Introduction
Breast cancer survival has increased continuously
during the last decades, and the 5-year survival rate in Sweden is
almost 90% (1,2). Improved and more individualized
treatment has contributed to this progress. However, a few patients
may be overtreated, possibly with undesired side effects. The
majority of patients with estrogen receptor (ER)-positive tumors
receive endocrine therapy. In a previous study, our group
demonstrated that patients with ER+/progesterone
receptor (PR)-positive tumors clearly did benefit from tamoxifen,
while the long-term benefit was lower for those with
ER+/PR- tumors (3). Nevertheless,
ER+/PR- tumors are a heterogeneous group, and
among them there may be subgroups of patients that do benefit from
tamoxifen. Therefore, one subject for further study was the
identification of such subgroups.
The ER+/PR- subgroup is
considered to have a more aggressive progression compared with that
of the ER+/PR+ subgroup, and according to the
St. Gallen criteria, patients with ER+/PR-
tumors are recommended chemotherapy as additional treatment,
despite the fact that in this subgroup certain patients may have
good prognosis without systemic treatment. Experimental studies
have shown that the Ras-related protein RAB6C (RAB6C) inhibits
proliferation, invasion and metastasis, suggesting that it acts as
a tumor suppressor (4). RAB6C also
interacts with p53, which is frequently mutated in breast cancer
(5-7). In
a concurrent study, it was revealed that systemically untreated
patients with ER+/PR- tumors and high RAB6C
expression (RAB6C+) had prolonged distant
recurrence-free survival compared with that of patients with low
RAB6C expression (RAB6C-) (26). The aim of the present study was to
investigate if RAB6C has a treatment predictive value for
tamoxifen. For this purpose, data for 486 patients from a
randomized clinical trial were used.
Patients and methods
Patients
Patients with operable invasive breast cancer were
entered in a previous study of adjuvant tamoxifen therapy conducted
by the Stockholm Breast Cancer Study Group (8). Postmenopausal women younger than 70
