Introduction
Non-small-cell lung carcinoma (NSCLC) with epidermal
growth factor receptor (EGFR) mutations responds well to epidermal
growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs).
However, most patients acquire resistance to EGFR-TKIs and
experience disease progression (1).
Although several resistance mechanisms to EGFR-TKIs have been
recently reported, small-cell lung carcinoma (SCLC) transformation
is a relatively rare resistance mechanism and mostly develops in
NSCLC patients with 1st and 2nd generation EGFR-TKIs such as
gefitinib, erlotinib, and afatinib (2,3). To
date, only a few cases of SCLC transformation during or after
treatment with osimertinib have been reported (4-8).
However, all previously reported cases of SCLC transformation have
been diagnosed by pathological tissues of primary lesion or
metastatic lesions, not pericardial effusion. Here, we report the
case of malignant pericarditis developed in SCLC transformed NSCLC
patient with a history of osimertinib treatment.
Case report
A 68-year-old man with a smoking history of 40 packs
per year was admitted to our hospital due to relapse of stage IA
lung adenocarcinoma in December 2014, which was previously
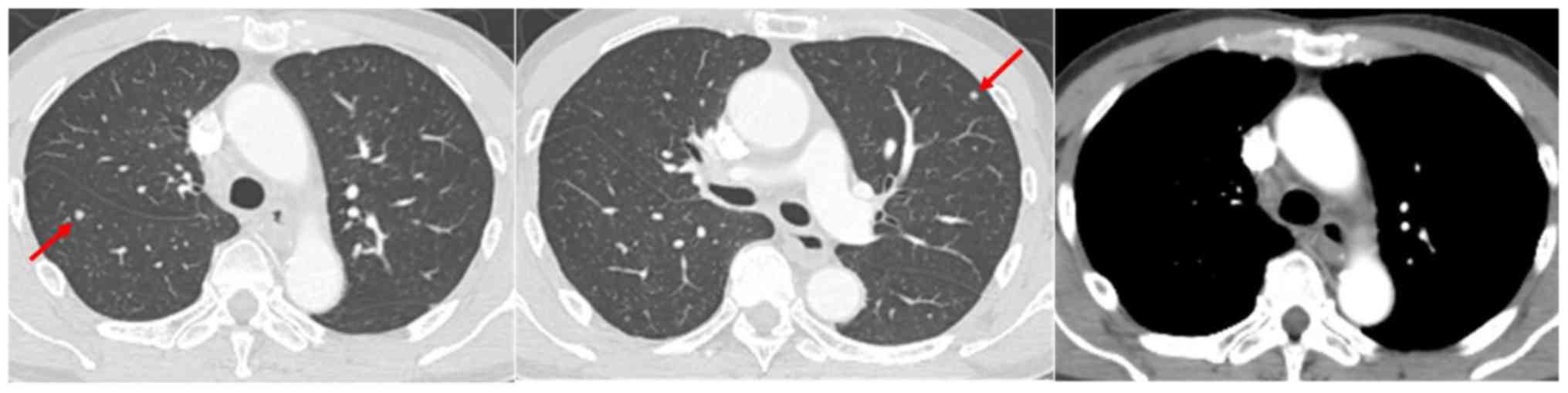
surgically resected in December 2012. Computed tomography (CT)
revealed mediastinal lymphadenopathy and multiple lung nodules
(Fig. 1). In addition, serum
carcinoembryonic antigen (CEA) level had increased to 16.0 ng/ml.
