Introduction
The incidence rate of colorectal cancer ranks fourth
worldwide, with about 0.8 million new cases each year, accounting
for 10% of all cancer types (1).
Approximately 20% of patients present with advanced metastatic
disease at initial diagnosis (2).
Approximately 50-60% of colorectal cancer patients may develop
local or distant metastases, mostly in liver and lungs, during the
course of the disease, thus seriously threatening their life
(3,4). Nowadays, oncologists are committed to
ensuring improved survival rates and quality of life for patients.
Thus, several studies have been conducted in recent years. Previous
studies conducted on patients with locally advanced rectal cancer
who achieved complete clinical remission (CCR) following
preoperative neoadjuvant therapy, revealed no statistical
significant differences in 3-year survival and local recurrence
rates between observation waiting and direct surgery (5-7).
Furthermore, the quality of life of the observation waiting
patients was improved. Based on these observations, many
researchers hypothesize that the active observation waiting method
may be considered the appropriate treatment for patients with
locally advanced rectal cancer.
Currently, the effect of active observation waiting
in CCR in advanced rectal cancer has not been reported. In the
present case report, the patient was actively observed and waited
for nearly 1 year following CCR achievement via chemoradiotherapy.
No sign of tumor recurrence was observed and the patient's quality
of life was not affected. These observations provide novel aspects
of advanced rectal cancer management.
Case report
A 59-year-old male patient, with rectal bleeding and
an Eastern Cooperative Oncology Group (ECOG) performance status
score of 0, was first admitted to the hospital in March 2018, and
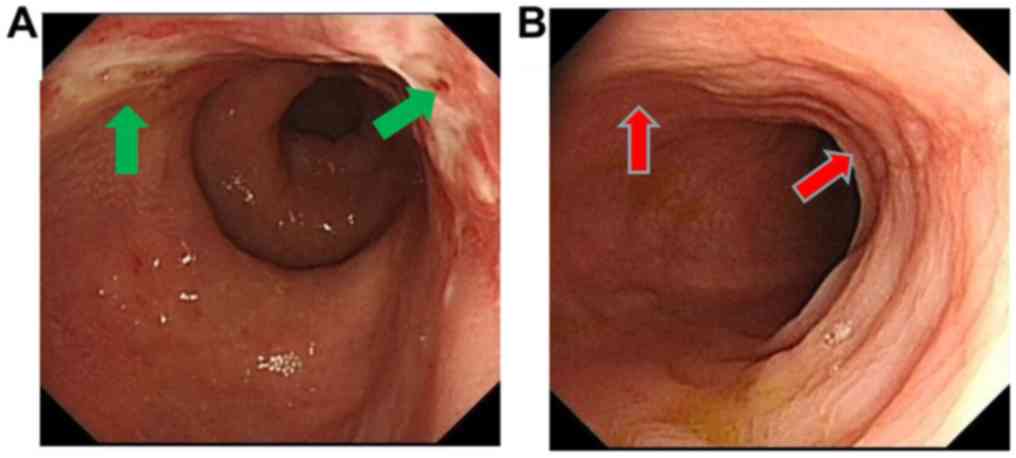
then followed up until May 2018. Electronic colonoscopy showed
multiple polyps in the colon and rectum (Fig. 1A), whereas pathological biopsy
indicated poorly differentiated rectal adenocarcinoma. The
screening for rectal cancer and the evaluation of T stage were
determined using positron emission tomography/computed tomography
(PET/CT) and pelvic magnetic resonance imaging (MRI), respectively.