years of age were randomly administered tamoxifen postoperatively
at a dose of 40 mg per day compared with no adjuvant endocrine
therapy. Between November 1976 and June 1990, 2,738 patients were
recruited into the trial. Among them, 1,780 patients (65%) with no
lymph node metastases and a tumor diameter ≤30 mm (established by
histological examination) were classified as ‘low risk’ and did not
receive cytotoxic chemotherapy. In this group, 432 patients were
treated with breast conserving surgery, including axillary
dissection plus radiation to the breast (50 Gy/5 weeks). The
remaining 1,348 patients had a modified radical mastectomy and no
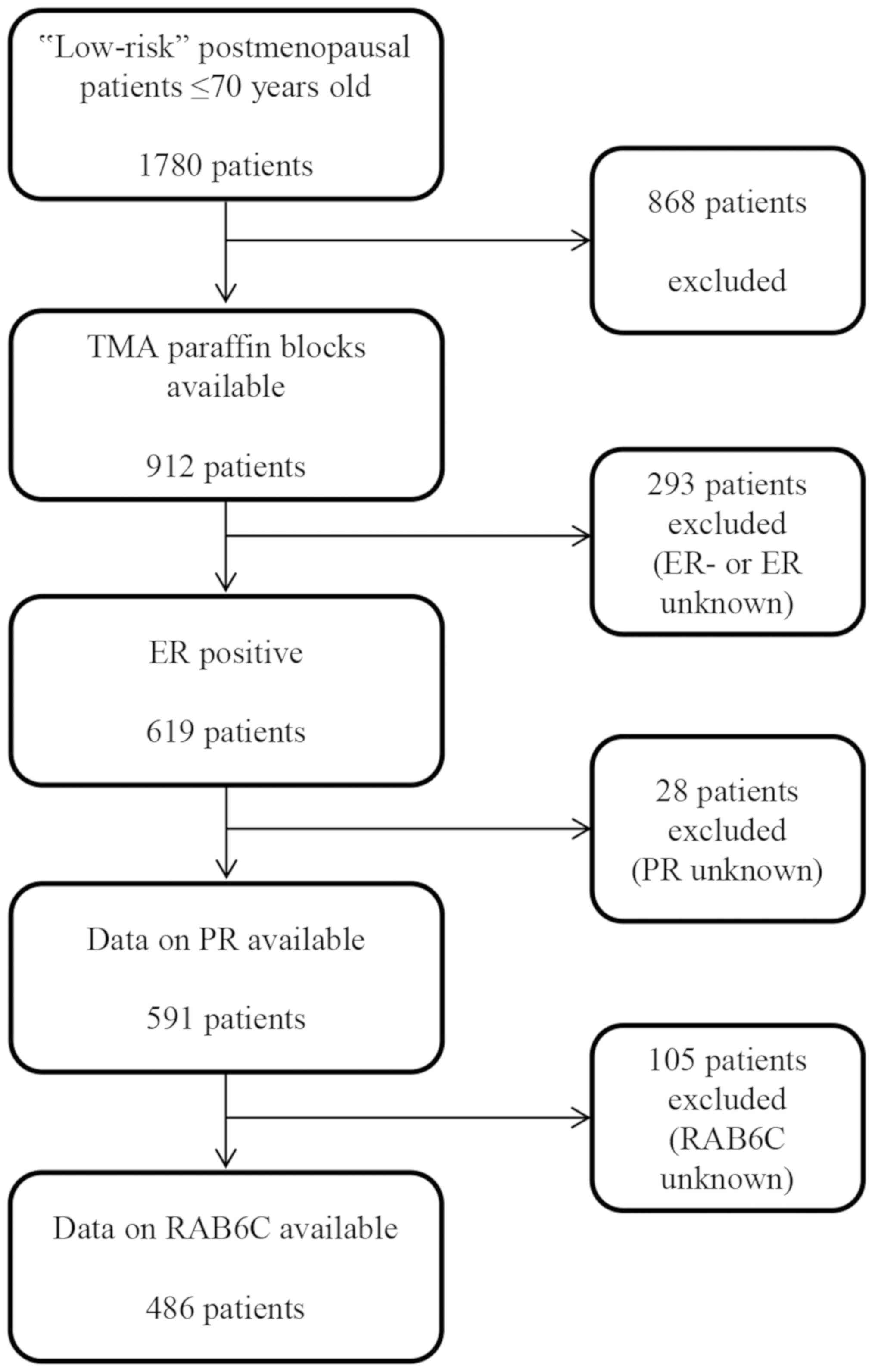
radiotherapy. Paraffin blocks from 912 low-risk patients were used
for the construction of tissue microarrays (TMAs). Of these, 619
tumors were ER+, whereof 591 also had data on PR status
(3). RAB6C expression could be
evaluated for 486 cases among these tumors (Fig. 1).
Hormone receptor status, human
epidermal growth factor receptor 2 (HER2) status, grade and RAB6C
expression
Data on ER, PR and HER2 was available from previous
studies. The status of ER and PR was assessed retrospectively with
immunohistochemistry (IHC) using the VENTANA® automated
slide stainer (Ventana Medical Systems, Inc.). The primary
monoclonal antibodies used were CONFIRM™ mouse anti-ER antibody
(clone 6F11) and CONFIRM™ mouse anti-PR antibody (clone 16)
(Ventana Medical Systems, Inc.). The cut-off level was set to 10%
positively stained tumor cells (9).
HER2 was analyzed with IHC as previously described (10). The Nottingham Histological Grade
(NHG) was analyzed retrospectively by the same investigator for all
tumor samples.
The protein expression of RAB6C was analyzed with
IHC, and the staining pattern was evaluated independently by two
investigators (JS and TB). The polyclonal rabbit antibody ab200396
(Abcam) was used. The intensity of RAB6C in the nucleus was
analyzed and scored as 0, 1, 2 or 3. If the nuclei had an intensity
≥2, the tumor was considered to have high expression of RAB6C
(RAB6C+). Otherwise, it was considered to have low RAB6C
expression (RAB6C-).