The specimen obtained by endobronchial ultrasound-guided
transbronchial needle aspiration to mediastinal lymph node (#4R)
confirmed lung adenocarcinoma with an EGFR mutation (exon 19
deletion) by therascreen® EGFR RGQ PCR Kit (Qiagen,
Hilden, Germany). Then, he was enrolled in a clinical trial (FLAURA
trial) comparing osimertinib and gefitinib (or erlotinib) as the
1st line chemotherapy and treated with osimertinib in March
2015(9). Although this treatment had
a significant response lasting 11 months and CEA fell to the normal
level, routine-follow-up CT in February 2016 showed slight
ground-glass attenuation (GGA) in the right lower lobe. The
treatment was discontinued on suspicion of drug-induced
pneumonitis. Although GGA was spontaneously regressed, mediastinal
lymphadenopathy and multiple lung nodules aggravated. Therefore,
erlotinib treatment as 2nd line chemotherapy was initiated with a
careful observation in May 2016. After 8 months treatment with
erlotinib, follow-up CT in January 2017 demonstrated re-progression
of the mediastinal lymph node and appearance of minor amounts of
pericardial effusion. With a re-biopsy of the lymph node (#4R),
lung adenocarcinoma was found the EGFR T790M mutation in addition
to the EGFR exon 19 deletion although liquid biopsy, a blood test
that detects evidence of cancer cells or tumor DNA, showed only
