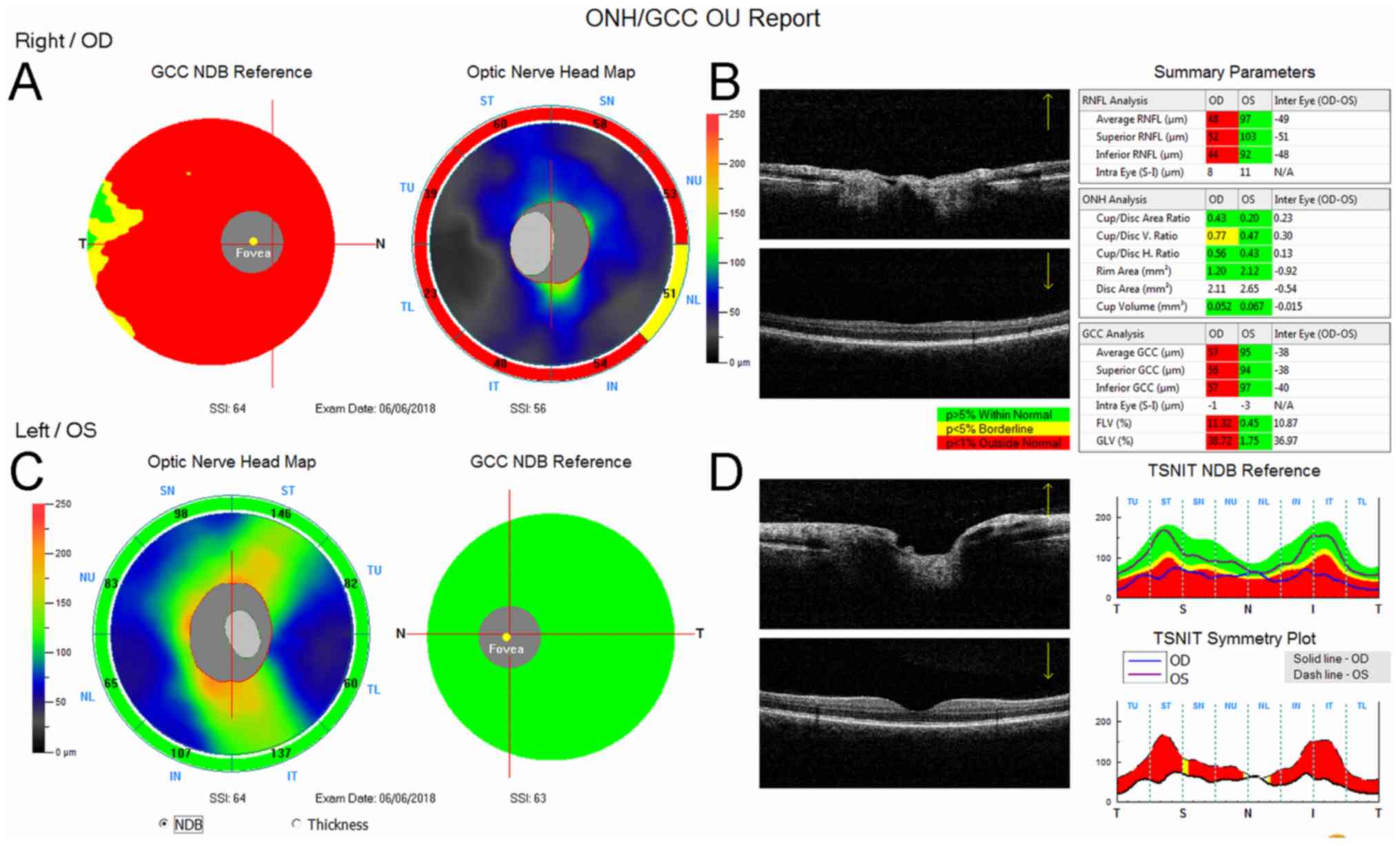
insensitivity. The RNFL and GCC were significantly changed on the
right side. The average value of RNFL was 48 µm on the right and 97
µm on the left. The average GCC value on the right was 57 µm and 95
µm on the left (Fig. 1).
After stimulation of the right eye, the PERG showed
decreased amplitude limit values (10 uV) and a prolongation of the
N95 component to 100 ms. On the left side, the amplitude values
were at the standard limit (13 uV), and the N95 latency was not
prolonged.
The PVEP amplitude values were affected bilaterally.
The response of the right side was minimal (0.5 uV); the left side
response was significantly decreased (6.5 uV), and the latency of
the P100 component was prolonged up to 118 ms.
The normal value for the PERG amplitudes for P50-N95
components is 14.80±2.51 uV; the normal value for PVEP amplitudes
for N70-P100 components is 12.22±3.22 uV (5).