Ethics approval and patient consent to
participate
The present study was performed in accordance with
the Declaration of Helsinki. Ethical approval for the use of tumor
material was approved by the local Ethical Committee at the
Karolinska University Hospital (approval no. KI 97-451 with
amendments 030201 and 171027). According to the approval, informed
consent from the patients was not required.
Statistical analysis
To compare the association between RAB6C and
clinical characteristics, the Pearson's χ2 test was
applied. Cumulative distant recurrence-free survival was estimated
using the Kaplan-Meier method. The end-point was defined as the
first distant recurrence from the patient's primary breast cancer
as described by Rutqvist and Johansson (8). Three patients had succumbed to breast
cancer but no date of distant recurrence was registered. For these
patients, the date of death was used as an event of distant
recurrence. The remaining patients were followed up until they had
a distant recurrence or succumbed, or for 25 years after
randomization, whichever occurred first. Patients were censored at
the last follow-up or at mortality due to causes other than breast
cancer.
The multivariable analyses with subgroups based on
RAB6C status included age, tumor size, HER2, NHG, tamoxifen and the
interaction term ‘RAB6C x tamoxifen’. Hazard ratios (HRs) and 95%
confidence intervals (CΙs) were estimated using the Cox's
proportional hazards model. To test whether there is an interaction
between RAB6C and tamoxifen, the Likelihood ratio χ2
test was applied. The statistical analyses were performed with
Stata/SE 13.1 (StataCorp LP).
Results
Patients with ER+ tumors did benefit from
tamoxifen (HR=0.47, 95% CI=0.31-0.71; P<0.001), which was more
evident in patients with ER+/PR+ tumors
(HR=0.39, 95% CI=0.23-0.67; P=0.001) than in those with
ER+/PR- tumors (HR=0.65, 95% CI=0.34-1.25;
P=0.19). However, dividing the ER+/PR- tumors
by RAB6C status showed that, for those with low RAB6C expression
levels, the tamoxifen effect on disease (HR=0.25, 95% CI=0.09-0.70;
P=0.008) was comparable to that in patients with
ER+/PR+ tumors (Fig. 2). For patients with
ER+/PR-/RAB6C+ tumors, prolonged
distant recurrence-free survival was not observed if they were
treated with tamoxifen (HR=1.82, 95% CI=0.69-4.79; P=0.23). The
different effect of the treatment between the RAB6C+ and
RAB6C- groups was indicated by a statistically
significant interaction (P=0.004; Table
I). In the ER+/PR+ subgroup, RAB6C
expression did not have a significant influence on benefit from
tamoxifen. Both patients with RAB6C- tumors (HR=0.63,
95% CI=0.29-1.37; P=0.24) and RAB6C+ tumors (HR=0.27,
95% CI=0.13-0.58; P=0.001) did benefit from tamoxifen, although it
was statistically significant only among those with
RAB6C+ tumors. Of note, the tamoxifen-treated patients
with ER+/PR+/RAB6C+ or
ER+/PR-/RAB6C- tumors had
excellent distant recurrence-free survival, with a 25-year
cumulative proportion of 87% (95% CI=75-93%) and 88% (95%
CI=72-95%), respectively. Together, these patients represent 60% of
all patients with ER+ disease treated with
tamoxifen.