exon 19 deletion by cobas EGFR Mutation test v2 (Roche Molecular
Systems, Pleasanton, CA, USA). Thus, re-challenge with osimertinib
as 3rd line treatment was initiated, and three month later, despite
a tentative response, enlargement of mediastinal tumors with an
elevated CEA level of 24.3 ng/ml was observed. Next, the patient
was treated with carboplatin in combination with paclitaxel,
docetaxel, and pemetrexed as 4th-6th line treatment, respectively,
which did not present a desired effect and CEA level remained
elevated. Thus, S-1 monotherapy was initiated in August 2018 as 7th
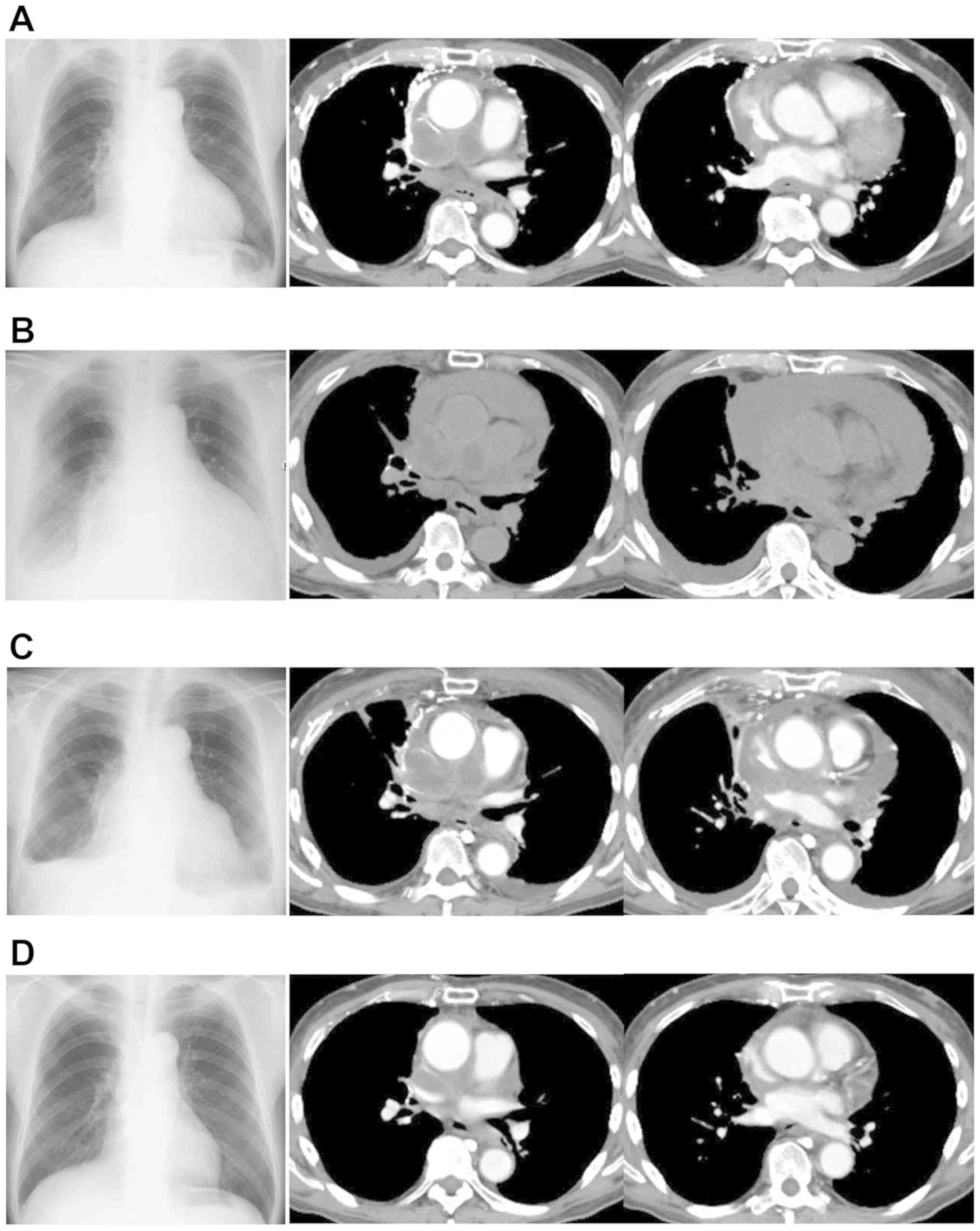
line treatment (Fig. 2A). One month
after this treatment was initiated, an apparent decrease in CEA
level from 100.5 ng/ml to 30 ng/ml was observed. However, dyspnea
gradually appeared, and CT showed apparent enlargement of
mediastinal tumors and increase of pericardial effusion, which
ultimately resulted in cardiac tamponade due to disease progression
(Fig. 2B). Then, a pericardial
paracentesis was performed, and the specimens obtained from
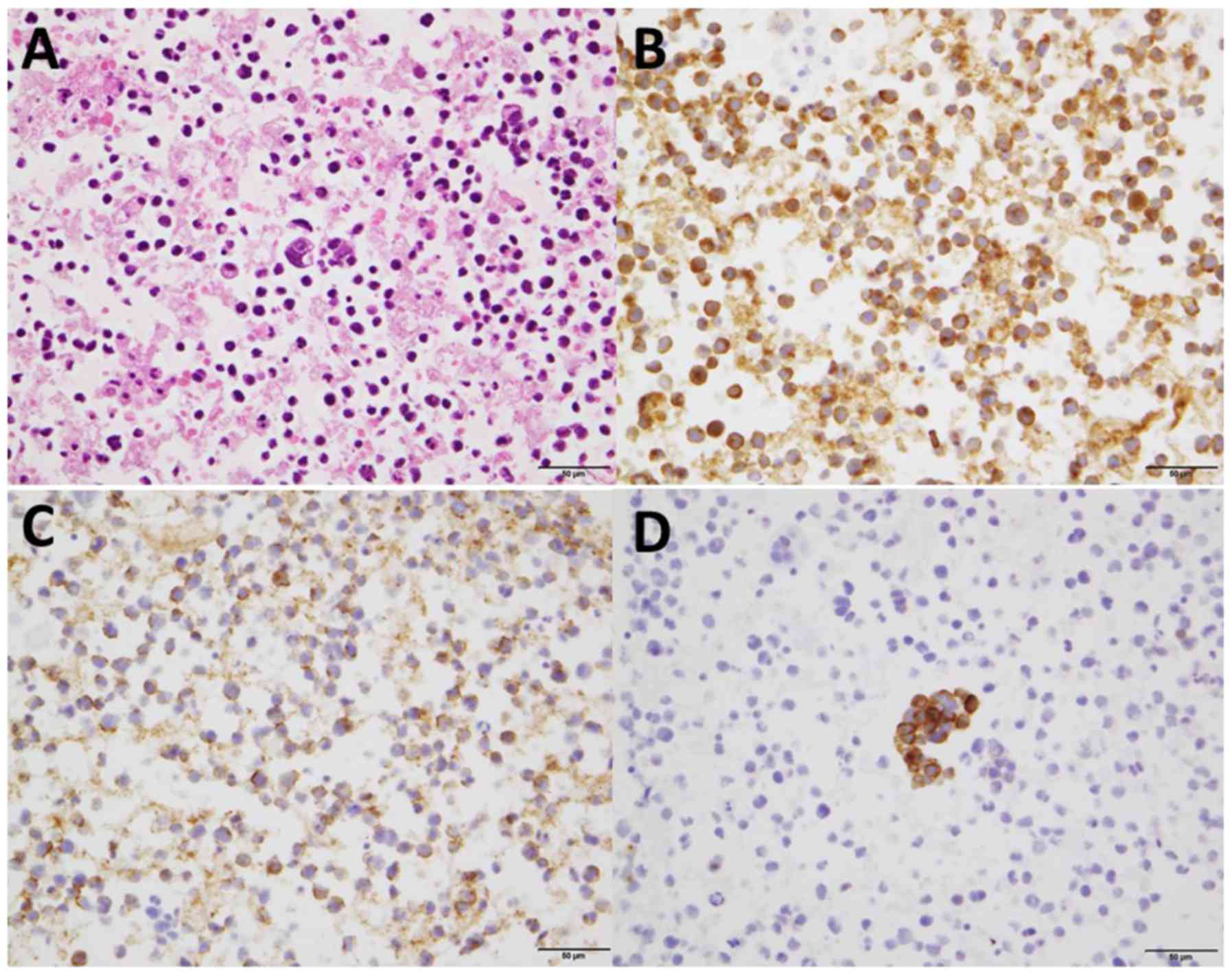
pericardial fluid showed poorly differentiated cells with a high
nuclear-to-cytoplasmic ratio. With the immunohistochemical
staining, neuroendocrine markers such as synaptophysin, NCAM and
chromogranin were positive (Fig. 3).
