1. Introduction
Gastric cancer is one of the most common types of
tumors worldwide, with a high incidence reported in East Asia,
Central Europe, Eastern Europe, and South America. According to the
International Agency for Research on Cancer; approximately 951,000
individuals were diagnosed with gastric cancer and the disease
caused approximately 723,000 deaths in 2012(1). In China in 2015, approximately 679,000
and 498,000 patients were newly diagnosed with gastric cancer and
expired due to the disease, respectively. According to these
numbers, gastric cancer ranks second after lung cancer in terms of
incidence and mortality (2). With
the prevention and treatment of Helicobacter pylori
infection and the improvement of diet, the rates of gastric
cancer-related morbidity and mortality have exhibited a downward
trend (3). Studies have shown that
the 5-year survival rate of patients with gastric cancer in most
European countries and regions was only 30% (4). This disease seriously threatens the
health of patients, severely affects their quality of life, and
brings a heavy burden to society (5).
In 1987, Johnstone et al discovered vesicles
secreted by sheep reticulocytes containing a variety of bioactive
macromolecules during maturation, which were later termed exosomes
(6). The main component of exosomes
is lipid, which is rich in cholesterol, diglyceride,
glycerophosphatide, phospholipid, and sphingomyelin or
glycosylceramide (including sphingomyelin and ceramide) (7). In addition to these lipids, exosomes
contain many types of bioactive lipids (7,8). They
also contain functional RNAs molecules, including messenger RNAs
(mRNAs) and other non-coding RNAs, such as microRNAs (miRNAs), long
non-coding RNAs (lncRNAs) (9,10), and
circular RNAs (circRNAs) (11). Many
previous studies have demonstrated that exosomes exist in various
fluids of the human body, including blood, amniotic fluid, urine,
malignant ascites, cerebrospinal fluid, breast milk, saliva, lymph,
and bile (12-14).
Exosomes were initially thought to be unnecessary material
discarded by cells (15). In
subsequent studies, it was shown that exosomes, which used to be
seen as molecular trash bins, carry molecules that can be absorbed
and utilized by other cells (16,17).
Exosomes play an important role in normal physiological functions,
such as lactation, inflammation, cell proliferation, immune
response, and neurological function (18-20).
In addition, they participate in thrombosis, diabetes,
atherosclerosis, liver disease, neurodegenerative diseases
(21-23),
cancer (24), and other pathological
processes.
In-depth studies have shown that gastric cancer is a
complex structure composed of cancer cells and the stroma around
them (25). Similar to normal cells,
cancer cells require the transmission of information (26). However, it has been widely reported
that RNAs (especially miRNAs in exosomes) closely participate in
the transmission of information (27,28).
Other studies have shown that exosomes may also be associated with
cancer cells, discarding their anti-cancer miRNAs and improving
their tumorigenicity (29). In
general, exosomes are closely involved in various changes in the
tumor microenvironment, promoting the proliferation and migration
of cancer cells (30).
Non-coding RNAs, which have been detected in
exosomes, include miRNAs, lncRNAs, and circRNAs (9-11).
They are related to many processes in tumor formation, including
tumor growth, metastasis and drug resistance. This fact renders
these non-coding RNAs potential targets for the diagnosis and
treatment of cancer (31). However,
the detailed mechanisms involved in these processes are unclear.
For example, Zhang et al revealed the diagnostic value of
exosomal lncRNA DLX6-AS1 in non-small cell lung cancer (32). Zhang et al found that the
expression levels of miRNAs in exosomes are significantly different
between patients with ovarian cancer and healthy individuals
(33). Compared with the
corresponding healthy state, the aforementioned differences are
also present in clear cell renal carcinoma (34) and invasive breast cancer (35).
These are only a few examples of the value of
non-coding RNAs in cancer. The following sections of this article
will elaborate on the relationship between the non-coding RNAs in
exosomes and gastric cancer.
2. miRNA
Exosomal miRNA of gastric cancer
The discovery of miRNAs is one of the most important
milestones in modern molecular biology. They were first discovered,
identified, and named ‘small temporal RNAs’ by Lee et al
(36) and Reinhart et al
(37). miRNAs have a small molecular
and mainly regulate the expression of mRNAs by binding to the
3'untranslated region (UTR) of the target gene. Their binding does
not have one-to-one characteristics, i.e., a single UTR may have
multiple miRNA binding sites, or a single miRNA can bind to
multiple sites. This further indicates that miRNAs play a
post-transcriptional regulatory role by mainly regulating mRNA
stability and protein translation (38). The results suggest that these
regulatory RNAs have complex post-transcriptional control
mechanisms for gene expression. In addition, the expression of
exosomal miRNAs varies between different cell and tissue types. As
the mechanism of their function is slowly explored, it is possible
for these molecules to be used as biomarkers for disease detection
and targets for therapeutic interventions.
The role of exosomal miRNA in the
diagnosis of gastric cancer
Although there are many biomarkers that can be used
for the diagnosis of gastric cancer, they do not meet the demand
for the early diagnosis of gastric cancer. New markers with better
performance in the diagnosis of gastric cancer at an early stage
are warranted. Since their detection in exosomes, several kinds of
miRNAs have been found to be promising markers.
According to studies utilizing quantitative reverse
transcriptase polymerase chain reaction, four kinds of miRNAs
(miR-19b-3p, miR-17-5p, miR-30a-5p, and miR-106a-5p) have been
related to the pathogenesis of gastric cancer. miR-19b and miR-106a
are significantly overexpressed in patients with gastric cancer
(P<0.0001). Receiver operating characteristic (ROC) analysis
revealed that the area under curve (AUC) values for miR-106a-5p and
miR19b-3p were 0.786 and 0.769, respectively. Combined with ROC
curve analysis, the highest AUC value in patients with gastric
cancer and healthy controls was 0.814. Further research showed that
the characteristics of two miRNAs (miR-19b-3p and miR-106a-5p)
correctly distinguished 19 of 20 gastric cancer serum samples (95%
sensitivity) and 18 of 20 healthy controls (90% specificity). In
addition, the above-mentioned miRNAs are associated with lymphatic
metastasis of gastric cancer (stage I and II: P<0.01; stage III
and IV: P<0.05). Hence, miR-19b-3p and miR-106a-5p in serum
exosomes are new potential biomarkers for the detection of gastric
cancer (39).