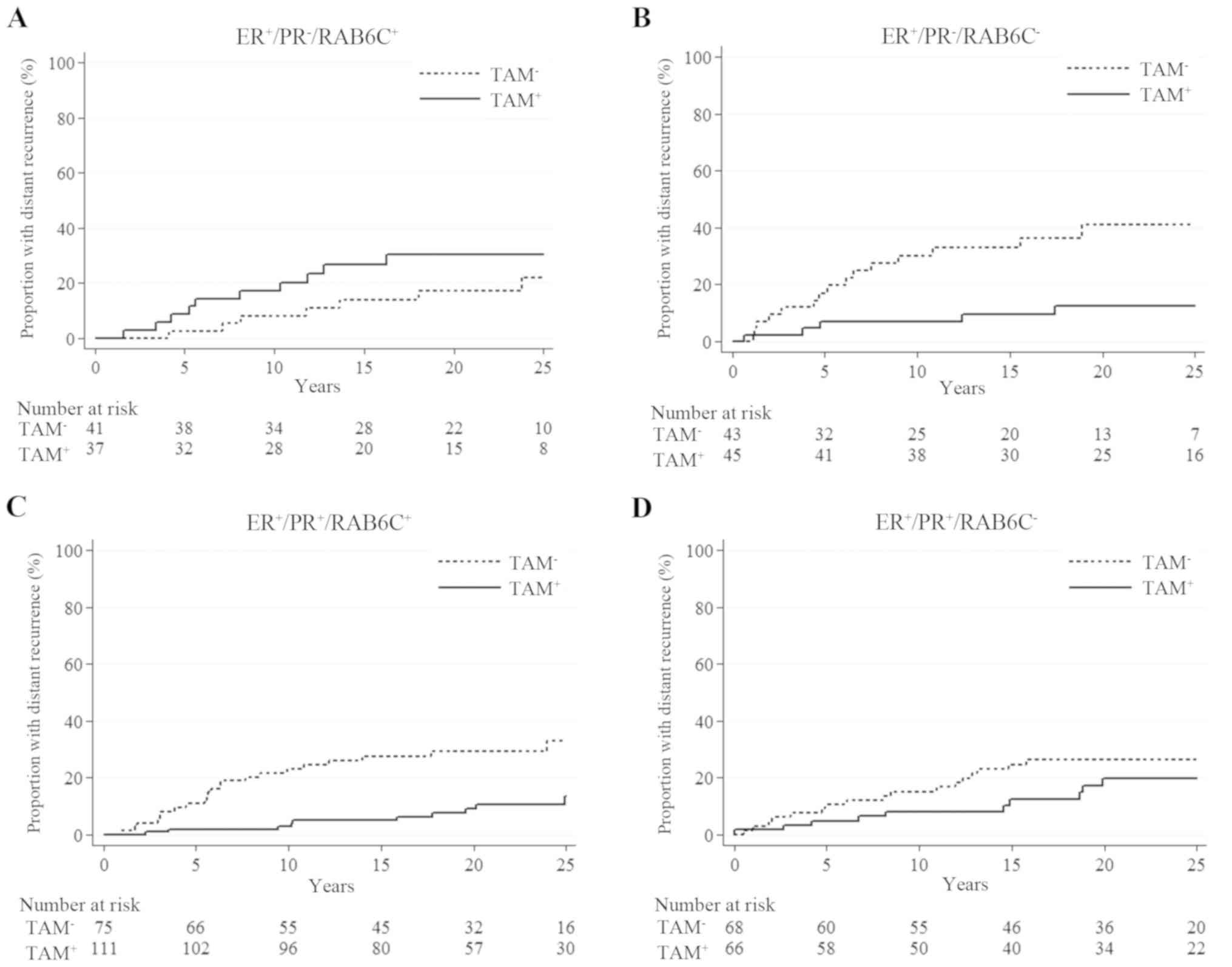 | Figure 2Cumulative distant recurrence risk in
relation to tamoxifen treatment in patients with (A)
ER+/PR-/RAB6C+ (HR, 1.82; 95% CI,
0.69-4.79; P=0.23), (B)
ER+/PR-/RAB6C- (HR, 0.25; 95% CI,
0.09-0.70; P=0.008), (C)
ER+/PR+/RAB6C+ (HR, 0.27; 95% CI,
0.13-0.58; P=0.001) and (D)
ER+/PR+/RAB6C- (HR, 0.63; 95% CI,
0.29-1.37; P=0.24) tumors. CI, confidence interval; ER, estrogen
receptor; HR, hazard ratio; PR, progesterone receptor; RAB6C,
Ras-related protein Rab-6C; TAM, tamoxifen. |
 | Table IUnivariable analysis of distant
recurrence rates for tamoxifen-treated patients compared with the
control group, stratified by hormonal receptor status. |
Table I
Univariable analysis of distant
recurrence rates for tamoxifen-treated patients compared with the
control group, stratified by hormonal receptor status.
