Introduction
Allogeneic hematopoietic stem cell transplantation
(HSCT) is a curative therapy for malignant and non-malignant
hematologic disorders. Haploidentical stem cell transplantation is
a treatment option for patients who do not have an HLA-matched
sibling or unrelated donor. The disadvantages of haploidentical
HSCT are high incidence of graft-versus-host disease (GVHD), graft
rejection, and delayed or incomplete immune reconstitution.
Currently, the source of stem cells includes bone marrow,
granulocyte colony-stimulating factor mobilized peripheral blood
stem cells and umbilical cord blood. Different compositions of the
grafts have different effects on the hematopoietic and immune
recovery, GVHD and overall survival (OS) (1). Mature donor CD3+ T cells in the graft
will recognize MHC molecules or peptides on the surface of host
cells and induce acute GVHD. However, depletion of T cells in the
graft results in graft failure, prolonged immunosuppression and
leukemia relapse, thus innovative strategies are needed to limit
the GVHD related pathological effects of donor alloreactive
CD3+ T cells while maintaining their graft versus
leukemia (GVL) effects. Impact of CD3+ T cells in the
grafts on the clinical outcomes in different type of HSCT is still
uncertain. It was reported that the incidence of the acute GVHD was
higher in the graft with high counts of CD3+ T cells in
HLA-matched HSCT, but it was also reported that high counts of
CD3+ T cells resulted in more intensive GVL without
producing more severe GVHD and resulted in better OS in
haploidentical bone marrow combined with peripheral stem cells
transplantation (2,3). To date, there was rare report about the
impacts of CD3+ T cells in grafts on the hematopoietic
recovery, GVHD and OS in haplo-PBSCT. In the present study, we
investigate the correlations between the CD3+ T cells in
the graft with hematopoietic recovery, GVHD, disease relapse,
progress free survival (PFS) and OS in haplo-PBSCT.
Patients and methods
The study included 30 patients who underwent
haploidentical HSCT between January 2015 and December 2017 at The
First Affiliated Ηospital of Xi'an Jiaotong University (Xi'an,
China). Only acute leukemia or myelodysplastic syndrome patients
were eligible for the study. Patients and donors were 5/10
HLA-mismatched using high-resolution typing at HLA-A/B/C/DRB1/
DQB1. All patients underwent haplo-PBSCT with the modified BuCy2
myeloablative conditioning regimen. All patients received ATG 2.5
mg/kg for continuous 4 days for GVHD prophylaxis, and cyclosporin,
short-term methotrexate and mycophenolate mofetil were also
included for GVHD prophylaxis.
This study was approved by the Ethics Comittee of
the First Affiliated Hospital of Xi'an Jiaotong University. All of
patients and donors, or their legal guardians, provided written
informed consent in accordance with the Declaration of
Helsiniki.
Mobilization and stem cell
collection
For donor stem cell mobilization, 10 µg/kg/day G-CSF
for consecutive four days was given to healthy donors by
subcutaneous injection and then stem cell collection was carried
out.
Graft content
The number of total nucleated, CD34+ and
CD3+ cells in the donor graft were assessed before stem
cell infusion. CD34+ and CD3+ cells were
calculated by flow cytometer, data were acquired and analyzed by
Flowjo 10 software (Tree Star Inc., Ashland, OR, USA).
Hematopoietic engraftment
Neutrophil, platelet and lymphocyte engraftment were
defined as the first three consecutive days with an absolute count
of >0.5, >20, >1x109/l respectively.
GVHD diagnosis and treatment
Acute and chronic GVHD was diagnosed according to
the Seattle criteria (4). All
patients with grade II or above acute GVHD were treated with 1 to 2
mg/kg/day methylprednisolone and chronic GVHD was initial treated
with 1 mg/kg/day prednisone.
Cytomegalovirus (CMV) infection and
CMV reactivation prophylaxis
Prophylaxis of CMV infection was instructed as
previous study and CMV reactivation was defined as detection of
CMV-DNA positive twice by PCR in serum.
Relapse, PFS and OS
Relapse was recorded as disease recurrence. Progress
free survival (PFS) was from the day of transplantation to the
disease relapse. Overall survival (OS) was from the disease onset
to death or last follow-up.
Statistical analysis
Chi-square test was used to compare categorical
variables and the Mann-Whitney rank-sum test was used to compare
the absolute cell counts. Hematopoietic recovery, GVHD, CMV
reactivation and relapse were assessed using cumulative incidence
with competing risk. OS and PFS were obtained using Kaplan-Meier
and compared by log-rank test. Multivariate analysis was performed
using the Logistic hazards. P<0.05 was considered statistically
significant. All analyses were performed using SPSS 13.0 (SPSS,
Inc.) software.
Results
Graft content
The median mononuclear cells and CD34+
cells transplanted was 10.9x108/kg (range 8.04-15.19)
and 7.2x106/kg (range 2.14-17.43) respectively.