In addition to the exosomal miRNAs, which have a
detailed statistical proof, many other exosomal miRNAs have been
found to be abnormally expressed in the exosomes of patients with
gastric cancer. The expression of miR-217(40), miR-27A (41), and miR-1290(42) is pathologically high in the exosomes
of patients with gastric cancer. miR-214, miR-221, and miR-222 are
highly expressed in gastric cancer and their expression levels are
closely related to lymph node metastasis, venous invasion, and
tumor-node-metastasis (TNM) stage (43). In a study assessing the relationship
between miR-130a and c-MYB mRNAs, Yang et al suggested that
the levels of miR-130a in exosomes may be a potential biomarker for
evaluating the invasion or metastasis of gastric cancer, although
there is no more clinical statistical proof (44). Of note, increases in the expression
of miRNAs are not the only observation in gastric cancer. The LET-7
family of miRNAs has an abnormal extracellular exosomal and
intracellular content of AZ-P7a (29). The expression levels of miR-101 in
gastric cancer tissues and gastric cancer cell lines are markedly
lower that those recorded in normal gastric mucosa. Moreover,
compared with healthy individuals, miR-101 in both exosomes and
serum of patients with gastric cancer is significantly
downregulated (45).
Above, we listed some miRNAs with significant
differences in expression in exosomes. Some of those have been
closely related to certain stages of gastric cancer. Relevant
information regarding the miRNAs mentioned in this article is
presented in Table I. Although some
exhibit abnormal expression levels, there was no statistical proof.
Moreover, these miRNAs have not been compared with the commonly
used diagnostic markers of gastric cancer. Perhaps in the near
future, these miRNAs can help to more accurately diagnose gastric
cancer, determine the stage of disease, and guide the clinical
treatment.
 | Table ImiRNAs in exosomes of patients with
gastric cancer. |
Table I
miRNAs in exosomes of patients with
gastric cancer.
| Accession no. | Gene ID | Name | Source of
exosomes | Expression | Related
target/molecule | (Refs.) |
|---|
| - | - | LET-7 family | GC cell | - | RAS, HMGA2 | (29) |
| NC_000013.11 | 406980 | miR-19b | Serum | UP | Unknown | (39) |
| NC_000023.11 | 406899 | miR-106a | Serum | UP | Inknown | (39) |
| NC_000002.12 | 406999 | miR-217 | GC cell | UP | CDH1 | (40) |
| NC_000019.10 | 407018 | miR-27a | GC cell | UP | CSRP2 | (41) |
| NC_000001.11 | 100302276 | miR-1290 | Serum | UP | NKD1 | (42) |
| NC_000001.11 | 406996 | miR-214 | GC cell | UP | Unknown | (43) |
| NC_000023.11 | 407006 | miR-221 | GC cell | UP | Unknown | (43) |
| NC_000023.11 | 407007 | miR-222 | GC cell | UP | Unknown | (43) |
| NC_000011.10 | 406919 | miR-130a | GC cell | UP | c-MYB | (44) |
| - | - | miR-101 | GC cell | DOWN | MCL1, ZEB1 | (45) |
| NC_000007.14 | 407014 | miR-25 | EAC cell | UP | PTEN, AIFM3 | (55) |
| NC_000011.10 | 406992 | miR-210 | EAC cell | UP | PTEN, AIFM3 | (55) |
Promoting mechanism and role of
exosomal miRNA in the treatment of gastric cancer
It is established that the development of gastric
cancer is not the result of a single factor, such as cell mutation,
growth, malignant maintenance, anti-apoptosis, vascular growth and
cell metastasis. These factors play an important role in the
occurrence and development of gastric cancer. With the gradual
discovery of exosomal miRNAs, scientists have found that some
affect the formation and development of gastric cancer in many
stages, through complex pathways.
Firstly, regarding the growth stage of gastric
cancer, there is a negative correlation between the expression of
miR-217 and cadherin 1 (CDH1; also known as E-cadherin). The former
contributes substantially to intercellular adhesions as a
transmembrane glycoprotein. It has also been proved to be a tumor
suppressor and its expression is decreased in gastric cancer.
Studies utilizing double luciferase analysis and immunoblotting
showed that miR-217 targets CDH1. Overexpression of miR-217 can
enhance the proliferation of gastric cancer cells. This leads to
the decrease of exosomal CDH1, and this effect is also observed in
the microenvironment. These findings reveal part of the function of
miR-217(40) and an important part
of the growth of gastric cancer.
The microenvironment is particularly important in
the growth of malignant tumors (46). As part of the tumor microenvironment,
cancer-related fibroblasts exert a negative effect on patients
(47). High miR-27A expression in
exosomes can induce the reprogramming of fibroblasts into
cancer-related fibroblasts and promote the proliferation,
migration, and metastasis of malignant cells. Overexpression of
miR-27A cancer-related fibroblasts significantly increased the
malignant degree of gastric cancer cells. Further investigation
revealed that cysteine and glycine-rich protein 2 (CSRP2) is the
downstream target gene of miR-27A, and its downregulation increases
the replication of gastric cancer cells (41).
Angiogenesis is involved in almost the entire course
of cancer, including occurrence (48,49),
progression (50), invasion, and
metastasis (51,52). Studies have shown that c-MYB is a
transcription factor affecting the growth of blood vessels
(53). The expression of miR-130a is
significantly increased in gastric cancer cells and their exosomes.
Bioinformatics analysis combined with luciferase analysis showed
that miR-130a directly targeted 30 UTR of c-MYB mRNA. Subsequently,
they also confirmed that the overexpression of miR-130a
significantly promotes the growth and angiogenesis of implanted
tumors in mice (44).
Anti-apoptosis is important for cancer cells. MCL1,
which belongs to the BCL2 family, is often highly expressed in
cancer cells and involved in anti-apoptosis (54). Zinc finger E-box binding homeobox 1
(ZEB1) can boost the invasiveness of epithelial tumors. It exerts
its effects by inhibiting the E-cadherin promoter and inducing
epithelial-stromal transformation. Studies have shown that restored
levels of miR-101, which exists in malignant cells and exosomes,
can induce apoptosis by inhibiting MCL1; moreover, they inhibit
cell migration and invasion by regulating ZEB1. Further research
suggested that the decrease of tumor suppressor miRNA-101 is
closely related to tumor progression (45). Exosomes from esophageal
adenocarcinoma can also affect the gastrointestinal tract,
assisting gastric cancer cells to fight apoptosis and create a
favorable environment. The gastrointestinal cells, which treated
with esophageal adenocarcinoma-derived extracellular vesicles
(C-EV), are more crowded, compact, and multilayered and contained
fewer lumens than control group. Further studies showed that, in
the control group treated with C-EV, the expression levels of
miR-25 and miR-210 were significantly higher, whereas those of
phosphatase and tensin homolog (PTEN) and apoptosis-inducing factor
mitochondria associated 3 (AIFM3) were significantly decreased.
