Introduction
Mucosal melanoma is subtype of melanoma which arise
from melanocytes in mucosal membranes. The major primary sites of
mucosal melanoma are head and neck, anorectal, and female genital
tract (1). Mucosal melanoma is
typically diagnosed at a more advanced stage compared to cutaneous
melanoma and generally carry a worse prognosis. Genome sequencing
of mucosal melanomas revealed that they are not markedly enriched
in ultraviolet signature mutations, different from cutaneous
melanoma (2).
Anorectal melanoma is a rare disease, accounting for
only 1% of all anorectal malignancies, 1% of all melanomas and 18%
of mucosal melanomas (3-5).
The 5-year survival rate of anorectal melanoma was reported to be
<20% in the era before immunotherapy (4,6), with
this poor prognosis being related to early disease dissemination
and a delay in diagnosis (7,8). Clinically, it is sometimes misdiagnosed
as hemorrhoids due to rectal bleeding. Furthermore, about 30% of
anorectal melanomas are amelanotic and pathological diagnosis is
difficult in those cases (9).
The only potentially curative option for mucosal
melanoma is complete surgical resection with negative margins.
Radiotherapy after surgery may be a treatment option, but there it
has not been evaluated in prospective study (10). Given the limited treatment options
available for individuals with metastatic mucosal melanoma, new
therapies are urgently needed to improve prognosis. Immune
checkpoint inhibitors (ICIs)-including the combination of nivolumab
and ipilimumab-have recently become the standard treatment option
for unresectable or metastatic melanoma regardless of tumor
subtype, although treatment data for mucosal melanoma are limited
compared with those for cutaneous melanoma (11-13).
Here we report a case of advanced anorectal melanoma that
progressed during nivolumab monotherapy but subsequently showed a
durable response to ipilimumab.
Case presentation
A 60-year-old woman with a history of hemorrhoids
for >30 years visited her local clinic with a complaint of
hemorrhoid enlargement that had persisted for >1 year. Blood
analysis revealed anemia, with a hemoglobin level of 6.5 g/dl. She
underwent a hemorrhoidectomy, and the resected tissue was submitted
for pathological examination because it had an atypical appearance.
The pathological findings were suggestive of primary malignant
melanoma. She was referred to our hospital 2 months after the
surgery. Her Eastern Cooperative Oncology Group performance status
was 0. Digital examination and anoscopy did not detect any residual