Besides, the additional blood tests indicated that neuron specific
enolase (NSE) was 39.4 ng/ml. Based on these results, SCLC
transformation was confirmed. Additionally, the molecular analysis
of the obtained specimens showed that the exon 19 deletion was
still positive despite negative T790M mutation by cobas EGFR
Mutation test v2. After pericardial paracentesis (Fig. 2C), a combination therapy of
carboplatin and etoposide was administered in September 2018. After
the 1st course of this regimen, mediastinal tumor and pericardial
effusion were dramatically improved with a substantial decrease of
NSE level, and therefore 4 cycles of this regimen were completed
(Fig. 2D). The disease related SCLC
transformation was considered to remain stable with normal level of
NSE in March 2019.
Discussion
We herein report the case of SCLC transformation
diagnosed by pericardial effusion after a long-term treatment with
EGFR-TKIs including osimertinib. All previously reported cases of
SCLC transformation have been diagnosed by pathological tissues of
primary lesion or metastatic lesions, not pericardial effusion. Our
results could provide the following two clinical implications.
First, SCLC transformation would occur after
treatment with osimertinib. Among several mechanisms of acquired
resistance to EGFR-TKIs, SCLC transformation reportedly accounts
for 3-14% and mostly developed as the acquired resistance to 1st-
or 2nd-generation EGFR-TKIs (2,3). To
date, there are limited cases of SCLC transformation after
osimertinib treatment (4-8).
Marcoux et al (10) reported
that median total time of treatment with EGFR-TKIs at the diagnosis
of SCLC transformation was 15.8 months. In our case, the duration
of total osimertinib treatment was 14 months and the total interval
of EGFR-TKI treatment was 22 months. Taking those reports together
with the present case, SCLC transformation would occur after
long-term use of EGFR-TKIs regardless of the generation of
EGFR-TKIs. With regard to mechanism of SCLC transformation, there
have been the following two hypotheses (11). Firstly, lung cancer consists of
combined SCLC and NSCLC histology at first diagnosis, and EGFR-TKI
treatment would cause a component of SCLC dominant. Secondary, type
II alveolar cells, the origin of some EGFR-mutant adenocarcinomas,
also have the potential to become SCLC. Lung adenocarcinoma arising
from these alveolar type II cells and harboring EGFR mutations
might transform to SCLC under the selective pressure of TKI
therapy. In our case, we did not detect a component of SCLC
histologically at first diagnosis. Of note, the molecular analysis
of the cell block from pericardial effusion showed that the exon 19
deletion was still positive despite negative T790M mutation, as
seen in some reports (4-6).
Based on those findings, we consider that this transformed SCLC
developed from a common precursor of adenocarcinoma.
Second, physicians should investigate whether SCLC
transformation occurs or not, especially in NSCLC patients who
develop pericardial effusion after long-term EGFR-TKI treatment.
Generally, malignant pericardial effusion develops in 2.5% of lung
cancer patients and is correlated with a poor prognosis with median
survival of 74.5 days (12,13). Of note, even minimal pericardial
effusion is reported as an independent prognostic factor for those
patients (14). Generally,
EGFR-mutant NSCLC patients who develop pericardial effusion after
the treatment failure with EGFR-TKIs therapy present very poor
prognosis due to lack of therapeutic options. On the other hand,
the median survival in patients with SCLC transformation reportedly
reached 10.9 months (10).
Therefore, it is crucial not to overlook SCLC transformation as a
treatable entity. Importantly, in our case, despite the decrease of
CEA level from 100.5 to 30.0 ng/ml during S-1 treatment, his
disease paradoxically progressed with high level of NSE. In fact,
some previous reports about SCLC transformation revealed that
pro-gastrin releasing peptide (pro-GRP) and NSE are reported to be
likely to increase (5,6). Therefore, it would be extremely
important to measure tumor markers of pro-GRP and NSE in NSCLC
patient with pericardial effusion after long-term EGFR-TKI
treatment.