PTEN and AIFM3 are apoptotic genes. When the expression is low,
abnormal proliferative cells may escape the fate of apoptosis.
However, the effects of C-EV on co-cultured gastrointestinal tract
could be reversed by inhibiting the high expression levels of
miR-25 and miR-210(55).
The development of gastric cancer is promoted
through the secretion of miRNAs by exosomes that are conducive to
the growth of cancer cells. In addition, gastric cancer cells can
also discard miRNAs that impede their growth through the exosomes.
The LET-7 family of miRNAs plays a major role as tumor suppressor
genes (56), targeting RAS and
high-mobility group AT-hook 2 (HMGA2) (57). A study showed that the LET-7 miRNAs
were abnormally expressed in exosomes of AZ-P7a cells. Further
research indicated that AZ-P7a cells maintain their tumorigenicity
and metastatic tendency by selectively secreting LET-7 miRNAs into
exosomes entering the extracellular environment (29).
Previous studies have interfered with the abnormal
expression of miRNAs in exosomes, which may play a role in the
development of gastric cancer cells, hindering or reversing the
growth of gastric cancer cells. The expression of miR-214, miR-221
and miR-222 in exosomes of patients with gastric cancer exhibits
high levels. A study showed that exosomes, which are secreted by
gastric cancer-derived mesenchymal stem cells, deliver miR-221 to
HGC-27 cells and promote malignancy. Inhibition of miR-221 can
block the tumor support provided by gastric cancer-derived
mesenchymal stem cells (43). In
addition, the high expression of miR-1290 in exosomes of patients
with gastric cancer has been confirmed. Subsequently, fluorescence
results showed that NKD inhibitor of WNT signaling pathway 1 (NKD1)
mRNA is the direct target of miR-1290. In contrast, overexpression
of NKD1 could attenuate the effect of miR-1290 on gastric cancer
cells. In summary, exosomal miR-1290 can enhance the malignancy of
gastric cancer cells by targeting NKD1 mRNA, to downregulate its
expression (42).
According to this evidence, exosomal miRNAs play an
important role in the formation and development of gastric cancer.
Moreover, there is a variety of mechanisms and molecular pathways
involved in this process. For example, these miRNAs can directly
assist cancer cells to grow, invade, and resist apoptosis.
Moreover, they affect the nearby microenvironment and discard
miRNAs that prevent cancer cell growth through exosomes. Relevant
information regarding the miRNAs mentioned in this article is
presented in Table I and Fig. 1. We hypothesized that such diverse
and complex mechanisms suggest the involvement of many alternative
pathways hindering the formation and development of gastric cancer.
For example, this idea has been confirmed in experiments targeting
miR-221(43) and miR-1290(42). We believe that the aforementioned
mechanisms and molecular pathways can be used as targets to hinder
the formation and development of gastric cancer cells, although
there is no conclusive research evidence. This may be the goal of
future research. Although these findings are preliminary, they can
be used as routes and guidance for follow-up research.
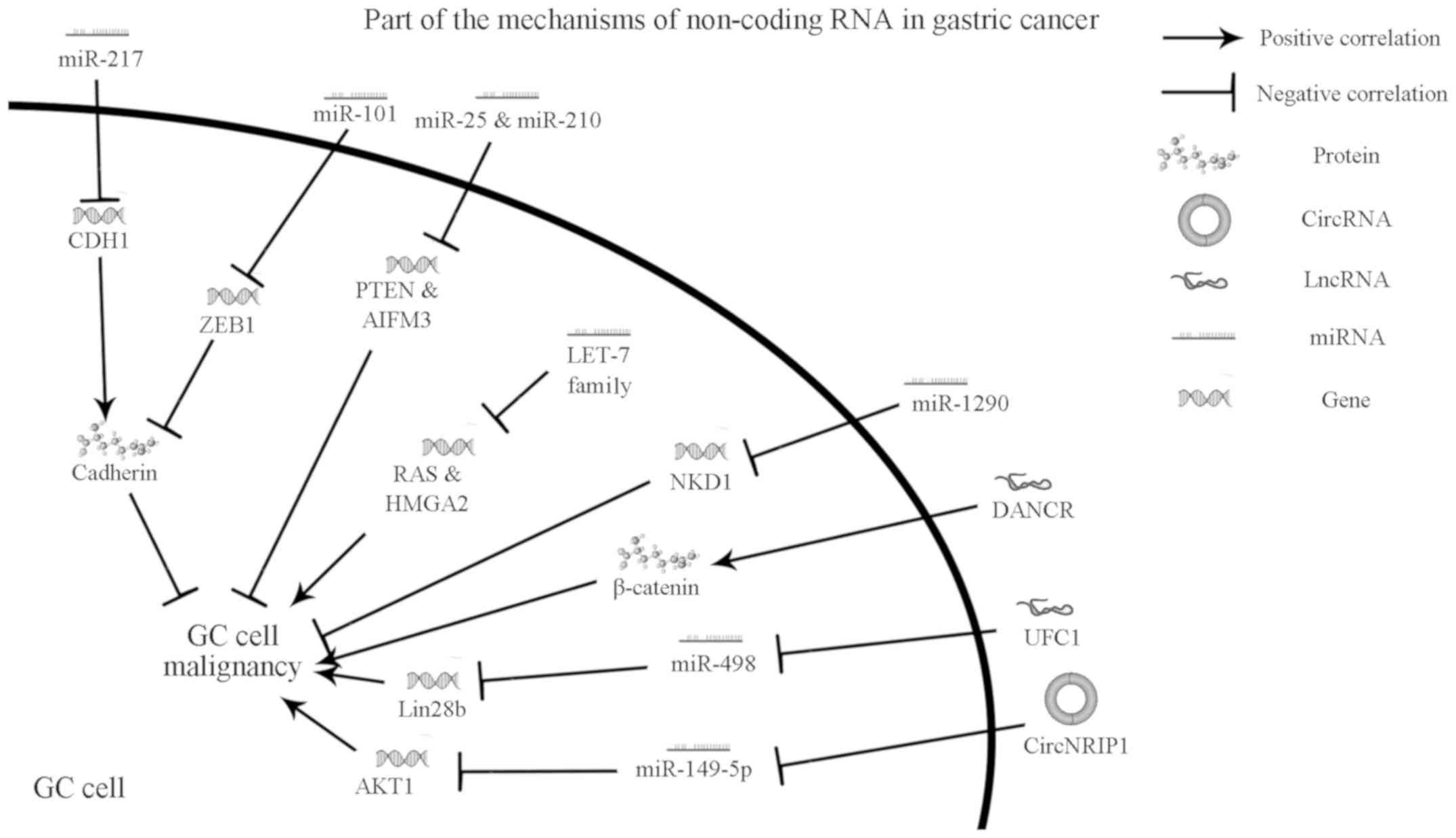 | Figure 1Mechanisms of non-coding RNA in GC.