The clinical stage was defined as T3N2M1a according to the TNM
classification of the American Joint Committee on Cancer (AJCC)
staging manual (version 8) (4). In
addition, the PET/CT scan showed non-regional lymph node metastasis
(subclavian). Finally, the expression of PD-L1 (-), NRAS (-), KRAS
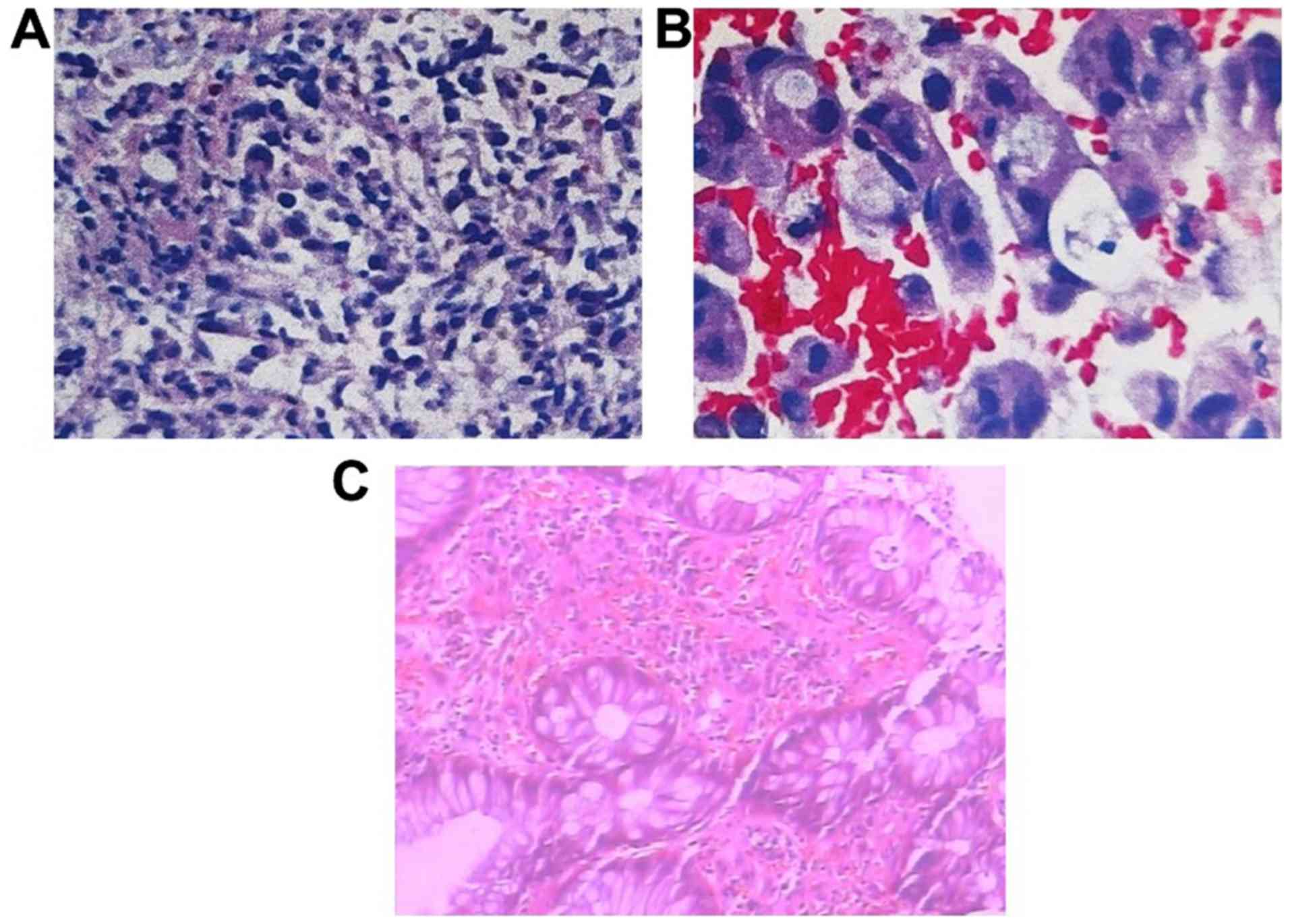
(-), HRAS (-), BRAF (-) and the microsatellite stability (MSS) were
detected in rectal cancer using molecular pathological examination
(Fig. 2A and B).