| | Number of
patients/events | | | |
|---|
| Subgroup | TAM+ | TAM- | HR (95% CI) TAM+
vs. TAM- | P-value | P for
interaction |
|---|
| ER+ | 259/35 | 227/61 | 0.47
(0.3-0.71) | <0.001 | |
|
ER+/PR+ | 177/20 | 143/39 | 0.39
(0.23-0.67) | 0.001 | 0.20 |
|
ER+/PR- | 82/15 | 84/22 | 0.65
(0.34-1.25) | 0.19 | |
|
ER+/PR+/RAB6C+ | 111/10 | 75/22 | 0.27
(0.13-0.58) | 0.001 | 0.14 |
|
ER+/PR+/RAB6C- | 66/10 | 68/17 | 0.63
(0.29-1.37) | 0.24 | |
|
ER+/PR-/RAB6C+ | 37/10 | 41/7 | 1.82
(0.69-4.79) | 0.23 | 0.004 |
|
ER+/PR-/RAB6C- | 45/5 | 43/15 | 0.25
(0.09-0.70) | 0.008 | |
Established clinical factors were similarly
distributed in the tamoxifen-treated and control groups (Table SI). In multivariable analyses
divided by PR status and adjusting for age, tumor size, HER2 status
and NHG, the results were similar to those obtained in univariable
analyses, indicating that the treatment effect of tamoxifen on
disease depending on the RAB6C expression was independent of these
factors (Tables I and II). In summary, the analyses revealed that
it was favorable to treat patients with
ER+/PR+ tumors regardless of RAB6C expression
status. In contrast to high RAB6C expression, low RAB6C expression
predicted benefit from tamoxifen for patients with
ER+/PR- tumors.
 | Table IIMultivariablea analysis of
distant recurrence rates for tamoxifen-treated patients compared
with the control group, stratified by hormonal receptor status. |
Table II
Multivariablea analysis of
distant recurrence rates for tamoxifen-treated patients compared
with the control group, stratified by hormonal receptor status.
| | Number of
patients/events | | | LR interaction term
RAB6C x TAM |
|---|
| Subgroup | TAM+ | TAM- | HR (95% CI) TAM+
vs. TAM- | P-value | χ2
value | P-value |
|---|
|
ER+/PR+/RAB6C+ | 88/7 | 69/20 | 0.17
(0.07-0.42) | <0.001 | 4.63 | 0.03 |
|
ER+/PR+/RAB6C- | 58/10 | 55/15 | 0.61
(0.27-1.37) | 0.23 | | |
|
ER+/PR-/RAB6C+ | 32/7 | 32/4 | 2.18
(0.61-7.78) | 0.23 | 10.17 | 0.001 |
|
ER+/PR-/RAB6C- | 40/4 | 40/15 | 0.19
(0.06-0.57) | 0.003 | | |
Discussion
The hormone receptors ER and PR are important
markers for breast cancer treatment. While tumors that are positive
for both receptors are associated with prolonged survival when
patients are treated with endocrine therapy,
ER+/PR- tumors are considered to have a more
aggressive phenotype (11). Patients
with this tumor subtype are mostly treated with both endocrine
therapy and chemotherapy, but there are probably subgroups of
patients with these tumors who have high survival rates with more
limited treatment. In a concurrent study, the results indicated
that RAB6C may identify such patients (26). The present study further investigated
the role of RAB6C in a retrospective study based on clinical data
with patients randomized to be treated with endocrine therapy
(tamoxifen) or included in the control group. Among patients with
ER+/PR- tumors, tamoxifen treatment showed
different effects depending on RAB6C expression, and patients with
low RAB6C expression did benefit from tamoxifen. For patients with
ER+/PR+ tumors, there was no statistically
significant difference in treatment effect on RAB6C+ or
RAB6C- disease.
The relevance of PR for tamoxifen benefit has been
discussed in various studies (3,12-16).