Various mechanisms of non-coding RNA act on GC cells via exosomes.
Related molecules are also described. miRNA, microRNA; GC, gastric
cancer; CDH1, cadherin 1; ZEB1, zinc finger E-box binding homeobox
1; PTEN, phosphatase and tensin homolog; AIFM3, apoptosis-inducing
factor mitochondria associated 3; RAS, rat sarcoma virus, here
refers to oncogenes firstly discovered from rat sarcoma virus;
HMGA2, high-mobility group AT-hook 2; NKD1, NKD inhibitor of WNT
signaling pathway 1; DANCR, anti-differentiation antagonizing
non-protein coding RNA; UFC1, ubiquitin-fold modifier conjugating
enzyme 1; lncRNA, long non-coding RNA; circRNA, circular RNA. |
3. LncRNA
Exosomal lncRNA of gastric cancer
LncRNAs are >200 base pairs long (i.e., longer
than miRNAs). Currently, there is limited research on lncRNAs.
Although >20,000 lncRNAs have already been annotated, our
knowledge of lncRNAs remains limited compared with that on miRNAs.
It is established that they play a role mainly by regulating the
transcription of genes that encode proteins (58). Notably, scientists have also found
some traces of lncRNAs in the exosomes of patients with gastric
cancer.
The role of exosomal lncRNA in the
diagnosis of gastric cancer
Some lncRNAs have shown excellent potential as
diagnostic markers of gastric cancer. Through the combinatorial
analysis of RNA sequencing results, lncUEGC1 and lncUEGC2 in
exosomes were shown to be highly expressed in patients with gastric
cancer. The same results were obtained in gastric cancer cell
culture medium. Of course, lncUEGC1 and lncUEGC2 are also present
in plasma. Studies have shown that almost all plasma lncUEGC1 is
wrapped in exosomes, and exosomes can protect them from degradation
by ribonuclease. The diagnostic accuracy of exosomal lncUEGC1 has
been evaluated. It exhibited AUC values of 0.8760 and 0.8406 in
discriminating patients with early gastric cancer from healthy
individuals and those with premalignant chronic atrophic gastritis,
respectively. Notably, this diagnostic accuracy is higher than that
of carcinoembryonic antigen (59).
In addition, the expression levels of anti-differentiation
antagonizing non-protein coding RNA (DANCR) targeting lncRNA-LET in
serum exosomes of patients with gastric cancer are high. The
results of a ROC curve analysis yielded a DANCR AUC value of 0.777.
As indicator, DANCR can indirectly reflect the levels of lncRNA-LET
and diagnose gastric cancer, and performs better than traditional
serological markers (carcinoembryonic antigen and carbohydrate
antigen 19-9 [CA19-9]). Based on this evidence, it was suggested
that DANCR may be a biomarker for the diagnosis of gastric cancer
(60). In addition, Hao et al
suggested that DANCR can be used as a prognostic index for the
growth and tumorigenicity of gastric cancer cells (61). Furthermore, through analysis of
lncRNA HOTTIP in the serum exosomes of 246 subjects, a study showed
that lncRNA HOXA distal transcript antisense RNA (HOTTIP) has
higher expression levels in exosomes of patients with gastric
cancer than in those of healthy individuals. The expression levels
were significantly related to the depth of invasion and TNM stage.
Moreover, the AUC of HOTTIP in exosomes was 0.827, indicating that
its diagnostic ability is higher than that of carcinoembryonic
antigen, CA19-9, and CA72-4. The Kaplan-Meier analysis showed a
correlation between increased levels of exosomal HOTTIP and poor
overall survival (log-rank P<0.001). Univariate and multivariate
COX (proportional hazards model) analyses revealed that
overexpression of exosomal HOTTIP is an independent prognostic
factor in patients with gastric cancer (P=0.027). These results
suggested that HOTTIP in exosomes may be a potential biomarker in
the diagnosis and prognosis of gastric cancer (62).
In conclusion, these lncRNAs demonstrated excellent
diagnostic performance in the laboratory. Although further research
is warranted to assess their utility in the clinical setting, these
lncRNAs show promise for the diagnosis of gastric cancer.
Promoting mechanism and role of
exosomal lncRNA in the treatment of gastric cancer
DANCR was first detected at high levels in liver
cancer (63). In subsequent studies,
DANCR was also proved to be expressed at high levels in exosomes of
patients with gastric cancer (60).
The expression of DANCR is closely related to tumor size, TNM
stage, lymph node metastasis, and depth of invasion. Knockout of
the DANCR gene can also inhibit epithelial–mesenchymal transition,
as well as the migration and invasion of gastric cancer cells.
Spalt-like transcription factor 4 (SALL4) activates DANCR.
Moreover, the β-catenin pathway is activated as a result of the
overexpression of DANCR (64).
Further studies have shown that lncRNA-LET is the target gene of
DANCR. DANCR binds to enhancer of zeste 2 polycomb repressive
complex 2 subunit (EZH2) and histone deacetylase 3 (HDAC3) to
silence lncRNA-LET, and subsequently regulate the migration and
invasion of gastric cancer. In summary, the DANCR-lncRNA-LET
mechanism plays an important role in the migration and invasion of
gastric cancer cells, revealing the new epigenetic mechanism of
lncRNA-LET silencing (65).
A study revealed that the prognosis of gastric
cancer patients with elevated levels of exosomal UFC1 is poor.