The male rectal cancer patient was treated with
three-dimensional conformal radiotherapy (3D-CRT; dose, 60 Gy/30
Fr) and XELOX chemotherapy (200 mg oxaliplatin at day 1 plus 1.5 g
capecitabine twice a day from day 1-14 for a total of 5 cycles)
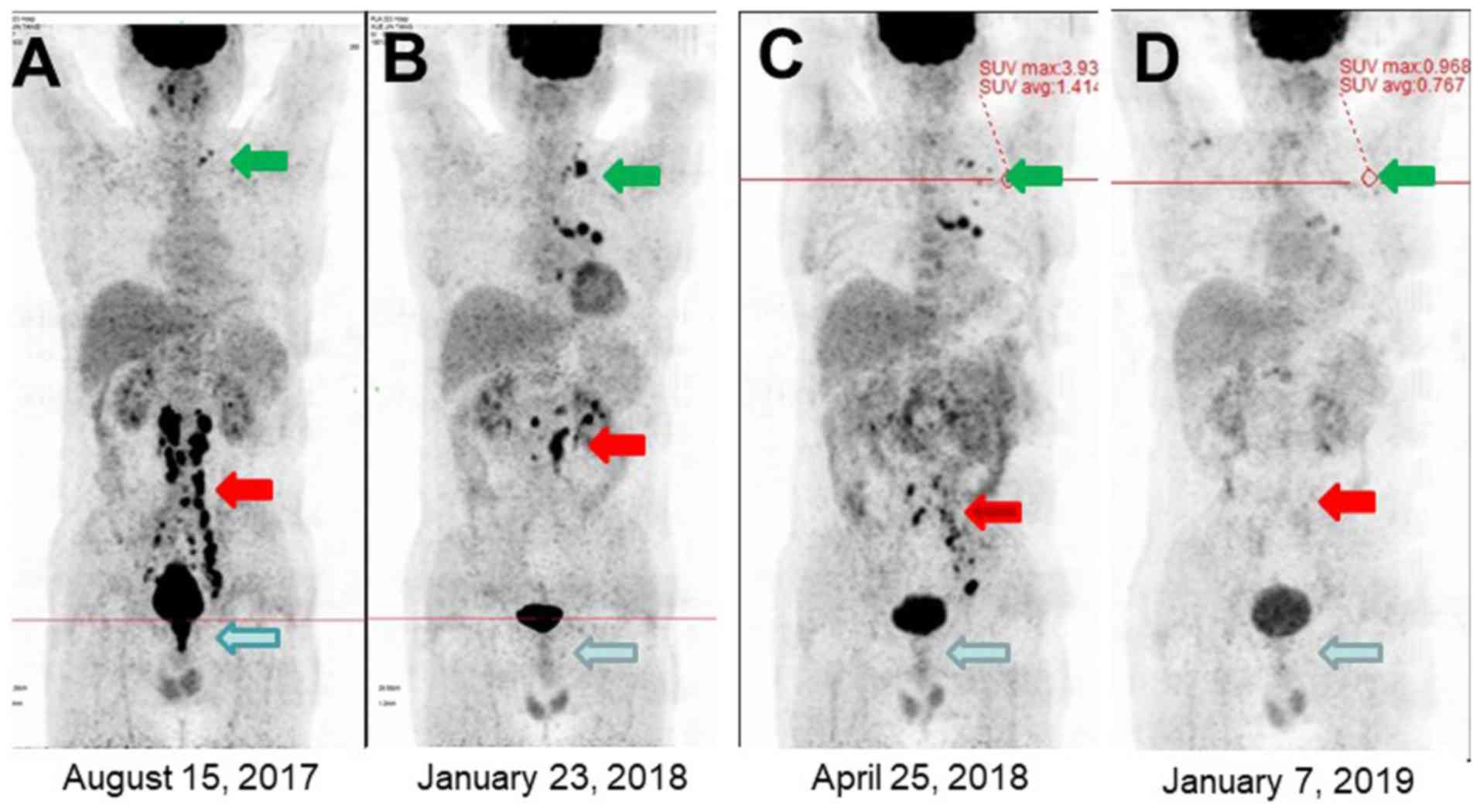
(Fig. 3). At the end of the first
cycle, blood was not observed in the stool, and the electronic
colonoscopy showed that the primary lesion was significantly shrunk
and pathological type transformed into inflammation (Figs. 1B and 2C). Following XELOX chemotherapy, the
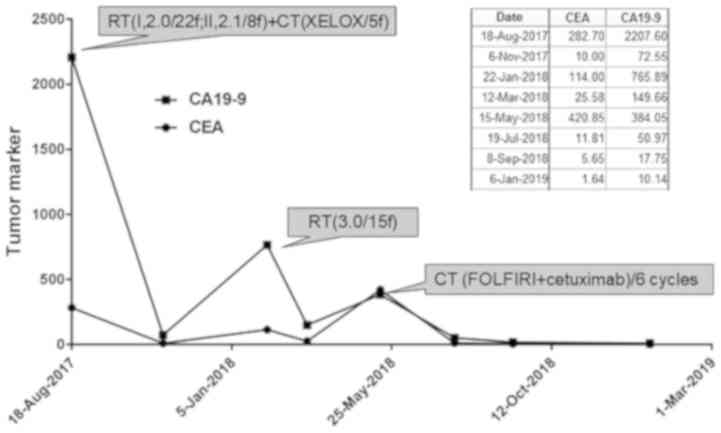
carcinoembryonic antigen (CEA) levels were decreased from 282.7 to
10.3 ng/ml. One month later, CEA increased to 49.79 ng/ml (Fig. 4) and PET/CT scan revealed that the
distant lymph nodes and the lymph nodes in the non-irradiated
target sites were still active. Subsequentially, the residual lymph
node underwent 3D-CRT (45 Gy/15 Fr) and the CEA level was further
decreased to 25.58 ng/ml after 1 month. After 3 months the CEA
levels were again increased to 154 ng/ml. The third PET/CT scan
showed that the retroperitoneal lesion was reduced in size;
however, recurrence of the pelvic lymph node was observed (Fig. 3). Therefore, the patient underwent
systemic chemotherapy with FOLFIRI plus cetuximab for a total of 6
cycles. The individualized scheme gradually reduced the CEA level
to 1.86 ng/ml and PET/CT re-examination indicated that the
metabolism levels of the lesion returned to normal while the lesion
was significantly reduced in size. Interestingly, his serum CEA
level remained within normal range.
The study was approved by the Ethics Committee of