CD3+ T cells accounted for a median of 23.1% (range
8-47.4%) with a median dose of 299.7x106/kg (range
104-623.4). There was no significant difference of CD3+
T cells proportion and counts between the first and the second day
harvest (P>0.05). There was no significant difference of
CD3+ T cell in the grafts between donors with different
sex and age (P>0.05). To test whether the CD3+ T cell
count in donor graft was associated with the hematopoietic
reconstitution, GVHD, PFS and OS. The recipients were divided into
two groups according to the CD3+ T cell count: Above the
median (high T cell group) and below the median CD3+ T
cell (low T cell group). The baseline characteristics of two groups
were comparable (Table I).
 | Table IBaseline characteristics of the two
study groups. |
Table I
Baseline characteristics of the two
study groups.
| Transplant
variables | Low CD3+ T cells
(n=15) | High CD3+ T cells
(n=15) | P-value |
|---|
| Recipient | | | |
|
Age (years),
median (range) | 33 (10-52) | 32 (14-55) | >0.05 |
|
CMV
serology, neg/pos | 1/14 | 2/13 | >0.05 |
|
Disease,
acute leuk/other | 14/1 | 12/3 | >0.05 |
|
Disease
status, early/other | 10/5 | 11/4 | >0.05 |
| Donor | | | |
|
Age (years),
median (range) | 28 (17-50) | 32 (22-47) | >0.05 |
|
CMV
serology, neg/pos | 0/15 | 1/15 | >0.05 |
| Recipient/donor | | | |
|
Sex (R:D),
M:F/other | 4/11 | 2/13 | >0.05 |
|
ABO
mismatch, minor/major | 1/0 | 1/1 | >0.05 |
| Transplant | | | |
|
Conditioning,
MAC/RIC | 14/1 | 12/3 | >0.05 |
|
ATG,
no/yes | 0/15 | 0/15 | >0.05 |
| PBSC graft | | | |
|
TNC
(x108/kg), median (range) | 358.53
(150.67-454.93) | 430.5
(260.9-619.87) | >0.05 |
|
CD34+
(x106/kg), median (range) | 9.17
(5.01-17.43) | 7.16
(2.14-10.91) | >0.05 |
|
CD3+
(x106/kg), median (range) | 244.7
(170.2-299.7) | 366.6
(326.2-623.4) | >0.05 |
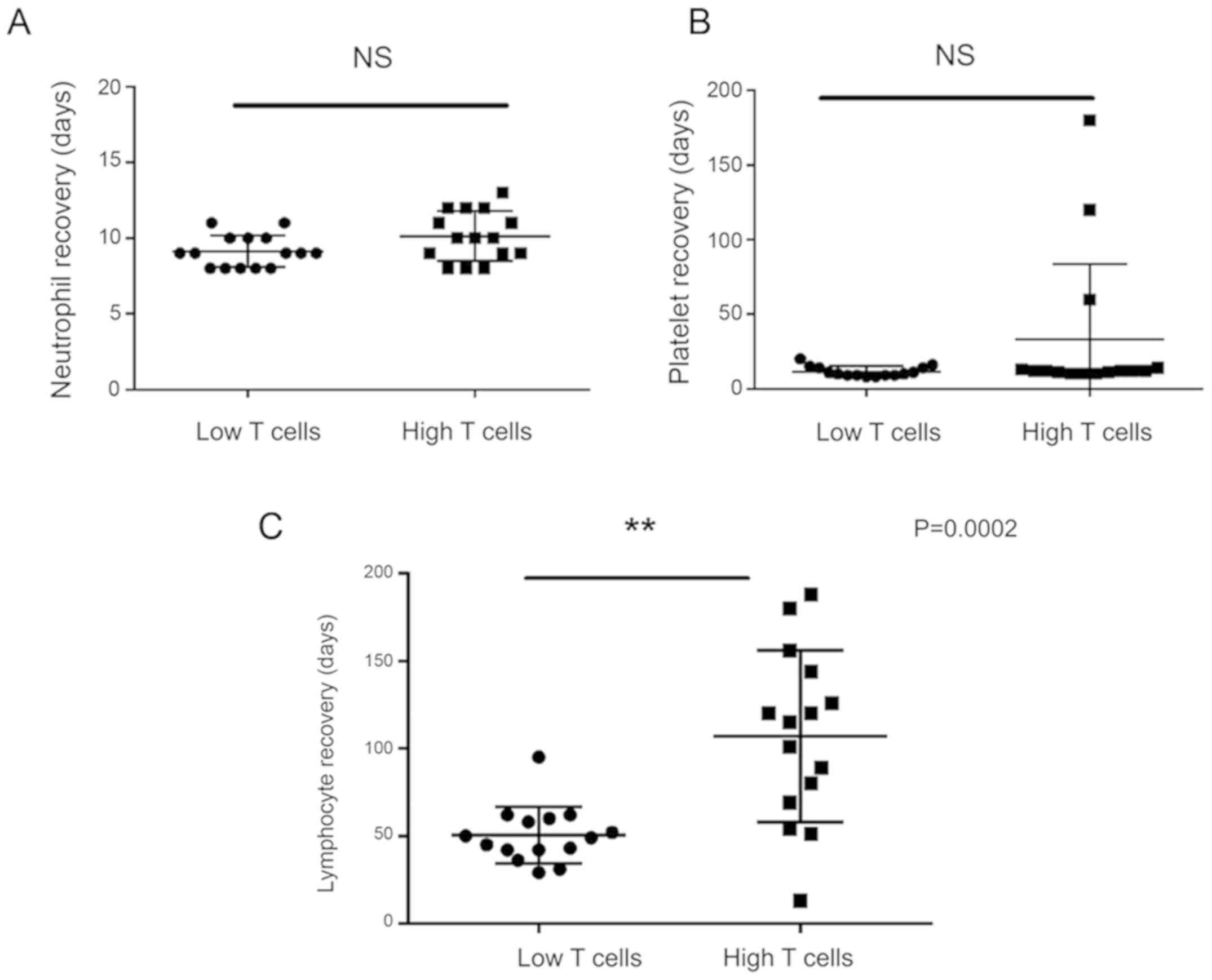
Neutrophil, platelet and lymphocyte
engraftment
The mean time to neutrophil engraftment was 10.13
days (95% CI 9.22-11.04) in high T cell group, and was 9.13 days
(95% CI 8.55-9.72) in low T cell group. The mean time to platelet
engraftment was 33.27 days (95% CI 5.41-61.12) in high T cell
group, and was 11.53 days (95% CI 9.59-13.47) in low T cell group.