Knockout of the UFC1 gene successfully hindered the proliferation,
migration, and invasion of gastric cancer cells, while its
overexpression promoted these processes. Although the mechanism are
not fully understood, it is clear that UFC1 can act on miR-498 and
downregulate the expression of LIN28B (66).
LncRNAs may be more complex than miRNAs in terms of
the mechanism. The impact of changes of lncRNAs may be achieved by
interfering with miRNAs, as well as other effects. The complex
mechanisms of lncRNAs need to be further investigated. Relevant
information regarding the lncRNAs mentioned in this article is
presented in Fig. 1. The abnormal
expression of lncRNAs in exosomes of patients with gastric cancer
and the possible mechanism provide a new idea for treatment. For
example, as mentioned above, regulation of UFC1 has a certain
effect on the growth of gastric cancer cells (66), although more in-depth and detailed
studies are needed to extrapolate these laboratory data to clinical
practice.
4. CircRNA
Exosomal circRNA of gastric
cancer
Covalently closed circRNAs were originally found in
plant viruses (67). Unlike lncRNAs
and miRNAs, circRNAs do not have a 5' head and 3' tail, forming a
ring in a covalently closed manner. They were previously considered
by-products of indirect errors and thus, their role in life was
ignored (68,69). High expression of circRNAs was first
found in the brains of humans and mice. Studies have shown that it
functions as a miR-7 sponge. The circular transcript was later
termed circRNA sponge for miR-7 (CIRS-7). More than 70 selectively
conserved miRNA targets are present in CIRS-7. It exerts its effect
by binding to the Argonaute protein in a miR-7-dependent manner.
Circular CIRS-7 may act as a miRNA sponge binding to miR-7,
downregulating the expression of miR-7 and regulating downstream
oncogenes to promote tumor cell proliferation and metastasis.
Moreover, research revealed that the testis-specific circRNA
sex-determining region Y (Sry) serves as a miR138 sponge. This
finding suggested that the miRNA sponge effect of circRNAs is not
unique to some kinds of circRNAs. This study is the first
functional analysis of naturally expressed circRNAs (70). Although the evidence related to
exosomal circRNAs in terms of specificity, conservatism, and
stability is not as rich as that for miRNAs and lncRNAs, the
confirmed existence of circRNAs in exosomes provides a new
direction for the diagnosis and targeted therapy of tumors.
The role of exosomal circRNA in the
diagnosis of gastric cancer
Analysis of circRNA hsa_circ_0065149 in exosomes
collected from individuals with gastric cancer reported differences
in the expression levels between four stages: Healthy stomach,
gastritis, intestinal metaplasia, and gastric cancer.
Hsa_circ_0065149 in exosomes, as a molecular tool for the screening
of early gastric field cancerization, has higher sensitivity and
specificity than traditional clinical biomarkers. Statistical
analysis showed that the survival time of gastric cancer patients
with low levels of hsa_circ_0065149 is longer (P<0.020).
Moreover, the levels of hsa_circ_0065149 in patients is
significantly correlated to tumor diameter and nerve invasion
(71).
In addition to the circRNAs that are abnormally
expressed in the exosomes of patients with gastric cancer, an
increasing number of circRNAs associated with gastric cancer are
being discovered, such as circFNDC3B (72) and hsa_circ_0000144(73), and hsa_circ_0005654(74). However, thus far, there is not enough
evidence to prove that they are present in the exosomes and helpful
in the diagnosis.
Promoting mechanism and role of
exosomal circRNA in the treatment of gastric cancer
The supply of energy is of critical importance for
the growth and proliferation of cancer cells. Some kinds of cancer
cells can alter several points of the phosphatidy linositide
3-kinases/protein kinase B (PI3K/AKT) signaling pathway to change
their metabolism, in order to gain more selective advantages than
other cells (75). The
AKT/mechanistic target of rapamycin kinase (AKT/MTOR) axis is a
classical signaling pathway, which can meet the needs of gastric
cancer cell proliferation through the Warburg effect (76). In addition, AKT/MTOR can also promote
anabolism (e.g., protein synthesis) and prevent catabolic activity
(e.g., autophagy), and the final effect is beneficial to the growth
of gastric cancer cells (77).
Further study showed that the AKT/MTOR axis plays a positive role
in epithelial-mesenchymal transition and is closely related to
tumor metastasis (78,79). A recent study showed that exosomal
circRNA circNRIP1 can sponge miR-149-5p and alter the expression of
AKT1. Also, inhibition of exosomal circNRIP1 can affect the
AKT1/MTOR signaling pathway and block the malignant behavior of
gastric cancer cells (80).
Research on circRNAs is at a preliminary stage
compared with that for miRNAs and lncRNAs. Relevant information
regarding the circRNAs mentioned in this article is presented in
Fig. 1. However, it is expected that
more mechanisms of circRNAs will be revealed in the future.
CircRNAs can also be involved in the occurrence and development of
gastric cancer. Therefore, it may be possible to treat gastric
cancer by targeting circRNAs. More in-depth research will provide
new directions for the treatment of gastric cancer.
5. Discussion
Gastric cancer poses a threat to human health and is
responsible for a substantial number of death worldwide. Patients
with gastric cancer are often diagnosed at a late stage of the
disease, missing the optimal period for treatment (2,81).
Therefore, investigation of the pathogenesis and discovery of
effective diagnostic and therapeutic approaches is of great
importance.
The exosomes are stable in vitro and can be
stored at 4˚C for 96 h or at -70˚C for longer periods of time
(12). In addition to serum,
exosomes are also found in various fluids of the human body,
including blood, amniotic fluid, urine, malignant ascites,
cerebrospinal fluid, breast milk, saliva, lymph, and bile (12-14).
Moreover, the number of RNAs in the exosomes of gastric cancer was
several-fold higher than that of normal gastric mucosal epithelial
cells (82). These excellent
properties render exosomes and exosomal non-coding RNAs ideal
biomarker candidates. An increasing number of studies on exosomal
non-coding RNAs obtained from patients with gastric cancer found
that some important indexes of non-coding RNAs are better than
those of traditional gastric cancer markers (e.g., lncRNA DANCR)
(60).
In addition to diagnosis, elucidation of the
mechanism of exosomal non-coding RNAs involved in promoting or
inhibiting the development of gastric cancer may influence the
therapeutic strategy. Intervention with exosomal non-coding RNAs is
expected to become a new direction for the treatment of gastric
cancer.
It is thought that effective methods for the
diagnosis and treatment of gastric cancer through the use of
exosomal non-coding RNAs will be developed in the near future.