the 986 Hospital of People's Liberation Army Air Force (file no. LZ
323-2018-07) and patient consent was obtained and provided.
Discussion
Currently, the median survival time of advanced
rectal cancer has increased from 10 to 30 months (8). In order to ensure an increased survival
time, patients and medical workers focus on improving the patient's
quality of life. Thus, observation and waiting method is a
challenging task for clinicians. This method may be considered a
potential treatment for rectal cancer patients with unresectable
concurrent metastasis, achieving CCR following preoperative
neoadjuvant therapy, and especially for patients with initial
diffuse lymph node metastasis.
The benefits of surgical excision of the primary
lesion in advanced non-resectable rectal cancer are controversial.
Faron et al reported that excision of primary lesions
increased overall survival (OS) and progression-free survival (PFS)
(9). However, a study by Cirocchi
et al revealed that primary lesion resection did not affect
OS, and lesion-related complications were not reduced in a
comprehensive analysis based on large sample size (10). In addition to OS and PFS, the risk of
surgery-related complications should not be ignored. Thus, previous
studies have investigated the effect of primary lesion surgical
resection on patients with advanced rectal cancer. The results
showed that the postoperative complications, colostomy and 1-month
mortality rates (for patients >65 years) were 30-50, 24 and 10%,
respectively (11,12). Therefore, the surgical treatment in
patients with non-resectable rectal cancer with initial
simultaneous metastasis should be cautiously performed.