The mean time to lymphocyte recovery was 107.07 days (95% CI
79.88-134.25) in high T cell group, and was 50.4 days (95% CI
41.42-59.38) in low T cell group. There was no significant
difference of neutrophil and platelet recovery time between two
groups (P>0.05). The lymphocyte recovery time in high T cell
group was longer than that in low T cell group (P=0.0002) (Fig. 1).
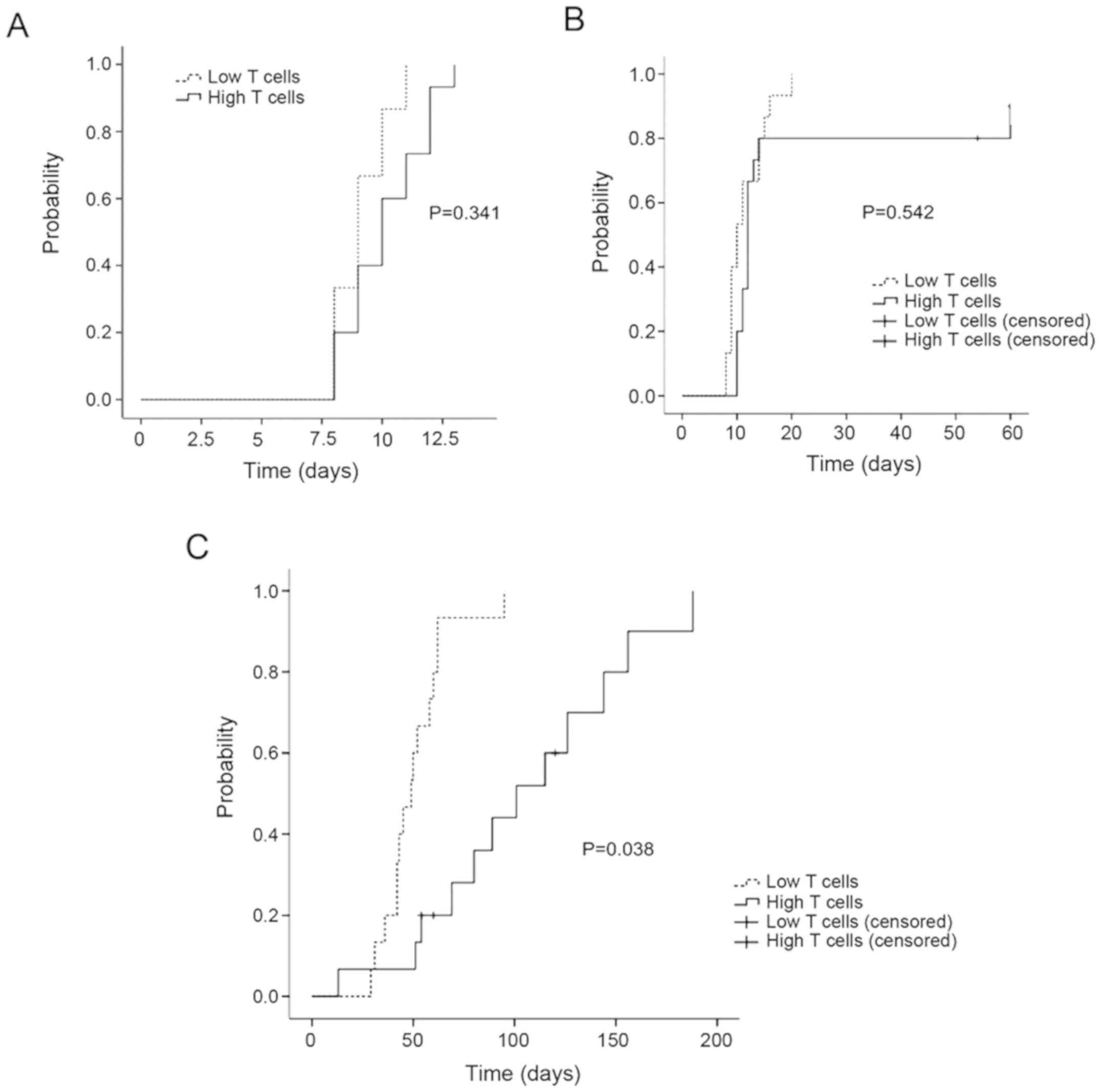
The cumulative incidence of sustained neutrophil
recovery (day 12) was 100% in low T groups and was 83.3% in high T
groups. The cumulative incidence of sustained platelet recovery
(day 15) was 100% in low T groups and was 90% in high T groups.
There was no significant difference of sustained neutrophil and
platelet recovery between two groups (P>0.05). Patients
receiving high T cell in grafts had a higher cumulative incidence
of sustained lymphocyte recovery (3 months) compared with low T
cells in grafts (100 vs. 44%) (P<0.05) (Fig. 2).