However, further research is warranted to translate these findings
from the laboratory to clinical practice.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Guangdong Province in China (grant no.
2016A030313602), Guangdong Provincial Science and Technology Plan
Projects (grant no. 2016B090913004) and the Science and Technology
Program of Guangzhou, China (grant no. 201604020007).
Availability of data and materials
Not applicable.
Authors' contributions
PG was responsible for writing and revising the
manuscript. DH was responsible for the collection and analysis of
relevant literature and contributed to the revision of the
manuscript. HWZ designed the project and acquired funding and
resources. JW and WL designed and drew of the table and figure, and
participated in the revision of the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu Qx and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Choi IJ, Kook MC, Kim YI, Cho JS, Lee JY,
Kim CG, Park B and Nam BH: Helicobacter pylori therapy for
the prevention of metachronous gastric cancer. New Engl J Med.
378:1085–1095. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sitarz R, Skierucha M, Mielko J, Offerhaus
GJA, Maciejewski R and Polkowski WP: Gastric cancer: Epidemiology,
prevention, classification, and treatment. Cancer Manag Res.
10:239–248. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu
S, Li Y, Wang L, Liu Y, Yin P, et al: Cause-specific mortality for
240 causes in China during 1990-2013: A systematic subnational
analysis for the Global Burden of disease study 2013. Lancet.
387:251–272. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Johnstone RM, Adam M, Hammond JR, Orr L
and Turbide C: Vesicle formation during reticulocyte maturation.
Association of plasma membrane activities with released vesicles
(exosomes). J Biol Chem. 262:9412–9420. 1987.PubMed/NCBI
|
|
7
|
Record M, Carayon K, Poirot M and
Silvente-Poirot S: Exosomes as new vesicular lipid transporters
involved in cell-cell communication and various pathophysiologies.
Biochim Biophys Acta. 1841:108–120. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Subra C, Grand D, Laulagnier K, Stella A,
Lambeau G, Paillasse M, Medina PD, Monsarrat B, Perret B, Poirot
SS, et al: Exosomes account for vesicle-mediated transcellular
transport of activatable phospholipases and prostaglandins. J Lipid
Res. 51:2105–2120. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Gezer U, Özgur E, Cetinkaya M, Isin M and
Dalay N: Long non-coding RNAs with low expression levels in cells
are enriched in secreted exosomes. Cell Biol Int. 38:1076–1079.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fanale D, Taverna S, Russo A and Bazan V:
Circular RNA in exosomes. Adv Exp Med Biol. 1087:109–117.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Taylor DD and Gercel-Taylor C: MicroRNA
signatures of tumor-derived exosomes as diagnostic biomarkers of
ovarian cancer. Gynecol Oncol. 110:13–21. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Simpson RJ, Lim JWE, Moritz RL and
Mathivanan S: Exosomes: Proteomic insights and diagnostic
potential. Expert Rev Proteomics. 6:267–283. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gallo A, Tandon M, Alevizos I and Illei
GG: The majority of MicroRNAs detectable in serum and saliva is
concentrated in exosomes. PLoS One. 7(e30679)2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Johnstone RM: The Jeanne Manery-fisher
memorial lecture 1991. Maturation of reticulocytes: Formation of
exosomes as a mechanism for shedding membrane proteins. Biochem
Cell Biol. 70:179–190. 1992.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Bang C and Thum T: Exosomes: New players
in cell-cell communication. Int J Biochem Cell Biol. 44:2060–2064.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tang MK and Wong AS: Exosomes: Emerging
biomarkers and targets for ovarian cancer. Cancer Lett. 367:26–33.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Simhadri VR, Reiners KS, Hansen HP,
Topolar D, Simhadri VL, Nohroudi K, Kufer TA, Engert A and von
Strandmann EP: Dendritic cells release HLA-B-associated
Transcript-3 positive exosomes to regulate natural Killer function.
PLoS One. 3(e3377)2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Admyre C, Johansson SM, Qazi KR, Filén JJ,
Lahesmaa R, Norman M, Neve EPA, Scheynius A and Gabrielsson S:
Exosomes with immune modulatory features are present in human
breast milk. J Immunol. 179:1969–1978. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gu Y, Li M, Wang T, Liang Y, Zhong Z, Wang
X, Zhou Q, Chen L, Lang Q, He Z, et al: Lactation-related MicroRNA
expression profiles of porcine breast milk exosomes. PLoS One.
7(e43691)2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pontes Azevedo LC, Janiszewski M, Pontieri
V, Pedro MDA, Bassi E, Tucci PJF and Laurindo FRM: Platelet-derived
exosomes from septic shock patients induce myocardial dysfunction.
Crit Care. 11(R120)2007.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Masyuk AI, Masyuk TV and LaRusso NF:
Exosomes in the pathogenesis, diagnostics and therapeutics of liver
diseases. J Hepatol. 59:621–625. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Vella LJ, Sharples RA, Nisbet RM, Cappai R
and Hill AF: The role of exosomes in the processing of proteins
associated with neurodegenerative diseases. Eur Biophys J.
37:323–332. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rak J and Guha A: Extracellular
vesicles-vehicles that spread cancer genes. Bioessays. 34:489–497.
2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sund M and Kalluri R: Tumor stroma derived
biomarkers in cancer. Cancer Metastasis Rev. 28:177–183.
2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kalluri R and Zeisberg M: Fibroblasts in
cancer. Nat Rev Cancer. 6:392–401. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Skog J, Wuerdinger T, van Rijn S, Meijer
DH, Gainche L, Esteves MS, Curry WT Jr, Carter BS, Krichevsky AM
and Breakefield XO: Glioblastoma microvesicles transport RNA and
proteins that promote tumour growth and provide diagnostic
biomarkers. Nat Cell Biol. 10:1470–1476. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Kogure T, Lin WL, Yan IK, Braconi C and
Patel T: Intercellular Nanovesicle-mediated microRNA transfer: A
mechanism of environmental modulation of hepatocellular cancer cell
growth. Hepatology. 54:1237–1248. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ohshima K, Inoue K, Fujiwara A, Hatakeyama
K, Kanto K, Watanabe Y, Muramatsu K, Fukuda Y, Ogura SI, Yamaguchi
K and Mochizuki T: Let-7 MicroRNA family is selectively secreted
into the extracellular environment via exosomes in a metastatic
gastric cancer cell line. PLoS One. 5(e13247)2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pan L, Liang W, Fu M, Huang ZH, Li X,
Zhang W, Zhang P, Qian H, Jiang PC, Xu WR and Zhang X:
Exosomes-mediated transfer of long noncoding RNA ZFAS1 promotes
gastric cancer progression. J Cancer Res Clini Oncol. 143:991–1004.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Melo SA, Sugimoto H, O'Connell JT, Kato N,
Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, et
al: Cancer exosomes perform cell-independent microRNA biogenesis
and promote tumorigenesis. Cancer Cell. 26:707–721. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang X, Guo H, Bao Y, Yu H, Xie D and
Wang X: Exosomal long non-coding RNA DLX6-AS1 as a potential
diagnostic biomarker for non-small cell lung cancer. Oncol Lett.