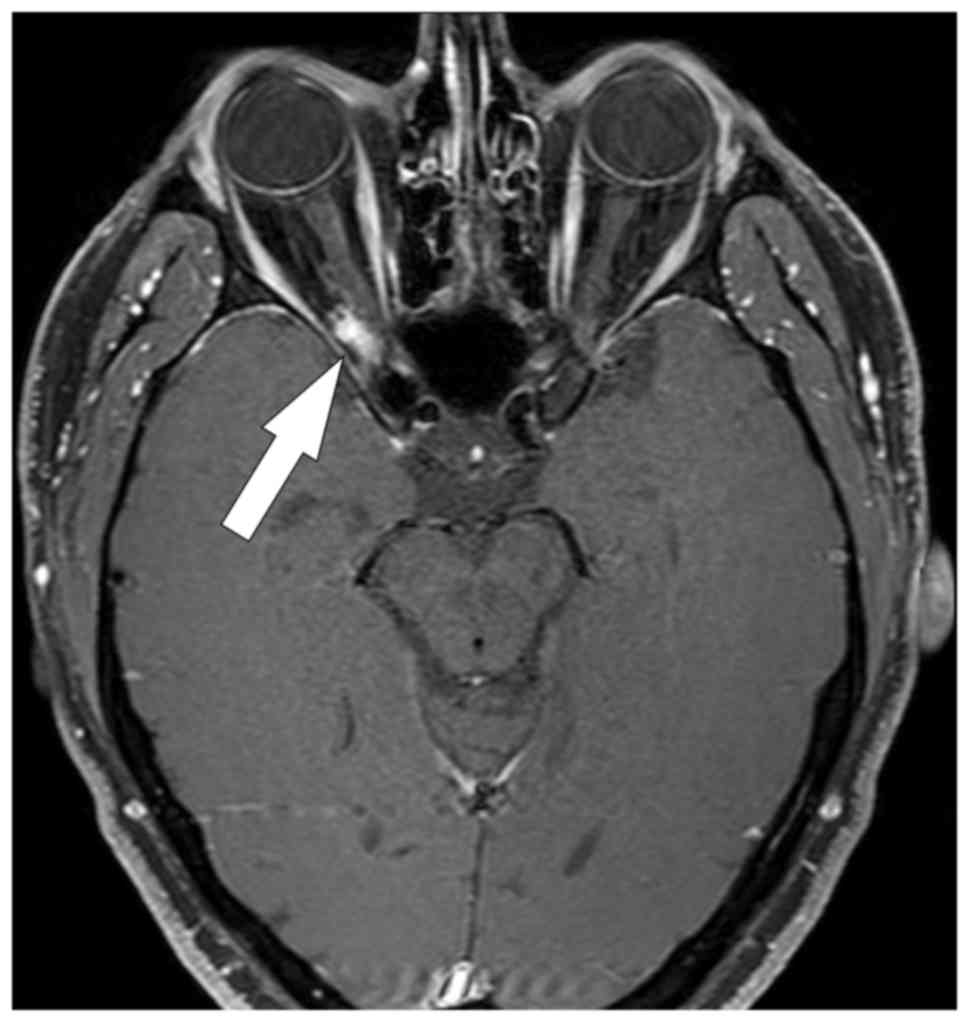
A structural MRI performed in June 2018 showed a
stationary, enhanced meningioma residue in the region of the
orbital apex area on the right side without intracranial ingrowth.
Intraorbital atrophy of the optic nerve on the right, together with
atrophy of the optic chiasma, was visible (Fig. 2). There was no infiltration of the
optic nerve chiasma.