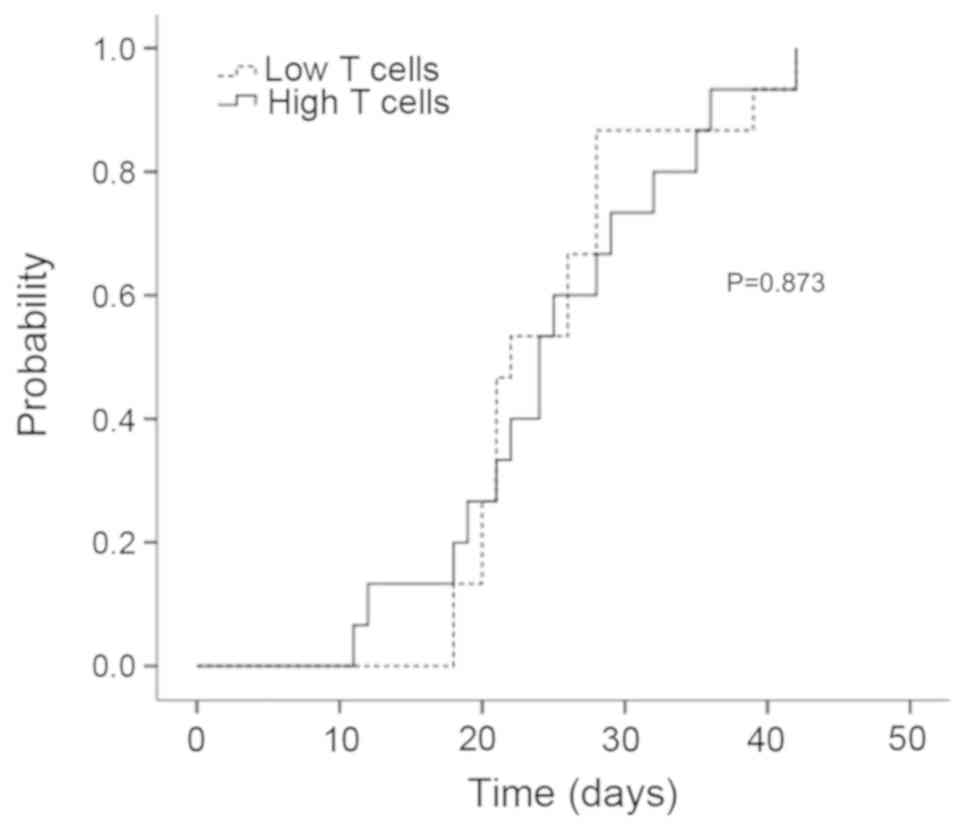
CMV reactivation
All patients had CMV reactivation at a median of 28
days (range, 14-42) (Fig. 3). In
multivariate analysis, donor sex and age as well as the CMV
seropositive status of the recipient and donor was not associated
with the CMV reactivation.
GVHD
The cumulative incidence of grade II or above acute
GVHD was higher in high T groups than that in low T groups
(P<0.05). There was no significant difference of the cumulative
incidence of chronic GVHD between two groups (P>0.05) (Fig. 4).
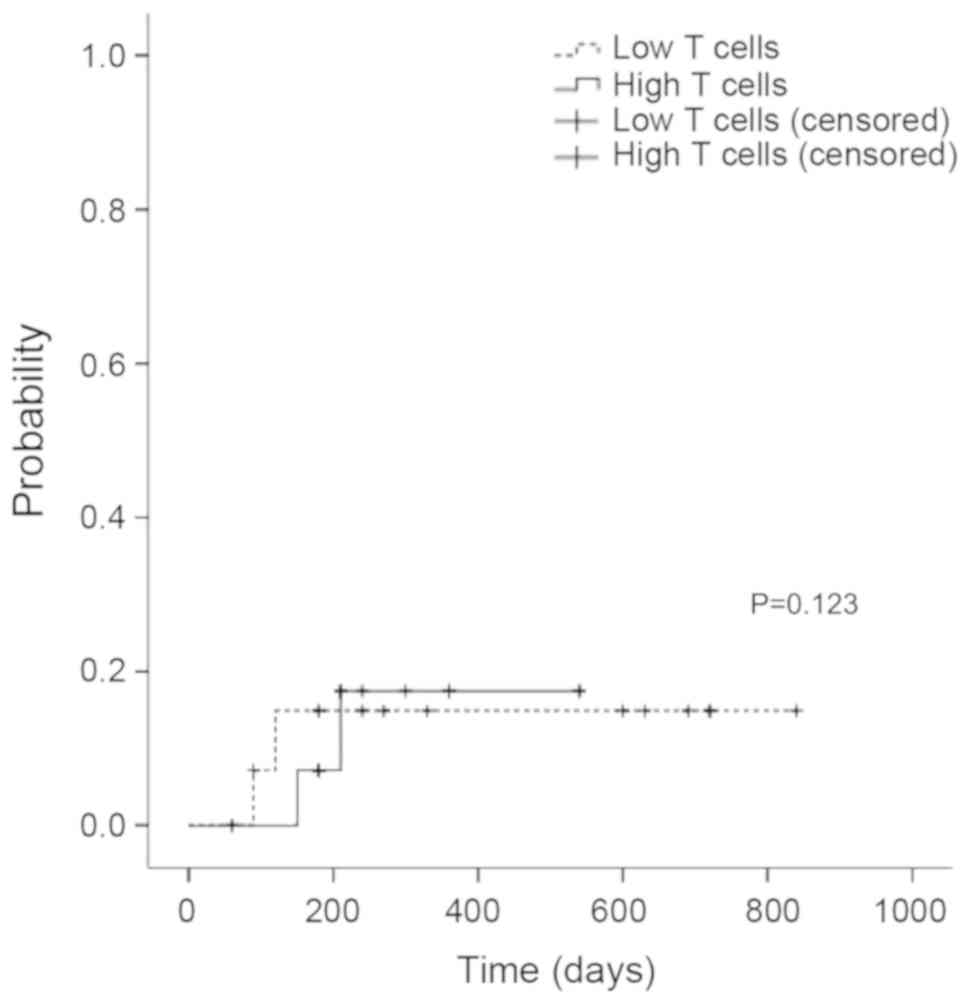
Disease relapse and TRM
Six patients relapsed, the cumulative incidence of
relapse at 1 year was 15% in low T cell group and 17.5% in high T
cell group, there was no significant difference of disease relapse
rate between two groups (P>0.05) (Fig. 5). Nine patients died, including five
died of primary disease relapse, two died of pulmonary infection,
and two died of refractory acute GVHD.