18:5197–5204. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang H, Xu S and Liu X: MicroRNA
profiling of plasma exosomes from patients with ovarian cancer
using high-throughput sequencing. Oncol Lett. 17:5601–5607.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Crentsil VC, Liu H and Sellitti DF:
Comparison of exosomal microRNAs secreted by 786-O clear cell renal
carcinoma cells and HK-2 proximal tubule-derived cells in culture
identifies microRNA-205 as a potential biomarker of clear cell
renal carcinoma. Oncol Lett. 16:1285–1290. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yoshikawa M, Iinuma H, Umemoto Y,
Yanagisawa T, Matsumoto A and Jinno H: Exosome-encapsulated
microRNA-223-3p as a minimally invasive biomarker for the early
detection of invasive breast cancer. Oncol Lett. 15:9584–9592.
2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Reinhart BJ, Slack FJ, Basson M,
Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR and Ruvkun G:
The 21-nucleotide let-7 RNA regulates developmental timing in
Caenorhabditis elegans. Nature. 403:901–906. 2000.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Shukla GC, Singh J and Barik S: MicroRNAs:
Processing, maturation, target recognition and regulatory
functions. Mol Cell Pharmacol. 3:83–92. 2011.PubMed/NCBI
|
|
39
|
Wang N, Wang L, Yang Y, Gong L, Xiao B and
Liu X: A serum exosomal microRNA panel as a potential biomarker
test for gastric cancer. Biochem Biophys Res Commun. 493:1322–1328.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Li W and Gao YQ: MiR-217 is involved in
the carcinogenesis of gastric cancer by down-regulating CDH1
expression. Kaohsiung J Med Sci. 34:377–384. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang J, Guan X, Zhang Y, Ge S, Zhang L, Li
H, Wang X, Liu R, Ning T, Deng T, et al: Exosomal miR-27a derived
from gastric cancer cells regulates the transformation of
fibroblasts into cancer-associated fibroblasts. Cell Physiol
Biochem. 49:869–883. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Jiying H, Manru S, Meizhu Y, Ying C,
Zhenjun G and Xin M: Exosome-mediated transfer of miR-1290 promotes
cell proliferation and invasion in gastric cancer via NKD1. Acta
Biochim Biophys Sin (Shanghai). 51:900–907. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wang M, Zhao C, Shi H, Zhang B, Zhang L,
Zhang X, Wang S, Wu X, Yang T, Huang F, et al: Deregulated
microRNAs in gastric cancer tissue-derived mesenchymal stem cells:
Novel biomarkers and a mechanism for gastric cancer. Br J Cancer.
110:1199–1210. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Yang H, Zhang H, Ge S, Ning T, Bai M, Li
J, Li S, Sun W, Deng T, Zhang L, et al: Exosome-derived miR-130a
activates angiogenesis in gastric cancer by targeting C-MYB in
vascular endothelial cells. Mol Ther. 26:2466–2475. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Imamura T, Komatsu S, Ichikawa D, Miyamae
M, Okajima W, Ohashi T, Kiuchi J, Nishibeppu K, Kosuga T, Konishi
H, et al: Low plasma levels of miR-101 are associated with tumor
progression in gastric cancer. Oncotarget. 8:106538–106550.
2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kessenbrock K, Plaks V and Werb Z: Matrix
Metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Hwang RF, Moore T, Arumugam T,
Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB and Logsdon CD:
Cancer-associated stroma fibroblasts promote pancreatic tumor
progression. Cancer Res. 68:918–926. 2008.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Albini A, Tosetti F, Li VW, Noonan DM and
Li WW: Cancer prevention by targeting angiogenesis. Nat Rev Clin
Oncol. 9:498–509. 2012.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Bergers G and Benjamin LE: Tumorigenesis
and the angiogenic switch. Nat Rev Cancer. 3:401–410.
2003.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Talasila KM, Soentgerath A, Euskirchen P,
Rosland GV, Wang J, Huszthy PC, Prestegarden L, Skaftnesmo KO,
Sakariassen PQ, Eskilsson E, et al: EGFR wild-type amplification
and activation promote invasion and development of glioblastoma
independent of angiogenesis. Acta Neuropathol. 125:683–698.
2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Cully M: Tumour vessel normalization takes
centre stage. Nat Rev Drug Discovery. 16:87. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Viallard C and Larrivee B: Tumor
angiogenesis and vascular normalization: Alternative therapeutic
targets. Angiogenesis. 20:409–426. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ramsay RG, Barton AL and Gonda TJ:
Targeting c-Myb expression in human disease. Expert Opin Ther
Targets. 7:235–248. 2003.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wang L, Liu Q, Zhou W, Shao H, Li F and Li
X: Prognostic role of myeloid cell Leukemia-1 protein (Mcl-1)
expression in human gastric cancer. J Surg Oncol. 100:396–400.
2009.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Ke X, Yan R, Sun Z, Cheng Y, Meltzer A, Lu
N, Shu X, Wang Z, Huang B, Liu X, et al: Esophageal
adenocarcinoma-derived extracellular vesicle MicroRNAs induce a
neoplastic phenotype in gastric organoids. Neoplasia. 19:941–949.
2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Roush S and Slack FJ: The let-7 family of
microRNAs. Trends Cell Biol. 18:505–516. 2008.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Boyerinas B, Park SM, Hau A, Murmann AE
and Peter ME: The role of let-7 in cell differentiation and cancer.