Several of them concluded that patients with
ER+/PR+ tumors did benefit from tamoxifen
treatment, whilst patients with ER+/PR-
tumors benefit less. There is a need to identify patients with
ER+/PR- tumors that do benefit from
tamoxifen. The results of the present study confirmed that patients
with ER+/PR+ tumors benefit from tamoxifen,
whereas for those with ER+/PR- tumors, the
effect depends on RAB6C status. Potential physical interactions
between RAB6C and the hormone receptors need to be evaluated in
further studies. A number of samples from the original cohort was
missing on the TMAs. However, in a previous study, the results
showed no bias in the missing cases with respect to tumor size, ER
status or tamoxifen treatment (17).
The knowledge of RAB6C is limited, although it is
known to be a member of the RAB6 family and consists of a single
exon. RAB6C shares 97% identity with the RAB6A' transcript,
leading to the hypothesis that RAB6C was generated by
retrotransposition of a fully processed RAB6A' mRNA, and
encodes a functional protein different from that coded by RAB6A'
(18). RAB6C is most highly
expressed in brain, prostate, testis, breast and cervical tissues,
and appears to participate not only in breast cancer (18,19). In
cervical cancer, RAB6C promoter methylation analysis has
been shown to have high sensitivity and specificity in
distinguishing between malignant, premalignant and normal tissue,
while in squamous cell carcinoma of the tongue, patients with low
RAB6C expression levels had poorer survival times than those
with high RAB6C expression (20,21).
This is also in line with our study on the prognostic value of
RAB6C in breast cancer (26).
Furthermore, the long non-protein coding RAB6C antisense RNA 1
(RAB6C-AS1) is frequently overexpressed in gastric and breast
cancer, and often deleted in prostate and pancreatic cancer and in
brain tumors. Its gene is located in the same chromosomal region as
RAB6C and both genes are often co-expressed (22). Results from previous experimental
studies on breast cancer cell lines indicated that RAB6C has
properties as a tumor suppressor with the capability to inhibit
proliferation, invasion and metastasis, and promote apoptosis
(4,18). It has also been suggested that
RAB6C may increase sensitivity to various drugs (23-25).
The present study investigated the predictive value of RAB6C in
relation to tamoxifen therapy with data from a randomized clinical
study. For untreated control patients with
ER+/PR- tumors, those with tumors expressing
low RAB6C levels had a higher recurrence rate than untreated
patients with tumors expressing high RAB6C levels. A higher number
of events in the control group with low RAB6C expression increased
the probability of observing differences between treated and
untreated patients. Therefore, the data showing a difference in
benefit from tamoxifen therapy in patients with
ER+/PR- tumors depending on the RAB6C
expression, should be interpreted with care. Additional data are
needed before clinical practice may be influenced. For patients
with ER+/PR- tumors, more tailored therapy is
required, and the results of the present study may contribute to
the identification of clinically relevant subgroups.
Supplementary Material
Table SI. Patient
characteristics.
Acknowledgements
The authors would like to thank Mrs. Ulla Johansson
(Regional Cancer Center of Stockholm Gotland, Stockholm, Sweden)
for updating the database and Mrs Birgitta Holmlund (Department of
Oncology, Linköping University Hospital, Linköping, Sweden) for
technical assistance. The authors would also like to thank Dr
Dennis Sgroi (Department of Pathology, Massachusetts General
Hospital, Boston, MA USA) for providing information on tumor
grading.
Funding
The present study was supported by grants from the
Swedish Cancer Society (grant no. 17-0479), The Cancer Research
Foundation of Radiumhemmet, Cancer Society in Stockholm and King
Gustav V Jubilee Clinical Research Foundation (grant no. 181093),
Onkologiska klinikerna i Linköpings forskningsfond (grant no.
2016-06-21), ALF grants Region Östergötland (grant no. LIO-795201)
and County Council of Östergötland (grant no. LIO-625491). The
funders did not have any role in the study design, collection,
analysis, interpretation of data, writing of the manuscript or the
decision to submit the manuscript for publication.