tumor. Chest and abdominal computed tomography (CT) as well as
positron emission tomography-CT also did not reveal residual tumor
tissue or distant metastasis. Blood analysis, including the level
of lactate dehydrogenase, showed no abnormalities. Histopathologic
reevaluation revealed that atypical cells with enlarged nuclei were
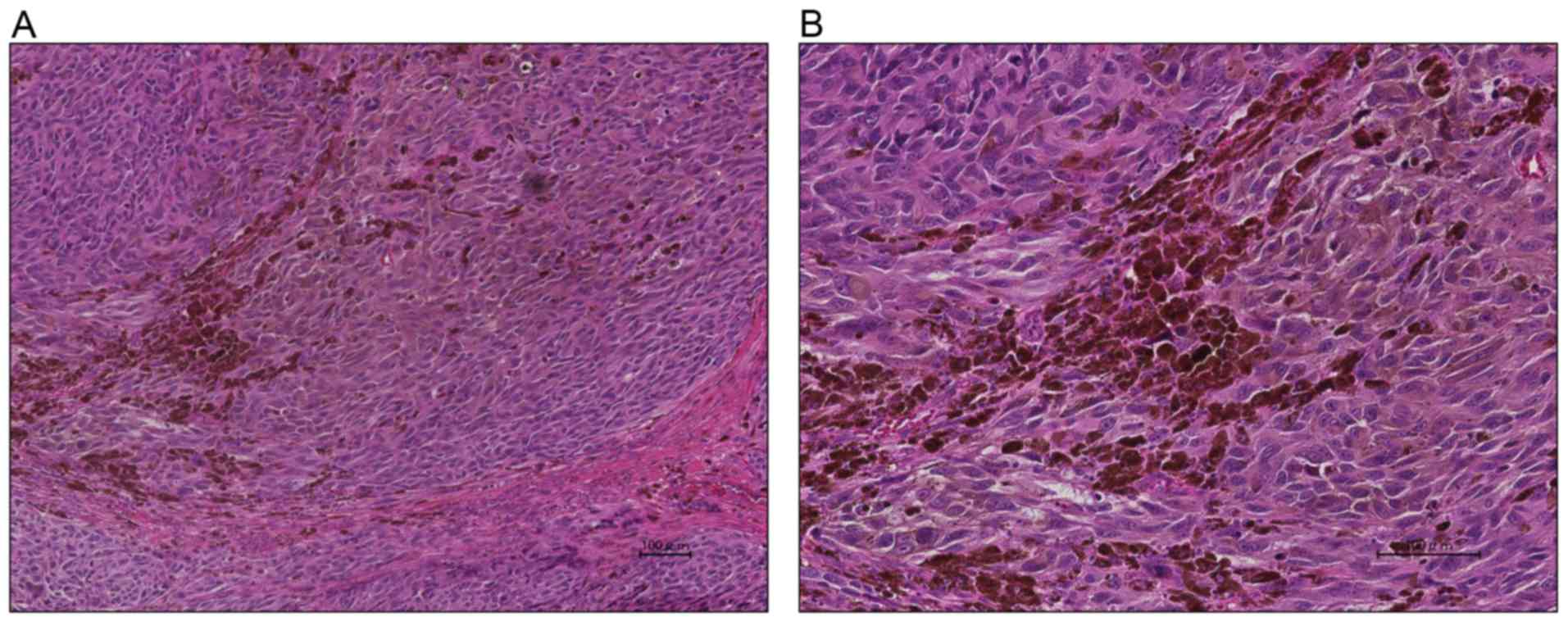
gathered in the stroma with a solid or alveolar pattern (Fig. 1) as well as the presence of brown
pigment granules in the tumor. Immunostaining showed the tumor to
be partly positive for vimentin, S-100, HBM-45, Melan A, and D2-40
as well as negative for cytokeratin, AE1/AE3, CD56, and
chromogranin A. These findings were thus consistent with melanoma.
No lymphatic invasion was evident by staining for the D2-40
lymphatic marker, and no vascular invasion was detected by CD31
immunostaining. The tumor proportion score (TPS) for programmed
cell death-ligand-1 (PD-L1) was <1% with PD-L1 antibody clone
28-8. The tumor was also negative for the V600E mutation of
BRAF. Next-generation sequencing analysis with the
FoundationOne CDx panel, which detects mutations in 324 genes,
select gene rearrangements, and genomic signatures including
microsatellite instability and tumor mutational burden, revealed