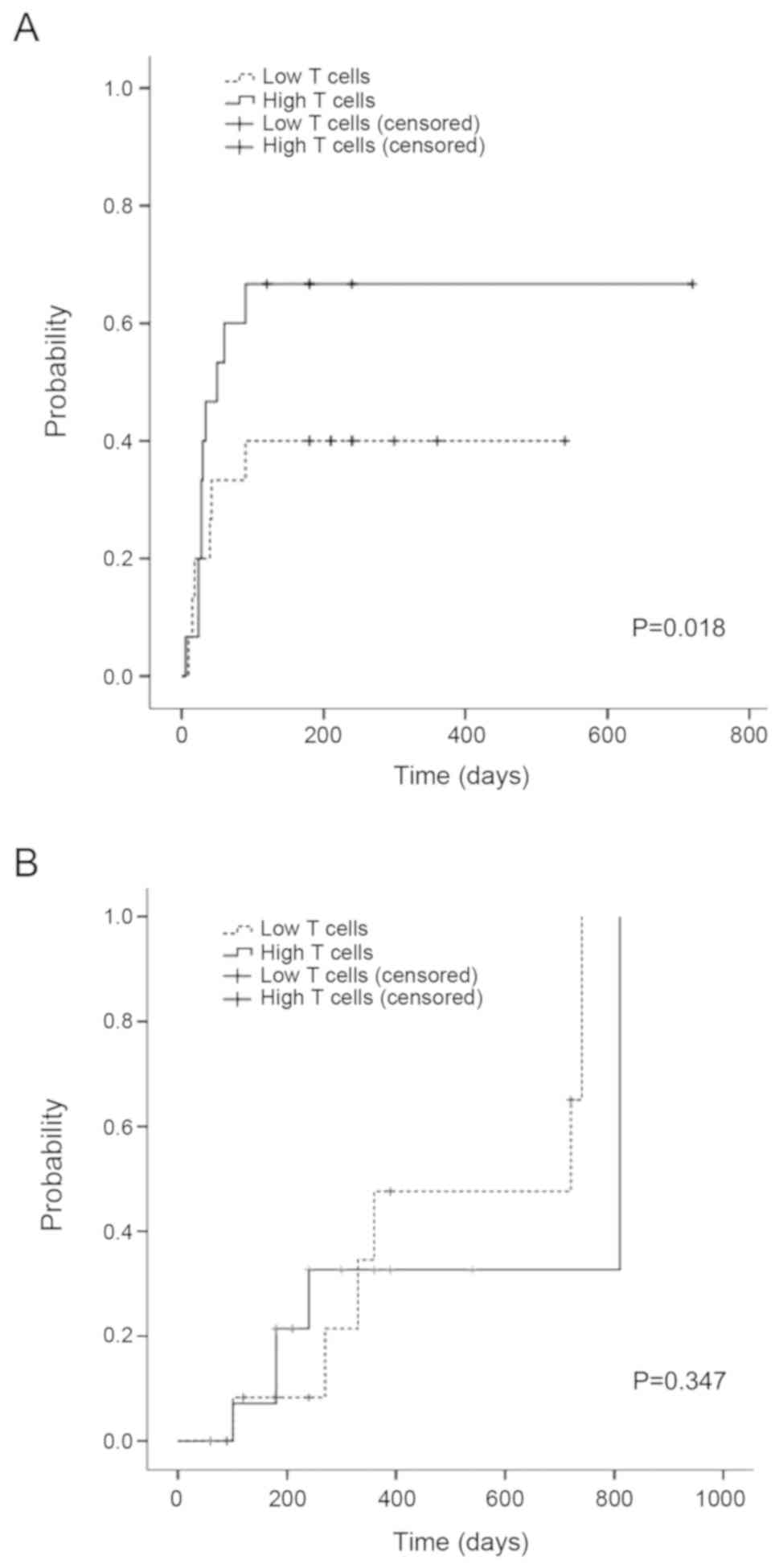
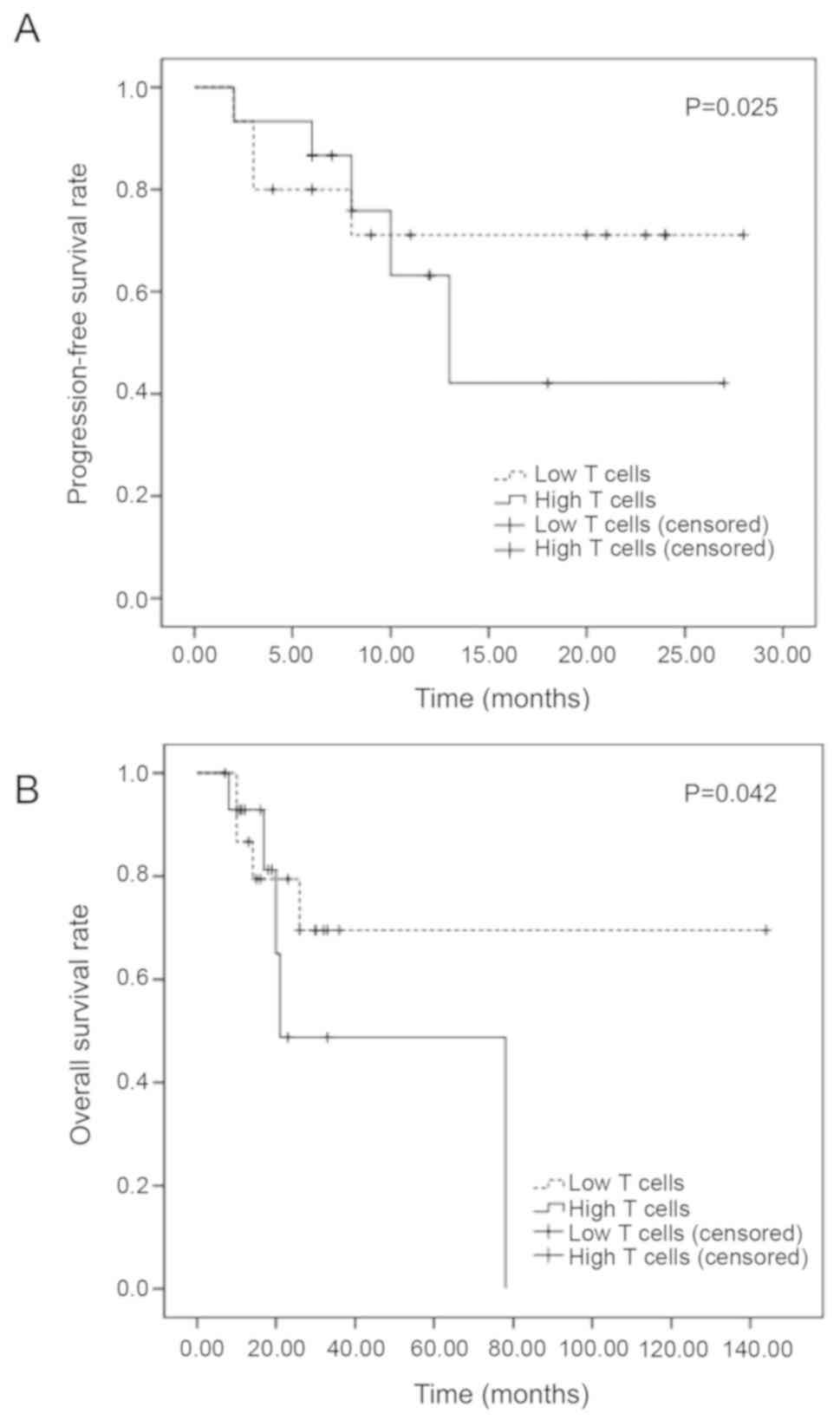
Progress free and overall
survival
The 1-year PFS was 71.1% in low T cell and 63.2% in
high T cell group. The 2 years PFS was 71.1% in low T cell and
42.1% in high T cell group. There was significant difference of the
one and two years of PFS between two groups (P=0.025). The
estimated OS at 1 year was 86.7% in low T cell and 92.9% in high T
cell group. The estimated OS at 3 years was 69.5% in low T cell and
48.8% in high T cell group. There was significant difference of OS
between two groups (P=0.042) (Fig.
6).
Discussion
HSCT remains the curative treatment for patients
with non-malignant and malignant hematological disorders. HSCT from
HLA-mismatched haploidentical donors was an option for patients
lacking HLA-matched donors. A major obstacle to haploidentical HSCT
was high rates of graft rejection and GVHD. Alloreactive
lymphocytes of the graft can mediate a potentially life-threatening
GVHD due to HLA dissimilarity (5-8).
In order to decrease the lethally acute GVHD induced by
alloreactive lymphocyte in the graft, various approaches to prevent
GVHD are being investigated, including in vitro T cell
depletion of bone marrow or peripheral blood stem cells (PBSCs) or,
more recently, in vivo T cell depletion approaches using
either granulocyte colony-stimulating factor (G-CSF)-mobilized bone
marrow in combination with PBSCs and anti-thymocyte globulin or the
administration of high-dose cyclophosphamide after transplantation
of haploidentical bone marrow-derived progenitor cells. These
trials in vitro or in vivo depletion of donor T cells
showed initially promising results by marked reduction of risk of
GVHD even without the use of post-transplant pharmacological GVHD