The rapid development of radiotherapy technology,
novel cytotoxic drugs and targeted therapies have gradually
weakened the therapeutic effect of surgery on advanced rectal
cancer. A study has shown that FOLFIRI treatment with or without
bevacizumab reduced primary lesion-related complications in
patients with advanced unresectable rectal concurrent metastases
without obvious obstruction or bleeding. Thus, surgical resection
of the primary lesions was not recommended (13). In addition, 10-20% of patients
achieved pathological complete response (pCR) following concurrent
chemotherapy-radiotherapy, whereas the local recurrence, 5-year
survival and disease-free survival rates were 4.6, 96 and 72%,
respectively (14,15). Based on previous studies (14) and this first present case report, we
hypothesize that patients exhibiting good response to systematic
treatment may be treated by observing and waiting method,
especially those >65 years of age. To the best of our knowledge,
this is the first case study to report colorectal cancer, using the
observation waiting method prior to non-surgical treatment.
Park et al have shown that the total survival
time of patients with CCR was significantly increased compared with
those without CCR (16). In the
present case study, several factors contributed to the CCR
achievement. Firstly, the ECOG score of the male patient case was 0
at the time of diagnosis. In addition, the immune function was
basically normal prior treatment. Particularly, the number and
proportion of CD8+ T and NK cells were within normal range,
suggesting that immune function was not completely collapsed. This
finding may explain the absence of liver or lung visceral
metastasis, with only multiple lymph node metastasis observed. In
addition, the patient did not suffer from other chronic diseases
that could affect treatment response, such as inflammatory bowel
disease, diabetes and the administration of immunosuppressive drugs
(tacrolimus and cyclosporin) which demonstrated the patient had
good compliance. Additionally, the treatment scheme was
adjusted.
According to the 2019 National Comprehensive Cancer
Network (NCCN) guidelines (version 2) (16), systemic translational treatment and
local long- or short-term radiotherapy are recommended for advanced
unresectable rectal cancer patients. In the present case study, the
patient underwent local 3D-CRT radiotherapy [rectal lesions and
pelvic lymph nodes; irradiation dose, DT, 60 Gy/30 Fr (17) followed by XELOX (18) systemic chemotherapy. The treatment
scheme reduced the local tumor load and eliminated rectal bleeding.
Following radiotherapy and chemotherapy, PET/CT examination
indicated that the metabolism of the primary lesion was basically
returned to normal and the pathological properties of the tumor
changed from poorly differentiated adenocarcinoma to high-grade
intraepithelial neoplasia. Following XELOX chemotherapy, the CEA
levels were decreased from 282.7 to 10.3 ng/ml. With progress of
the disease, local lymph nodes recurred and distant lymph nodes
still exhibited increased metabolism levels. However, primary
lesions were well controlled, reflecting the beneficial effect of
radiotherapy in primary rectal cancer lesions. Following
chemotherapy with FOLFIRI plus cetuximab regimen (19), the residual metastatic lymph nodes
were completely relieved, and CEA and metabolism levels returned to
normal. Furthermore, treatment was well tolerated by the patient,
with no perforation, radiation proctitis and other complications,
whereas only grade II hematology and gastrointestinal toxicity was
observed. In addition, in terms of tumor characteristics, the
pathologic features of the patient indicated common poorly
differentiated adenocarcinoma, rather than mucinous adenocarcinoma
characterized by poor prognosis or hepatoid adenocarcinoma
characterized by AFP expression (18,20,21). At
present, there is no consensus on the prognostic impact of RAS/RAF
mutations in localized disease. Douillard et al (22) provided new information on different
aspects of the RAS/BRAF mutations, together with a combination of
BRAF mutations in serum and tumor MMR status. Neoadjuvant
chemotherapy may be an option in the near future and plasma
mutations may serve as a tool for relevant selection. Finally,
whole-body PET/CT scan and CEA levels detection was performed
during the treatment process in order for disease changes to be
monitored (23). When the CEA levels
were abnormally elevated, a PET/CT scan was performed in order for
the tumor changes to be accurately evaluated in a timely manner.