the tumor to be microsatellite stable (MSS) and to have a tumor
mutation burden (TMB) of 8 mutations/Mb, a deletion of exons 28 to
37 of NF1, an S37Y mutation of CTNNB1, and an R625H
mutation of SF3B1. These findings supported a diagnosis of
BRAF mutation-negative anorectal melanoma. Given the
operation report and that hematoxylin-eosin staining of the excised
tissue confirmed a surgical margin of 10 mm, no additional surgery
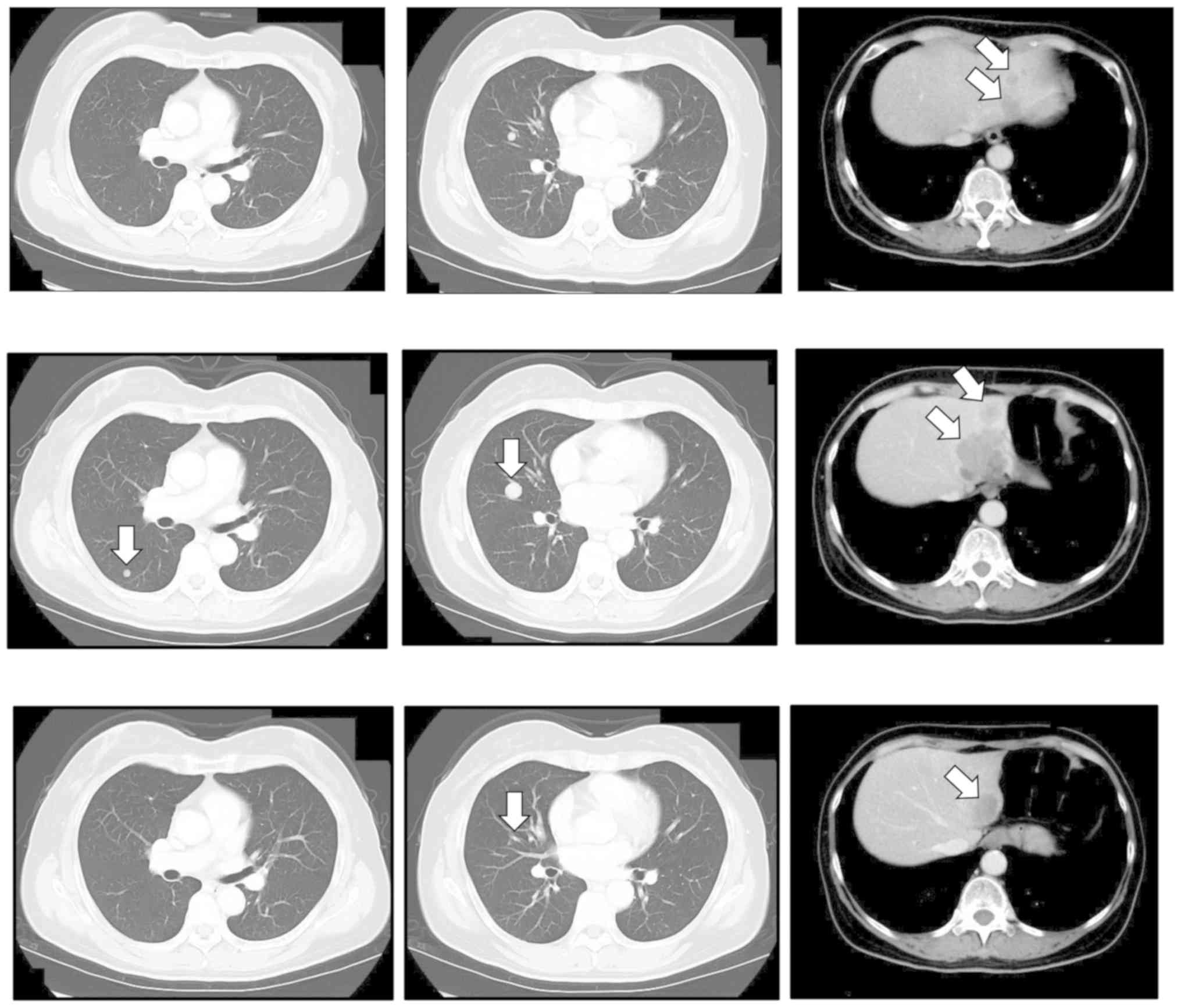
was performed. Six months after her first visit to our hospital, CT
revealed that the patient had developed multiple lung and liver
metastases (Fig. 2). Nivolumab
monotherapy (3 mg every 2 weeks) was initiated. After six treatment
cycles, chest and abdominal CT showed progression of liver
metastasis (Fig. 2). Ipilimumab
monotherapy (3 mg/kg every 3 weeks) was then started. After four
cycles, the maximum number allowed, chest and abdominal CT
confirmed a partial response. The treatment was well tolerated,
with no immune-related adverse events. The response has been
persisted over 32 months at the time of this writing (november
2019) (Fig. 2). The
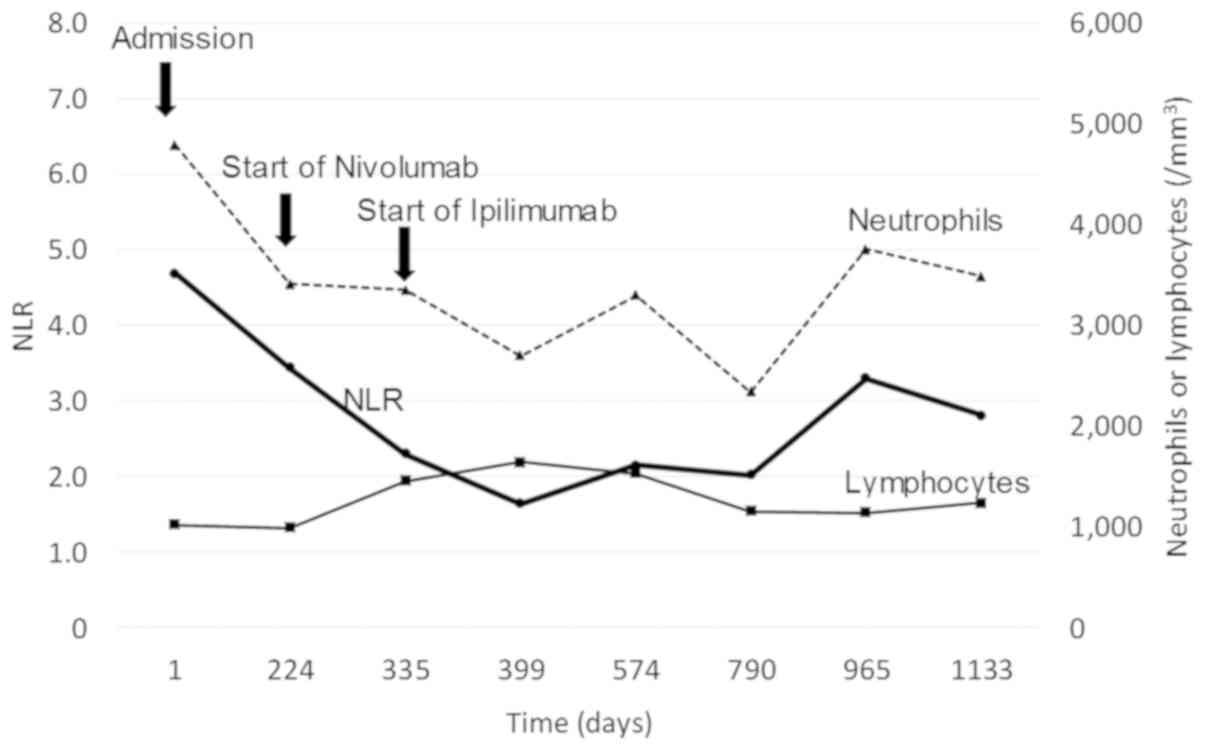
neutrophil-to-lymphocyte ratio (NLR) in peripheral blood has
remained <5 throughout the disease course (Fig. 3).