Availability of data and materials
The datasets used and/or analyzed during the present
study is available from the corresponding author on reasonable
request.
Authors' contributions
HF, JC and OS conceived the study and participated
in the study design and coordination. TF and BN provided the study
materials, collected clinical follow-up data and hormone receptor
data from patients and performed clinical interpretations. TB and
JS performed the laboratory experiments and scored RAB6C. HF
performed the statistical analysis and drafted the manuscript. HF,
JC, OS, TB, JS, BN and TF interpreted the results, provided
critical revision. All authors read and approved the final
manuscript.
Ethics approval and patient consent to
participate
The present study was performed in accordance with
the Declaration of Helsinki. Ethical approval for the use of tumor
material was approved by the local Ethical Committee at the
Karolinska University Hospital (Stockholm, Sweden) (approval no. KI
97-451 with amendments 030201 and 171027). According to the
approval, informed consent from the patients was not required.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sant M, Chirlaque Lopez MD, Agresti R,
Sánchez Pérez MJ, Holleczek B, Bielska-Lasota M, Dimitrova N, Innos
K, Katalinic A, Langseth H, et al: Survival of women with cancers
of breast and genital organs in Europe 1999-2007: Results of the
EUROCARE-5 study. Eur J Cancer. 51:2191–2205. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fredholm H, Magnusson K, Lindström LS,
Garmo H, Fält SE, Lindman H, Bergh J, Holmberg L, Pontén F, Frisell
J and Fredriksson I: Long-term outcome in young women with breast
cancer: A population-based study. Breast Cancer Res Treat.
160:131–143. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nordenskjöld A, Fohlin H, Fornander T,
Löfdahl B, Skoog L and Stål O: Progesterone receptor positivity is
a predictor of long-term benefit from adjuvant tamoxifen treatment
of estrogen receptor positive breast cancer. Breast Cancer Res
Treat. 160:313–322. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gan L, Zuo G, Wang T, Min J, Wang Y, Wang
Y and Lv G: Expression of WTH3 in breast cancer tissue and the
effects on the biological behavior of breast cancer cells. Exp Ther
Med. 10:154–158. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cancer Genome Atlas Network: Comprehensive
molecular portraits of human breast tumours. Nature 490: 61-70,
2012.
|
|
6
|
Haverty PM, Fridlyand J, Li L, Getz G,
Beroukhim R, Lohr S, Wu TD, Cavet G, Zhang Z and Chant J:
High-resolution genomic and expression analyses of copy number
alterations in breast tumors. Genes Chromosomes Cancer. 47:530–542.
2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tian K, Wang Y and Xu H: WTH3 is a direct
target of the p53 protein. Br J Cancer. 96:1579–1586.
2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rutqvist LE and Johansson H: Stockholm
Breast Cancer Study Group: Long-term follow-up of the randomized
Stockholm trial on adjuvant tamoxifen among postmenopausal patients
with early stage breast cancer. Acta Oncol. 46:133–145.
2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Khoshnoud MR, Löfdahl B, Fohlin H,
Fornander T, Stål O, Skoog L, Bergh J and Nordenskjöld B:
Immunohistochemistry compared to cytosol assays for determination
of estrogen receptor and prediction of the long-term effect of
adjuvant tamoxifen. Breast Cancer Res Treat. 126:421–430.
2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jerevall PL, Jansson A, Fornander T, Skoog
L, Nordenskjöld B and Stål O: Predictive relevance of HOXB13
protein expression for tamoxifen benefit in breast cancer. Breast
Cancer Res. 12(R53)2010.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Thakkar JP and Mehta DG: A review of an
unfavorable subset of breast cancer: Estrogen receptor positive
progesterone receptor negative. Oncologist. 16:276–285.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dowsett M, Houghton J, Iden C, Salter J,
Farndon J, A'Hern R, Sainsbury R and Baum M: Benefit from adjuvant
tamoxifen therapy in primary breast cancer patients according
oestrogen receptor, progesterone receptor, EGF receptor and HER2
status. Ann Oncol. 17:818–826. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bartlett JM, Brookes CL, Robson T, van de
Velde CJ, Billingham LJ, Campbell FM, Grant M, Hasenburg A, Hille
ET, Kay C, et al: Estrogen receptor and progesterone receptor as
predictive biomarkers of response to endocrine therapy: A
prospectively powered pathology study in the Tamoxifen and
Exemestane adjuvant multinational trial. J Clin Oncol.