prophylaxis (9-13).
Therefore, however, this was associated with an increased risk of
disease relapse, impaired immune recovery and also an increased
incidence of graft failure was observed, in both matched and
unmatched donors, which suggesting that donor T cells in graft act
as a double-edged sword, it may help eradicate malignant clones and
counter balance the ability of residual recipient T cells
(surviving conditioning regimen) to reject the graft (14).
How to balance the function of donor T cells in the
graft and how many T cells in the graft is better for recipients,
it is still uncertain. One study found that more than
0.2x106/kg CD3+ T cells in the graft was an
important factor for sustained engraftment in CD34 positive
selected G-PBSC transplantation (1).
Several studies found that higher CD3+ T cell dose is independently
associated with more severe acute GVHD in HLA matched HSCT
(15-18),
but Kałwak et al (3) reported
that pediatric patients received ≥4x108 CD3+
T cells/kg had a better overall survival, without an increased risk
of severe GVHD in HLA matched HSCT. In haploidentical stem cell
transplantation, Pastore et al (19) found that patients with doses of CD3+
T cells above the median 177x106/kg in bone marrow combined with
peripheral blood stem cell grafts had a significantly better
overall survival without increase the acute GVHD. But rare study
was reported about the effects of CD3+ T cells in the
peripheral blood stem cell grafts only on the hematopoietic
reconstitution, GVHD and overall survival. In this study, we found
that high CD3+ T cells in peripheral blood stem cell
graft will increase the rate of acute GVHD, delay the lymphocyte
recovery and decrease the overall survival in haploidentical stem
cell transplantation.