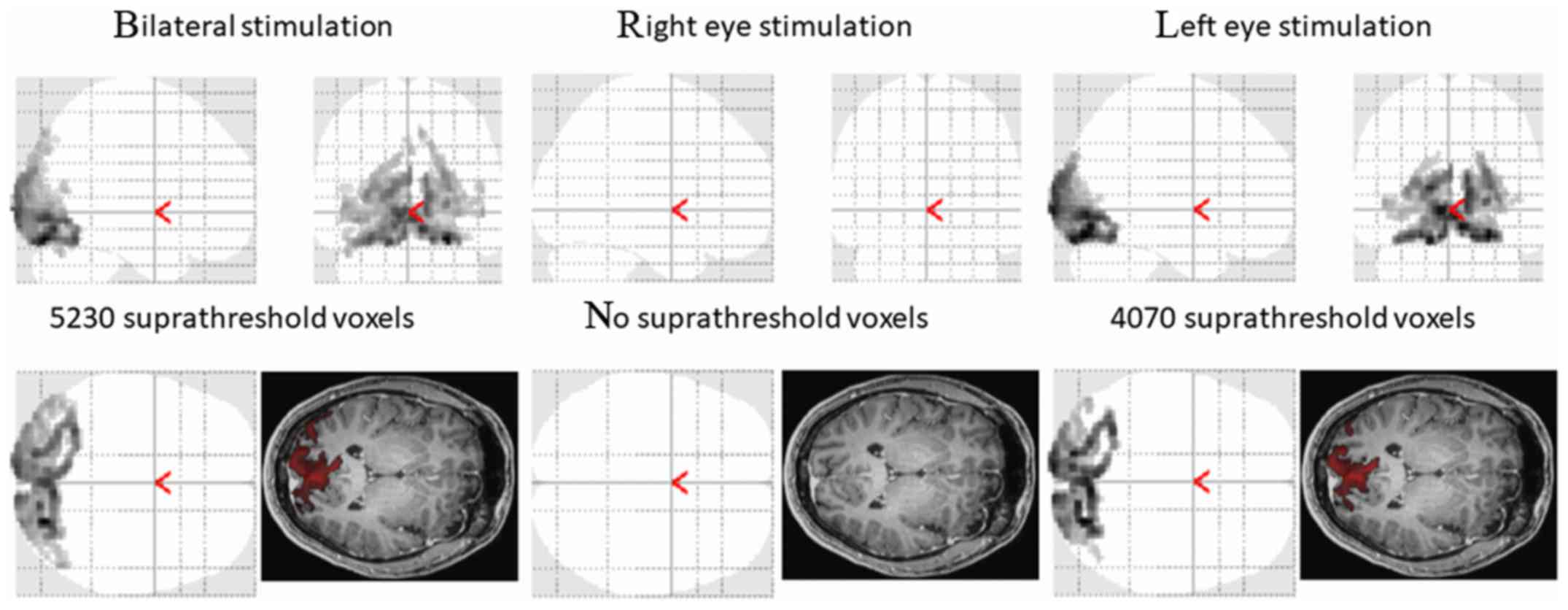
A functional magnetic resonance scan was performed
two months after the first ophthalmic examination (Fig. 3).
The average healthy population values of responsive
values, according to our method, are as follows: When stimulating
on the right, 7,508±2,018 voxels; when stimulating on the left,
7,340±2,775 voxels; and after bilateral stimulation, 7,898±2,579
voxels.
With regard to the blindness in the right eye and
with regard to the fact that the tumour has not grown, we decided
on a conservative approach with monitoring.
Discussion
Retinal glial activation on both the ipsilateral and
the contralateral sides is well known from the literature on
experimental models (1-3).
These experimental conclusions made us ask whether the processes of
bilateral ganglial activation are also occurring at the level of
ganglial retinal activation in the human visual pathway. In our
recent work, which focused on TON (4), we showed that with unilateral damage to
the optic nerve, there are significant changes in the contralateral
eye pathways, including functional retinal changes.
In this case, our patient had a normal visual field
and colour perception on the contralateral side and did not show
any abnormalities relative to the ONSM. This might be because there
must be a loss of 25-30% of the ganglia retinal cells to register
any perimetric changes when examining a patient using static
automatic perimetry, and the same is with respect to colour
perception.
RNFL and GCC examinations showed a loss on the
ipsilateral side only. Although these changes can occur on the
contralateral side, they cannot be detected by any method currently
available. Optic coherence tomography only measures the GCC
thickness, which can be affected by glial proliferation. GCC is
altered only when structural changes are present. The functional
outcome of the PERG and PVEP that was pathological on the
contralateral eye is essential.
ONSM is a lesion that is sensitive to gadolinium
contrast. On MRI axial images, it presents with a characteristic
‘tram-track’ sign, which corresponds to enhancement of the outer
ONSM encircling the inner non-enhanced optic nerve. On coronal
images, this pattern will have the appearance of a ‘doughnut.’
One issue related to contralateral eye nerve damage
or, rather, damage to the contralateral eye pathway after ONSM, is
not mentioned in the human medical literature. Using PERG in a
mouse model, Liu et al (6)
found changes both in the ipsilateral and in the contralateral eye
in situations with unilateral damage to the optic nerve; these
findings preceded morphologic changes in the retinal nerve fibre
layer. The PERG results in our patient support this finding.
We are not aware of any available PVEP or fMRI
findings related to ONSM in published experimental or human
studies. Our results show changes to the entire visual pathway not
only on the damaged side but also on the contralateral side.