Discussion
We here report a case of anorectal melanoma that has
shown a long-lasting response to ipilimumab after failure of
nivolumab treatment. The tumor was found to be MSS, to have an
intermediate TMB, and to be negative for PD-L1 expression. Given
that the combination of nivolumab and ipilimumab had not been
approved for melanoma in Japan at the time the patient presented at
our hospital, we initiated nivolumab monotherapy followed by
ipilimumab monotherapy. A pooled analysis of patients with
cutaneous (n=665) or mucosal (n=86) melanoma revealed
a longer progression-free survival (PFS) and higher objective
response rate for nivolumab in combination with ipilimumab than for
nivolumab monotherapy (11). Among
patients who received the combination therapy, the median PFS was
5.9 months for mucosal melanoma and 11.7 months for cutaneous
melanoma, with objective response rates of 37.1 and 60.4%,
respectively. One reason for the poorer response of mucosal
melanoma may be a lower TMB. The TMB, the total number of somatic
mutations in a defined region of a tumor genome, is currently the
most reliable predictive marker for ICI treatment in melanoma
(14,15). Two studies have shown that TMB as
determined by whole-exome sequencing is related to the clinical
benefit rate for ipilimumab monotherapy in melanoma (16,17). A
study based on next-generation sequencing with FoundationOne CDx
for patients with advanced melanoma revealed the TMB to be high
(>23.1 mutations/Mb), intermediate (3.3-23.1 mutations/Mb), or
low (<3.3 mutations/Mb) in 27 (41.5%), 24 (36.9%), and 14
(21.5%) patients, respectively, with the TMB correlating with
benefit from therapy targeted to the programmed cell death-1
(PD-1)-PD-L1 checkpoint (18).
Furthermore, TMB in mucosal melanoma was found to be markedly lower
than that in cutaneous melanoma, likely because of the contribution
of ultraviolet-induced mutagenesis to cutaneous melanoma (2,19,20).
PD-L1 expression has also been investigated as a
potential biomarker for ICI therapy in melanoma. PD-L1 expression
on tumor cells did not tend to be related to the response rate in
melanoma patients treated with the combination of nivolumab and
ipilimumab (21). As far as we are
aware, the relation between PD-L1 expression and response to
ipilimumab monotherapy has not been examined. With regard to PD-L1
positivity in mucosal melanoma, a small study found that, with a
TPS of ≥5% as the cutoff, the proportion of tumors positive for
PD-L1 was 44% (16/36), a value similar to that for cutaneous
melanoma at 35% (19/54) (22,23).
The present case was found to be MSS, with an
intermediate TMB, and negative for PD-L1 expression. These
characteristics of an ‘immunologically cold tumor’ would be
expected to confer a low sensitivity to ICIs. A recent report
showed that many mucosal melanoma cases had PD-L1 low/negative
disease, and average mutational load of 4 cases was much lower than
that of cutaneous melanoma (24).
Consistent with these findings, our tumor immune profile showed low
PD-L1 expression as well as low TMB; however, there is a
discrepancy the efficacy of ICI. The reason for the discrepancy
between the biological features of the tumor and the clinical
benefit conferred by ipilimumab in the current case is unclear.
However, a high NLR in peripheral blood has been shown to be
strongly associated with a poor outcome of ipilimumab treatment in
patients with advanced melanoma (25-28).
An NLR of ≥4 before initiation of ipilimumab treatment was thus
associated with a worse overall survival compared with a ratio of
<4 in patients with metastatic melanoma (25). An NLR of ≥5 at each time point
examined was also associated with a worse overall survival, PFS,
and response to ipilimumab treatment (26). In the present case, the NLR was <5
at baseline and remained so during and after ipilimumab treatment.
The potential of the NLR as a biomarker for ipilimumab treatment in
melanoma patients thus warrants further investigation.
In conclusion, the present case shows that
ipilimumab is a potentially effective treatment option for patients
with metastatic mucosal melanoma including anorectal melanoma, even
though mucosal melanoma in general has been found to have a less
favorable outcome during ipilimumab treatment compared with
cutaneous melanoma. Even if tumor histology, past treatment
history, and certain biomarkers suggest that a tumor is
immunologically cold, it might still respond to ICI treatment.
Evaluation of the NLR should be considered before excluding ICI
therapy as an option.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
HS, KS, KNa, KNi and MT conceived the current study.