Which subset of T cells was the keycells that
induces a GVHD. It is reported that low CD3+/Treg ratio in graft
will decrease the incidence of acute GVHD in HLA matched HSCT,
because Treg cells was able to mediate protective effects against
acute GVHD and to maintain an optimal microenviroment for the
reconstitution of functional immunity and will improved the
hematopoietic reconstitution and improve the overall survival of
the patients (20). In this regard,
Rezvani et al (21)
determined that increased frequencies of
CD4+Foxp3+ Treg cells in the peripheral blood
of the donor negatively correlated with the incidence of GVHD in
the graft recipient. Several subsequent studies confirmed this
correlation in recipients of HLA-identical sibling and unrelated
donor stem cell grafts indicating that hematopoietic stem cell
graft content appears to modulate GVHD severity (21). In a prospective study found that in
haploidentical HSCT patients with infusion of
>0.22x108
CD4+CD45RA+CD62L+ cells infused/kg
had an increased risk of grade II-IV acute GVHD and the risk of
chronic GVHD was increased in the patients receiving
>0.45x108CD4+CD45RA+ cells
infused/kg (22). It suggested that
allo-depletion of naïve CD4+ T cells contributes to
alleviating GVHD, especially in patients receiving haploidentical
allografts. Preclinical models showed that both CD4+ and CD8+ T
cells are capable of mediating lethal GVHD in HLA-incompatible
transplants. Mohty et al (22) reported that the CD8+ T cell dose
infused was the only parameter associated with the risk of a GVHD
(P=031; RR=1.96) after a multivariate analysis. But in
haploidentical HSCT, Chang et al (23) reported that researchers from China
found that patients with a higher CD4/CD8 ratio in the G-CSF
mobilized marrow harvests (≥1.16) had a survival disadvantage, a
significantly increased risk of aGVHD grades II-IV and a trend
towards relapse (23). In this
study, patients infused with higher CD3+ T cells had
higher incidence of acute GVHD, it may due to high CD3+
T cells contains more CD4+ and CD8+ T cells
which would induce acute GVHD. When patients had acute GVHD, the
immunosuppression drugs will be used and lymphocyte recovery
delayed which resulted in high incidence of infection and
non-relapse mortality.
This study has many limitations that cell subsets in
the graft cell is not detected and different subsets such as NK, B
and DC cells may also have different impacts on hematopoietic
engraftment, immune recovery and long-term outcome.
Overall, there are still controversial about
different effects of the CD3+ T cells in the grafts on
transplant outcomes, because patients with different conditioning
regimens, allografts origin, GVHD prophylaxis, and different
underlying disease may have different complications and outcomes of
HSCT. These variables should be considered to carefully assess the
CD3+ T graft content and tailor the cell dose infused in
order to reduce complications and improve the overall survival and
disease progress survival.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Nature Science Fund of China (grant no. 81600179) and the Natural
Science Basic Research of Shaanxi Province (grant no.
2019JM-564).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and XW designed the study and wrote the initial
draft of the manuscript. XW and PH contributed to refining the
figures. CG, CS, YC, JX, HZ and MZ contributed to the analysis and
interpretation of data. YC, JX and HZ verified the analytical
methods. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Comittee of
the First Affiliated Hospital of Xi'an Jiaotong University (Xi'an,
China). All of patients and donors, or their legal guardians,
provided written informed consent in accordance with the
Declaration of Helsiniki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gu G, Yang JZ and Sun LX: Correlation of
graft immune composition with outcomes after allogeneic stem cell
transplantation: Moving towards a perfect transplant. Cell Immunol.
323:1–8. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Urbano-Ispizua A, Rozman C, Pimentel P,
Solano C, de la Rubia J, Brunet S, Pérez-Oteiza J, Ferrá C, Zuazu
J, Caballero D, et al: The number of donor CD3(+) cells is the most
important factor for graft failure after allogeneic transplantation
of CD34(+) selected cells from peripheral blood from HLA-identical
siblings. Blood. 97:383–387. 2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kałwak K, Porwolik J, Mielcarek M,
Gorczyńska E, Owoc-Lempach J, Ussowicz M, Dyla A, Musiał J,
Paździor D, Turkiewicz D and Chybicka A: Higher CD34(+) and CD3(+)
cell doses in the graft promote long-term survival, and have no
impact on the incidence of severe acute or chronic
graft-versus-host disease after in vivo T cell-depleted unrelated
donor hematopoietic stem cell transplantation in children. Biol
Blood Marrow Transplant. 16:1388–1401. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sakiyama M, Kami M, Hori A, Imataki O,
Hamaki T, Murashige N, Kobayashi K, Kishi Y, Kojima R, Kim SW, et
al: Regimen-related toxicity following reduced-intensity stem-cell
transplantation (RIST): Comparison between seattle criteria and
national cancer center common toxicity criteria (NCI-CTC) version
2.0. Bone Marrow Transplant. 34:787–794. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Blazar BR, Murphy WJ and Abedi M: Advances
in graft-versus-host disease biology and therapy. Nat Rev Immunol.