ONSM is not a malignant tumour; nevertheless, the
consequences of ONSM can lead to blindness. A patient with ONSM
usually visits an ophthalmologist due to a temporary unilateral
fogging of eyesight, changes in the visual field, and worsening
vision. These visual problems, on closer inspection, are often
accompanied by edema of the optic nerve. A slight proptosis of the
affected bulb can also be accompanied by a loss in ocular mobility.
An afferent pupillary defect is also obvious. The gold standard for
diagnosis is magnetic resonance imaging using gadolinium as a
contrast substance. Sequences with fat signal reduction are
preferable. An MRI examination can show the extent of damage, the
degree of optic nerve channel infiltration, and possible extension
into the optic chiasma and other intracranial structures. In terms
of therapy and management of ONSM, the recommended methods are
similar. Monitoring remains a suitable conservative treatment when
visual functions remain stable, especially in patients whose
central visual acuity is 0.4 or better. Neuro-ophthalmologists
should carefully monitor patients and perform a thorough
neuro-ophthalmic examination, including regular monitoring of the
visual field and the peri-papillary RNFL. Repeated MRI examinations
are also justified (7-9).
In cases where visual function deteriorates,
fractionated stereotactic radiotherapy has become the preferred
treatment since it provides sufficient radiation of the tumour in a
very localized way. This method can preserve visual functions in
most patients, although risks from radiation retinopathy or optic
neuropathy certainly exist (7-9).
Tumour resection is almost impossible without
significant loss of vision due to the close proximity of the ONSM
to the optic nerve. Nevertheless, surgical resection can be
justified in cases of increased proptosis that has significantly
reduced visual functions or in cases of intracranial enlargement
(7,8).
Our previous work, together with this case study,
shows that unilateral damage to the optic nerve associated with
ONSM can lead to significant changes in the contralateral visual
pathways, including changes in retinal function. Clinical trials
with companion ophthalmologic correlative studies of optic nerve
pathways and neurotransmissions are required to help inform about
mechanism of both side visual pathway affection after one side
optic nerve damage.
Acknowledgements
Not applicable.
Funding
Supported by Ministry of Health, Czech Republic,
conceptual development of research organization, Motol University
Hospital, Prague, Czech Republic 00064203 (Progress Q35). The
present study was supported by the Charles University research
program PROGRES Q35.
The present study was supported by the EU Structural
Funds OPP competitiveness (grant no. CZ.2.16/3.1.00/21532).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
All the authors were involved in conceiving and
designing the present study. JL drafted and wrote the manuscript.
MK, JL and JT were responsible for collecting and analyzing patient
data. PH drafted the manuscript and revised it critically for
important intellectual content. All authors have read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient prior to enrolment.
Patient consent for publication
The patient gave written consent; however, the
authors also made efforts to remove any identifying information to
protect the privacy of the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bodeutsch N, Siebert H, Dermon C and
Thanos S: Unilateral injury to the adult rat optic nerve causes
multiple cellular responses in the contralateral site. J Neurobiol.
38:116–128. 1999.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cen LP, Han M, Zhou L, Tan L, Liang JJ,
Pang CP and Zhang M: Bilateral retinal microglial response to
unilateral optic nerve transection in rats. Neuroscience.
311:56–66. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Panagis L, Thanos S, Fischer D and Dermon
CR: Unilateral optic nerve crush induces bilateral retinal glial
cell proliferation. Eur J Neurosci. 21:2305–2309. 2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kyncl M, Lestak J, Tintera J and Haninec
P: Traumatic optic neuropathy-a contralateral finding: A case
report. Exp Ther Med. 17:4244–4248. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lestak J, Nutterova E, Pitrova S, Krejcova
H, Bartosova L and Forgacova V: High tension versus normal tension
glaucoma. A comparison of structural and functional examinations. J
Clin Exp Ophthalmol S5. 6:2011.
|
|
6
|
Liu Y, McDowell CM, Zhang Z, Tebow HE,
Wordinger RJ and Clark AF: Monitoring retinal morphologic and
functional changes in mice following optic nerve crush. Invest
Ophthalmol Vis Sci. 55:3766–3774. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Patel BC, Najem K and Margolin E: Optic
nerve sheath meningioma. 2019 Jun 3. StatPearls Treasure Island
(FL): StatPearls Publishing, 2020.
|
|
8
|
Miller NR: New concepts in the diagnosis
and management of optic nerve sheath meningioma. J Neuroophthalmol.
26:200–208. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hamilton SN, Nichol A, Truong P, McKenzie
M, Hsu F, Cheung A, Dolman P, Gete E and Ma R: Visual outcomes and
local control after fractionated stereotactic radiotherapy for
optic nerve sheath meningioma. Ophthalmic Plast Reconstr Surg.
34:217–221. 2018.PubMed/NCBI View Article : Google Scholar
|