HS drafted the current study HS, MT, KS, KNa and KNi acquired and
analyzed the data. HS, MT, KS, KNi and KNa wrote, reviewed and
edited the manuscript. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards. Informed consent was obtained from the patient.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the present case report and accompanying
images prior to receiving chemotherapy.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lian B, Cui CL, Zhou L, Song X, Zhang XS,
Wu D, Si L, Chi ZH, Sheng XN, Mao LL, et al: The natural history
and patterns of metastases from mucosal melanoma: An analysis of
706 prospectively-followed patients. Ann Oncol. 28:868–873.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Furney SJ, Turajlic S, Stamp G, Nohadani
M, Carlisle A, Thomas JM, Hayes A, Strauss D, Gore M, van den Oord
J, et al: Genome sequencing of mucosal melanomas reveals that they
are driven by distinct mechanisms from cutaneous melanoma. J
Pathol. 230:261–269. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
van Schaik PM, Ernst MF, Meijer HA and
Bosscha K: Melanoma of the rectum: A rare entity. World J
Gastroenterol. 14:1633–1635. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chang AE, Karnell LH and Menck HR: The
national cancer data base report on cutaneous and noncutaneous
melanoma: A summary of 84,836 cases from the past decade. The
American college of surgeons commission on cancer and the American
cancer society. Cancer. 83:1664–1678. 1998.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Haanen JBAG: Converting cold into hot
tumors by combining immunotherapies. Cell. 170:1055–1056.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Brady MS, Kavolius JP and Quan SH:
Anorectal melanoma. A 64-year experience at memorial
sloan-kettering cancer center. Dis Colon Rectum. 38:146–151.
1995.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Perez DR, Trakarnsanga A, Shia J, Nash GM,
Temple LK, Paty PB, Guillem JG, Garcia-Aguilar J, Bello D, Ariyan
C, et al: Locoregional lymphadenectomy in the surgical management
of anorectal melanoma. Ann Surg Oncol. 20:2339–2344.
2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hicks CW, Pappou EP, Magruder JT, Gazer B,
Fang S, Wick EC, Gearhart SL, Ahuja N and Efron JE:
Clinicopathologic presentation and natural history of anorectal
melanoma: A case series of 18 patients. JAMA Surg. 149:608–611.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hillenbrand A, Barth TF, Henne-Bruns D and
Formentini A: Anorectal amelanotic melanoma. Colorectal Dis.
10:612–615. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Malaguarnera G, Madeddu R, Catania VE,
Bertino G, Morelli L, Perrotta RE, Drago F, Malaguarnera M and
Latteri S: Anorectal mucosal melanoma. Oncotarget. 9:8785–8800.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
D'Angelo SP, Larkin J, Sosman JA, Lebbé C,
Brady B, Neyns B, Schmidt H, Hassel JC, Hodi FS, Lorigan P, et al:
Efficacy and safety of nivolumab alone or in combination with
ipilimumab in patients with mucosal melanoma: A pooled analysis. J
Clin Oncol. 35:226–235. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Del Vecchio M, Di Guardo L, Ascierto PA,
Grimaldi AM, Sileni VC, Pigozzo J, Ferraresi V, Nuzzo C, Rinaldi G,
Testori A, et al: Efficacy and safety of ipilimumab 3 mg/kg in
patients with pretreated, metastatic, mucosal melanoma. Eur J
Cancer. 50:121–127. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Postow MA, Luke JJ, Bluth MJ, Ramaiya N,
Panageas KS, Lawrence DP, Ibrahim N, Flaherty KT, Sullivan RJ, Ott
PA, et al: Ipilimumab for patients with advanced mucosal melanoma.