12:443–458. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Kosugi-Kanaya M, Ueha S, Abe J, Shichino
S, Shand FHW, Morikawa T, Kurachi M, Shono Y, Sudo N, Yamashita A,
et al: Long-lasting graft-derived donor T cells contribute to the
pathogenesis of chronic graft-versus-host disease in mice. Front
Immunol. 8(1842)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Danby RD, Zhang W, Medd P, Littlewood TJ,
Peniket A, Rocha V and Roberts DJ: High proportions of regulatory T
cells in PBSC grafts predict improved survival after allogeneic
haematopoietic SCT. Bone Marrow Transplant. 51:110–118.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li Pira G, Malaspina D, Girolami E,
Biagini S, Cicchetti E, Conflitti G, Broglia M, Ceccarelli S,
Lazzaro S, Pagliara D, et al: Selective depletion of αβ T cells and
b cells for human leukocyte antigen-haploidentical hematopoietic
stem cell transplantation. A three-year follow-up of procedure
efficiency. Biol Blood Marrow Transplant. 22:2056–2064.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Oevermann L, Lang P, Feuchtinger T, Schumm
M, Teltschik HM, Schlegel P and Handgretinger R: Immune
reconstitution and strategies for rebuilding the immune system
after haploidentical stem cell transplantation. Ann N Y Acad Sci.
1266:161–170. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Oevermann L and Handgretinger R: New
strategies for haploidentical transplantation. Pediatric Res.
7:418–426. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Handgretinger R: New approaches to graft
engineering for haploidentical bone marrow transplantation. Semin
Oncol. 39:664–673. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vadakekolathu J and Rutella S: T-cell
manipulation strategies to prevent graft-versus-host disease in
haploidentical stem cell transplantation. Biomedicines. 5:
pii(E33)2012.
|
|
13
|
Prentice HG, Blacklock HA, Janossy G,
Gilmore MJ, Price-Jones L, Tidman N, Trejdosiewicz LK, Skeggs DB,
Panjwani D, Ball S, et al: Depletion of T lymphocytes in donor
marrow prevents significant graft-versus-host disease in matched
allogeneic leukaemic marrow transplant recipients. Lancet.
1:472–476. 1984.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Saad A and Lamb LS: Ex vivo T-cell
depletion in allogeneic hematopoietic stem cell transplant: past,
present and future. Bone Marrow Transplant. 52:1241–1248.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Urbano-Ispizua A, Rozman C, Pimentel P,
Solano C, de la Rubia J, Brunet S, Pérez-Oteyza J, Ferrá C, Zuazu
J, Caballero D, et al: Risk factors for acute graft-versus-host
disease in patients undergoing transplantation with CD34+ selected
blood cells from HLA-identical siblings. Blood. 100:724–727.
2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gaziev J, Isgrò A, Marziali M, Daniele N,
Gallucci C, Sodani P, Simone MD, Adorno G, Paciaroni K, Andreani M,
et al: Higher CD3(+) and CD34(+) cell doses in the graft increase
the incidence of acute GVHD in children receiving BMT for
thalassemia. Bone Marrow Transplant. 47:107–114. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Czerw T, Labopin M, Schmid C, Cornelissen
JJ, Chevallier P, Blaise D, Kuball J, Vigouroux S, Garban F, Lioure
B, et al: High CD3+ and CD34+ peripheral blood stem cell grafts
content is associated with increased risk of graft-versus-host
disease without beneficial effect on disease control after
reduced-intensity conditioning allogeneic transplantation from
matched unrelated donors for acute myeloid leukemia-an analysis
from the acute leukemia working party of the European society for
blood and marrow transplantation. Oncotarget. 7:27255–27266.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Anderson BE, Taylor PA, McNiff JM, Jain D,
Demetris AJ, Panoskaltsis-Mortari A, Ager A, Blazar BR, Shlomchik
WD and Shlomchik MJ: Effects of donor T-cell trafficking and
priming site on graft-versus-host disease induction by naive and
memory phenotype CD4 T cells. Blood. 111:5242–5251. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pastore D, Delia M, Mestice A, Carluccio
P, Perrone T, Gaudio F, Curci P, Rossi AR, Ricco A and Specchia G:
CD3+/Tregs ratio in donor grafts is linked to acute
graft-versus-host disease and immunologic recovery after allogeneic
peripheral blood stem cell transplantation. Biol Blood Marrow
Transplant. 18:887–893. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pabst C, Schirutschke H, Ehninger G,
Bornhäuser M and Platzbecker U: The graft content of donor T cells
expressing gamma delta TCR+ and CD4+foxp3+ predicts the risk of
acute graft versus host disease after transplantation of allogeneic
peripheral blood stem cells from unrelated donors. Clin Cancer Res.
13:2916–2922. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rezvani K, Mielke S, Ahmadzadeh M, Kilical
Y, Savani BN, Zeilah J, Keyvanfar K, Montero A, Hensel N, Kurlander
R and Barrett AJ: High donor FOXP3-positive regulatory T-cell
(Treg) content is associated with a low risk of GVHD following
HLA-matched allogeneic SCT. Blood. 108:1291–1297. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mohty M, Bagattini S, Chabannon C, Faucher
C, Bardou VJ, Bilger K, Vey N, Gaugler B, Stoppa AM, Coso D, et al:
CD8+ T cell dose affects development of acute graft-vs-host disease
following reduced-intensity conditioning allogeneic peripheral
blood stem cell transplantation. Exp Hematol. 32:1097–1102.
2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chang YJ, Zhao XY, Huo MR and Huang XJ:
Expression of CD62L on donor CD4(+) T cells in allografts:
Correlation with graft-versus-host disease after unmanipulated
allogeneic blood and marrow transplantation. J Clin Immunol.
29:696–704. 2009.PubMed/NCBI View Article : Google Scholar
|