In conclusion, we presented the case of SCLC
transformation diagnosed by pericardial effusion after long-term
EGFR-TKI treatment including osimertinib. In NSCLC patients who
develop pericardial effusion after long-term EGFR-TKI therapy
including osimertinib, it is important to investigate whether SCLC
transformation occurs or not, and measuring tumor markers.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RO and AS analysed and interpreted the data and
wrote the manuscript. RO, AS, MA, YS, SI, TB, SK, EH and TO
evaluated the patient and participated in the therapy. KO evaluated
the pathological specimens. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the publication of the case details and any associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yu HA, Arcila ME, Rekhtman N, Sima CS,
Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M and Riely GJ:
Analysis of tumor specimens at the time of acquired resistance to
EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers.
Clin Cancer Res. 19:2240–2247. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sequist LV, Waltman BA, Dias-Santagata D,
Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger
S, Cosper AK, et al: Genotypic and histological evolution of lung
cancers acquiring resistance to EGFR inhibitors. Sci Transl Med.
3(75ra26)2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Minari R, Bordi P, Del Re M, Facchinetti
F, Mazzoni F, Barbieri F, Camerini A, Comin CE, Gnetti L, Azzoni C,
et al: Primary resistance to osimertinib due to SCLC
transformation: Issue of T790M determination on liquid re-biopsy.
Lung Cancer. 115:21–27. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Iijima Y, Hirotsu Y, Mochizuki H, Amemiya
K, Oyama T, Uchida Y, Kobayashi Y, Tsutsui T, Kakizaki Y, Miyashita
Y and Omata M: Dynamic changes and drug-induced selection of
resistant clones in a patient with EGFR-mutated adenocarcinoma that
acquired T790M mutation and transformed to small-cell lung cancer.
Clin Lung Cancer. 19:e843–e847. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Taniguchi Y, Horiuchi H, Morikawa T and
Usui K: Small-cell carcinoma transformation of pulmonary
adenocarcinoma after osimertinib treatment: A case report. Case Rep
Oncol. 11:323–329. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kim TM, Song A, Kim DW, Kim S, Ahn YO,
Keam B, Jeon YK, Lee SH, Chung DH and Heo DS: Mechanisms of
acquired resistance to AZD9291: A mutation-selective, irreversible
EGFR inhibitor. J Thorac Oncol. 10:1736–1744. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ham JS, Kim S, Kim HK, Byeon S, Sun JM,
Lee SH, Ahn JS, Park K, Choi YL, Han J, et al: Two cases of small
cell lung cancer transformation from EGFR mutant adenocarcinoma
during AZD9291 treatment. J Thorac Oncol. 11:e1–e4. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Soria JC, Ohe Y, Vansteenkiste J,
Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura
F, Nogami N, Kurata T, et al: Osimertinib in untreated EGFR-mutated
advanced non-small-cell lung cancer. N Engl J Med. 378:113–125.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Marcoux N, Gettinger SN, O'Kane G, Arbour
KC, Neal JW, Husain H, Evans TL, Brahmer JR, Muzikansky A, Bonomi
PD, et al: EGFR-mutant adenocarcinomas that transform to small-cell
lung cancer and other neuroendocrine carcinomas: Clinical outcomes.
J Clin Oncol. 37:278–285. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Oser MG, Niederst MJ, Sequist LV and
Engelman JA: Transformation from non-small-cell lung cancer to
small-cell lung cancer: Molecular drivers and cells of origin.
Lancet Oncol. 16:e165–e172. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hayano M, Hokamura Y, Kimura Y, Kimura T
and Tokube K: Clinical studies of 16 cases of carcinomatous
pericarditis. Kokyu To Junkan. 39:683–686. 1991.PubMed/NCBI(In Japanese).
|
|
13
|
Wang PC, Yang KY, Chao JY, Liu JM, Perng
RP and Yen SH: Prognostic role of pericardial fluid cytology in
cardiac tamponade associated with non-small cell lung cancer.
Chest. 118:744–749. 2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kato R, Hayashi H, Chiba Y, Tanaka K,
Takeda M and Nakagawa K: Prognostic impact of minimal pericardial
effusion in patients with advanced non-small-cell lung cancer. Clin
Lung Cancer. 18:e449–e455. 2017.PubMed/NCBI View Article : Google Scholar
|