Thus, an effective local and whole-body antitumor treatment was
performed, which significantly improved the antitumor effects.
In summary, there are many factors that affect the
efficacy of advanced rectal cancer treatment and permanently
eliminate interfering factors. Individualized and accurate
treatment under the suggestion of multi-disciplinary specialist is
an effective strategy to improve the CCR rate. Observation and
waiting method may be a new treatment approach, rather than direct
sequential surgical resection of the primary lesion, for patients
with advanced rectal cancer who achieved CCR after non-surgical
therapy. Thus, the differences between observation and waiting
method, and direct sequential surgical resection on OS and QoS need
to be further verified by multicenter clinical trials.
Acknowledgements
Not applicable.
Funding
There is no funding information.
Availability of data and materials
Data and materials are available from correspondence
author.
Authors' contributions
YD, HG, SH designed the study and contributed to the
study. SH reviewed and edited the manuscript, and TQ analyzed the
data and supervised the study. All authors read and agreed to the
final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the 986 Hospital of People's Liberation Army Air Force (file no. LZ
323-2018-07).
Patient consent for publication
Patient consent was obtained and provided.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Isbister WH: Audit of definitive colorectal
surgery in patients with early and advanced colorectal cancer. ANZ
J Surg. 72:271–274. 2002.PubMed/NCBI
|
|
3
|
Lee WS, Yun SH, Chun HK, Lee WY, Yun HR,
Kim J, Kim K and Shim YM: Pulmonary resection for metastases from
colorectal cancer: Prognostic factors and survival. Int J
Colorectal Dis. 22:699–704. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yoo PS, Lopez-Soler RI, Longo WE and Cha
CH: Liver resection for metastatic colorectal cancer in the age of
neoadjuvant chemotherapy and bevacizumab. Clin Colorectal Cancer.
6:202–207. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bernier L, Balyasnikova S, Tait D and
Brown G: Watch-and-wait as a therapeutic strategy in rectal cancer.
Curr Colorectal Cancer Rep. 14:37–55. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yahya J, Herzig D, Farrell M, Degnin C,
Chen Y, Holland J, Brown S, Binder C, Jaboin J, Tsikitis VL, et al:
Survey results of US radiation oncology providers' contextual
engagement of watch-and-wait beliefs after a complete clinical
response to chemoradiation in patients with local rectal cancer. J
Gastrointest Oncol. 9:1127–1132. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Habr-Gama A, Perez RO, Nadalin W, Sabbaga
J, Ribeiro U Jr, Silva e Sousa AH Jr, Campos FG, Kiss DR and
Gama-Rodrigues J: Operative versus nonoperative treatment for stage
0 distal rectal cancer following chemoradiation therapy: Long-term
results. Ann Surg. 240:711–717. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mayer RJ, Van Cutsem E, Falcone A, Yoshino
T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero
J, Komatsu Y, et al: Randomized trial of TAS-102 for refractory
metastatic colorectal cancer. N Engl J Med. 372:1909–1919.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Faron M, Pignon JP, Malka D, Bourredjem A,
Douillard JY, Adenis A, Elias D, Bouché O and Ducreux M: Is primary
tumor resection associated with survival improvement in patients
with colorectal cancer and unresectable synchronous metastases? A
pooled analysis of individual data from four randomized trials. Eur
J Cancer. 51:166–176. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cirocchi R, Trastulli S, Abraha I,
Vettoretto N, Boselli C, Montedori A, Parisi A, Noya G and Platell
C: Non-resection versus resection for an asymptomatic primary tumor
in patients with unresectable stage IV colorectal cancer. Cochrane
Database Syst Rev. 8(CD008997)2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
McCahill LE, Yothers G, Sharif S, Petrelli
NJ, Lai LL, Bechar N, Giguere JK, Dakhil SR, Fehrenbacher L, Lopa
SH, et al: Primary mFOLFOX6 plus bevacizumab without resection of
the primary tumor for patients presenting with surgically
unresectable metastatic colon cancer and an intact asymptomatic
colon cancer: Definitive analysis of NSABP trial C-10. J Clin
Oncol. 30:3223–3228. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Temple LK, Hsieh L, Wong WD, Saltz L and
Schrag D: Use of surgery among elderly patients with stage IV
colorectal cancer. J Clin Oncol. 22:3475–3484. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Poultsides GA, Servais EL, Saltz LB, Patil
S, Kemeny NE, Guillem JG, Weiser M, Temple LK, Wong WD and Paty PB:
Outcome of primary tumor in patients with synchronous stage IV
colorectal cancer receiving combination chemotherapy without
surgery as initial treatment. J Clin Oncol. 27:3379–3384.