Oncologist. 18:726–732. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Büttner R, Longshore JW, López-Ríos F,
Merkelbach-Bruse S, Normanno N, Rouleau E and Penault-Llorca F:
Implementing TMB measurement in clinical practice: Considerations
on assay requirements. ESMO Open. 4(e000442)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hugo W, Zaretsky JM, Sun L, Song C, Moreno
BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G,
et al: Genomic and transcriptomic features of response to Anti-PD-1
therapy in metastatic melanoma. Cell. 165:35–44. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Van Allen EM, Miao D, Schilling B, Shukla
SA, Blank C, Zimmer L, Sucker A, Hillen U, Foppen MHG, Goldinger
SM, et al: Genomic correlates of response to CTLA-4 blockade in
metastatic melanoma. Science. 350:207–211. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Snyder A, Makarov V, Merghoub T, Yuan J,
Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et
al: Genetic basis for clinical response to CTLA-4 blockade in
melanoma. N Engl J Med. 371:2189–2199. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Johnson DB, Frampton GM, Rioth MJ, Yusko
E, Xu Y, Guo X, Ennis RC, Fabrizio D, Chalmers ZR, Greenbowe J, et
al: Targeted next generation sequencing identifies markers of
response to PD-1 blockade. Cancer Immunol Res. 4:959–967.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Berger MF, Hodis E, Heffernan TP, Deribe
YL, Lawrence MS, Protopopov A, Ivanova E, Watson IR, Nickerson E,
Ghosh P, et al: Melanoma genome sequencing reveals frequent PREX2
mutations. Nature. 485:502–506. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hayward NK, Wilmott JS, Waddell N,
Johansson PA, Field MA, Nones K, Patch AM, Kakavand H, Alexandrov
LB, Burke H, et al: Whole-genome landscapes of major melanoma
subtypes. Nature. 545:175–180. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Larkin J, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M,
Rutkowski P, et al: Combined nivolumab and ipilimumab or
monotherapy in untreated melanoma. N Engl J Med. 373:23–34.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kaunitz GJ, Cottrell TR, Lilo M, Muthappan
V, Esandrio J, Berry S, Xu H, Ogurtsova A, Anders RA, Fischer AH,
et al: Melanoma subtypes demonstrate distinct PD-L1 expression
profiles. Lab Invest. 97:1063–1071. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Taube JM, Anders RA, Young GD, Xu H,
Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL
and Chen L: Colocalization of inflammatory response with B7-h1
expression in human melanocytic lesions supports an adaptive
resistance mechanism of immune escape. Sci Transl Med.
4(127ra37)2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dodds TJ, Wilmott JS, Jackett LA, Lo SN,
Long GV, Thompson JF and Scolyer RA: Primary anorectal melanoma:
Clinical, immunohistology and DNA analysis of 43 cases. Pathology.
51:39–45. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zaragoza J, Caille A, Beneton N, Bens G,
Christiann F, Maillard H and Machet L: High neutrophil to
lymphocyte ratio measured before starting ipilimumab treatment is
associated with reduced overall survival in patients with melanoma.
Br J Dermatol. 174:146–151. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cassidy MR, Wolchok RE, Zheng J, Panageas
KS, Wolchok JD, Coit D, Postow MA and Ariyan C: Neutrophil to
lymphocyte ratio is associated with outcome during ipilimumab
treatment. EBioMedicine. 18:56–61. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ferrucci PF, Gandini S, Battaglia A,
Alfieri S, Di Giacomo AM, Giannarelli D, Cappellini GC, De Galitiis
F, Marchetti P, Amato G, et al: Baseline neutrophil-to-lymphocyte
ratio is associated with outcome of ipilimumab-treated metastatic
melanoma patients. Br J Cancer. 112:1904–1910. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ferrucci PF, Ascierto PA, Pigozzo J, Del
Vecchio M, Maio M, Antonini Cappellini GC, Guidoboni M, Queirolo P,
Savoia P, Mandalà M, et al: Baseline neutrophils and derived
neutrophil-to-lymphocyte ratio: Prognostic relevance in metastatic
melanoma patients receiving ipilimumab. Ann Oncol. 27:732–738.
2016.PubMed/NCBI View Article : Google Scholar
|