Endocrine Related Cancer. 17:F19–F36. 2010.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Kolat D, Hammouz R, Bednarek AK and
Pluciennik E: Exosomes as carriers transporting long noncoding
RNAs: Molecular characteristics and their function in cancer
(Review). Mol Med Rep. 20:851–862. 2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Lin LY, Yang L, Zeng Q, Wang L, Chen ML,
Zhao ZH, Ye GD, Luo QC, Lv PY, Guo QW, et al: Tumor-originated
exosomal lncUEGC1 as a circulating biomarker for early-stage
gastric cancer. Mol Cancer. 17(84)2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Yang L, Lei P, Wei L, Peng Z, Hui Q,
Wenrong X, Zhijian Z, Pengcheng J and Xu Z: Detection and clinical
value of serum exosomal DANCR in gastric cancer patients. Chin J
Clin Lab Sci. 35:171–174. 2017.(In Chinese).
|
|
61
|
Hao YP, Qiu JH, Zhang DB and Yu CG: Long
non-coding RNA DANCR, a prognostic indicator, promotes cell growth
and tumorigenicity in gastric cancer. Tumour Biol.
39(1010428317699798)2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Zhao R and Zhang Y, Zhang X, Yang Y, Zheng
X, Li X, Liu Y and Zhang Y: Exosomal long noncoding RNA HOTTIP as
potential novel diagnostic and prognostic biomarker test for
gastric cancer. Mol Cancer. 17(68)2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Yuan SX, Wang J, Yang F, Tao QF, Zhang J,
Wang LL, Yang Y, Liu H, Wang ZG, Xu QG, et al: Long noncoding RNA
DANCR increases stemness features of hepatocellular carcinoma by
derepression of CTNNB1. Hepatology. 63:499–511. 2016.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Pan L, Liang W, Gu J, Zang X, Huang Z, Shi
H, Chen J, Fu M, Zhang P, Xiao X, et al: Long noncoding RNA DANCR
is activated by SALL4 and promotes the proliferation and invasion
of gastric cancer cells. Oncotarget. 9:1915–1930. 2017.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Mao Z, Li H, Du B, Cui K, Xing Y, Zhao X
and Zai S: LncRNA DANCR promotes migration and invasion through
suppression of lncRNA-LET in gastric cancer cells. Biosci Rep.
37(BSR20171070)2017.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Zhang X, Liang W, Liu J, Zang X, Gu J, Pan
L, Shi H, Fu M, Huang Z, Zhang Y, et al: Long non-coding RNA UFC1
promotes gastric cancer progression by regulating miR-498/Lin28b. J
Exp Clin Cancer Res. 37(134)2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Sanger HL, Klotz G, Riesner D, Gross HJ
and Kleinschmidt AK: Viroids are single-stranded covalently closed
circular RNA molecules existing as highly base-paired rod-like
structures. Proc Natl Acad Sci USA. 73:3852–3856. 1976.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Cocquerelle C, Mascrez B, Hetuin D and
Bailleul B: Mis-splicing yields circular RNA molecules. FASEB J.
7:155–160. 1993.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Pasman Z, Been MD and Garcia-Blanco MA:
Exon circularization in mammalian nuclear extracts. RNA. 2:603–610.
1996.PubMed/NCBI
|
|
70
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Shao Y, Tao X, Lu R, Zhang H, Ge J, Xiao
B, Ye G and Guo J: Hsa_circ_0065149 is an indicator for Early
Gastric cancer screening and prognosis prediction. Pathol Oncol
Res: Aug 20, 2019 (Epub ahead of print).
|
|
72
|
Hong Y, Qin H, Li Y, Zhang Y, Zhuang X,
Liu L, Lu K, Li L, Deng X, Liu F, Shi S and Liu G: FNDC3B circular
RNA promotes the migration and invasion of gastric cancer cells via
the regulation of E-cadherin and CD44 expression. J Cell Physiol.
234:19895–19910. 2019.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Wei J, Wang J, Gao X and Qi F:
Identification of differentially expressed circRNAs and a novel
hsa_circ_0000144 that promote tumor growth in gastric cancer.
Cancer Cell Int. 19(268)2019.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Wang Y, Xu S, Chen Y, Zheng X, Li T and
Guo J: Identification of hsa_circ_0005654 as a new early biomarker
of gastric cancer. Cancer Biomark. 26:403–410. 2019.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Mosca E, Barcella M, Alfieri R, Bevilacqua
A, Canti G and Milanesi L: Systems biology of the metabolic network
regulated by the Akt pathway. Biotechnol Adv. 30:131–141.
2012.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Giguere V: Canonical signaling and nuclear
activity of mTORa teamwork effort to regulate metabolism and cell
growth. FEBS J. 285:1572–1588. 2018.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Heras-Sandoval D, Perez-Rojas JM,
Hernandez-Damian J and Pedraza-Chaverri J: The role of
PI3K/AKT/mTOR pathway in the modulation of autophagy and the
clearance of protein aggregates in neurodegeneration. Cell Signal.
26:2694–2701. 2014.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Yang Y, Gao M, Lin Z, Chen L, Jin Y, Zhu
G, Wang Y and Jin T: DEK promoted EMT and angiogenesis through
regulating PI3K/AKT/mTOR pathway in triple-negative breast cancer.
Oncotarget. 8:98708–98722. 2017.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Wu ZH, Lin C, Liu CC, Jiang WW, Huang MZ,
Liu X and Guo WJ: MiR-616-3p promotes angiogenesis and EMT in
gastric cancer via the PTEN/AKT/mTOR pathway. Biochem Biophys Res
Commun. 501:1068–1073. 2018.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Zhang X, Wang S, Wang H, Cao J, Huang X,
Chen Z, Xu P, Sun G, Xu J, Lv J and Xu Z: Circular RNA circNRIP1
acts as a microRNA-149-5p sponge to promote gastric cancer
progression via the AKT1/mTOR pathway. Mol Cancer.
18(20)2019.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Allemani C, Weir HK, Carreira H, Harewood
R, Spika D, Wang XS, Bannon F, Ahn JV, Johnson CJ, Bonaventure A,
et al: Global surveillance of cancer survival 1995-2009: Analysis
of individual data for 25 676 887 patients from 279
population-based registries in 67 countries (CONCORD-2). Lancet.
385:977–1010. 2015.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Ren J, Zhou Q, Li H, Li J, Pang L, Su L,
Gu Q, Zhu Z and Liu B: Characterization of exosomal RNAs derived
from human gastric cancer cells by deep sequencing. Tumor Biol.
39(1010428317695012)2017.PubMed/NCBI View Article : Google Scholar
|