2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Huang S, Dang Y, Li F, Wei W, Ma Y, Qiao S
and Wang Q: Biological intensity-modulated radiotherapy plus
neoadjuvant chemotherapy for multiple peritoneal metastases of
ovarian cancer: A case report. Oncol Lett. 9:1239–1243.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Glynne-Jones R and Hughes R: Critical
appraisal of the ‘wait and see’ approach in rectal cancer for
clinical complete responders after chemoradiation. Br J Surg.
99:897–909. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Park IJ, You YN, Agarwal A, Skibber JM,
Rodriguez-Bigas MA, Eng C, Feig BW, Das P, Krishnan S, Crane CH, et
al: Neoadjuvant treatment response as an early response indicator
for patients with rectal cancer. J Clin Oncol. 30:1770–1776.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Appelt AL, Pløen J, Harling H, Jensen FS,
Jensen LH, Jørgensen JC, Lindebjerg J, Rafaelsen SR and Jakobsen A:
High-dose chemoradiotherapy and watchful waiting for distal rectal
cancer: A prospective observational study. Lancet Oncol.
16:919–927. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Goldberg RM, Sargent DJ, Morton RF, Fuchs
CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC and Alberts
SR: A randomized controlled trial of fluorouracil plus leucovorin,
irinotecan, and oxaliplatin combinations in patients with
previously untreated metastatic colorectal cancer. J Clin Oncol.
22:23–30. 2004.
|
|
19
|
Bokemeyer C, Van Cutsem E, Rougier P,
Ciardiello F, Heeger S, Schlichting M, Celik I and Köhne CH:
Addition of cetuximab to chemotherapy as first-line treatment for
KRAS wild-type metastatic colorectal cancer: Pooled analysis of the
CRYSTAL and OPUS randomised clinical trials. Eur J Cancer.
48:1466–1475. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
McCawley N, Clancy C, O'Neill BD, Deasy J,
McNamara DA and Burke JP: Mucinous rectal adenocarcinoma is
associated with a poor response to neoadjuvant chemoradiotherapy: A
systematic review and meta-analysis. Dis Colon Rectum.
59:1200–1208. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ren F, Weng W, Zhang Q, Tan C, Xu M, Zhang
M, Wang L, Sheng W, Ni S and Huang D: Clinicopathological features
and prognosis of AFP-producing colorectal cancer: A single-center
analysis of 20 cases. Cancer Manag Res. 11:4557–4567.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Douillard JY, Oliner KS, Siena S,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Panitumumab-FOLFOX4 treatment and RAS mutations
in colorectal cancer. N Engl J Med. 369:1023–1034. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Perez RO, Habr-Gama A, Gama-Rodrigues J,
Proscurshim I, Julião GP, Lynn P, Ono CR, Campos FG, Silva e Sousa
AH Jr, Imperiale AR, et al: Accuracy of positron emission
tomography/computed tomography and clinical assessment in the
detection of complete rectal tumor regression after neoadjuvant
chemoradiation: Long-term results of a prospective trial (national
clinical trial 00254683). Cancer. 118:3501–3511. 2012.PubMed/NCBI View Article : Google Scholar
|