29:1531–1538. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), Davies C, Godwin J, Gray R, Clarke M,
Cutter D, Darby S, McGale P, Pan HC, Taylor C, et al:
Relevance of breast cancer hormone receptors and other factors to
the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of
randomised trials. Lancet 378: 771-784, 2011.
|
|
15
|
Bardou VJ, Arpino G, Elledge RM, Osborne
CK and Clark GM: Progesterone receptor status significantly
improves outcome prediction over estrogen receptor status alone for
adjuvant endocrine therapy in two large breast cancer databases. J
Clin Oncol. 21:1973–1979. 2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Viale G, Regan MM, Maiorano E,
Mastropasqua MG, Dell'Orto P, Rasmussen BB, Raffoul J, Neven P,
Orosz Z, Braye S, et al: Prognostic and predictive value of
centrally reviewed expression of estrogen and progesterone
receptors in a randomized trial comparing letrozole and tamoxifen
adjuvant therapy for postmenopausal early breast cancer: BIG 1-98.
J Clin Oncol. 25:3846–3852. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bostner J, Skoog L, Fornander T,
Nordenskjöld B and Stål O: Estrogen receptor-alpha phosphorylation
at serine 305, nuclear p21-activated kinase 1 expression, and
response to tamoxifen in postmenopausal breast cancer. Clin Cancer
Res. 16:1624–1633. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Young J, Ménétrey J and Goud B: RAB6C is a
retrogene that encodes a centrosomal protein involved in cell cycle
progression. J Mol Biol. 397:69–88. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Uhlén M, Fagerberg L, Hallström BM,
Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C,
Sjöstedt E, Asplund A, et al: Proteomics. Tissue-based map of the
human proteome. Science. 347(1260419)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bhat S, Kabekkodu SP, Varghese VK,
Chakrabarty S, Mallya SP, Rotti H, Pandey D, Kushtagi P and
Satyamoorthy K: Aberrant gene-specific DNA methylation signature
analysis in cervical cancer. Tumour Biol.
39(1010428317694573)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bhat S, Kabekkodu SP, Jayaprakash C,
Radhakrishnan R, Ray S and Satyamoorthy K: Gene promoter-associated
CpG island hypermethylation in squamous cell carcinoma of the
tongue. Virchows Arch. 470:445–454. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Salavaty A, Motlagh FM, Barabadi M,
Cheshomi H, Esmatabadi MJD, Shahmoradi M and Soleimanpour-Lichaei
HR: Potential role of RAB6C-AS1 long noncoding RNA in different
cancers. J Cell Physiol. 234:891–903. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tian K, Wang Y, Huang Y, Sun B, Li Y and
Xu H: Methylation of WTH3, a possible drug resistant gene, inhibits
p53 regulated expression. BMC Cancer. 8(327)2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shan J, Mason JM, Yuan L, Barcia M, Porti
D, Calabro A, Budman D, Vinciguerra V and Xu H: Rab6c, a new member
of the rab gene family, is involved in drug resistance in MCF7/AdrR
cells. Gene. 257:67–75. 2000.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shan J, Yuan L, Budman DR and Xu HP: WTH3,
a new member of the Rab6 gene family, and multidrug resistance.
Biochim Biophys Acta. 1589:112–123. 2002.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fohlin H, Bekkhus T, Sandström J,
Fornander T, Nordenskjöld B, Carstensen J and Stål O: RAB6C is an
independent prognostic factor of estrogen
receptor-positive/progesterone receptor-negative breast cancer.
Oncol Lett. 19:52–60. 2020.PubMed/NCBI View Article : Google Scholar
|