Introduction
A total of >180 million people are chronically
infected with hepatitis C virus (HCV) worldwide and chronic HCV
infection may lead to the development of chronic hepatitis, liver
cirrhosis and hepatocellular carcinoma (HCC) (1). Once patients have progressed to liver
cirrhosis, there is an annual 3-5% risk of developing HCC (2). Recently, treatment of HCV infection
has been revolutionized by the development of direct-acting
antiviral agents (DAAs) and sustained virologic response (SVR)
rates of >90% have been achieved, regardless of the HCV genotype
(3). In addition, interferon
(IFN)-free regimens have enabled patients with HCV who are
ineligible for conventional IFN therapy owing to depression,
cytopenia, and autoimmune diseases to be cured of HCV infection
without any severe adverse events (4).
Lymphoproliferative disorders, including B-cell
non-Hodgkin's lymphomas (NHLs), are important extrahepatic
manifestations associated with chronic HCV infection (5). The most common B-cell NHLs in
HCV-infected patients are marginal zone lymphomas (MZLs), diffuse
large B-cell lymphomas (DLBCLs) and lymphoplasmacytic lymphoma
(6). The pathogenesis of
HCV-related lymphomagenesis is still under investigation; however,
chronic antigen stimulation and genetic mutations arising from
HCV-induced replication proteins are the most accepted mechanisms
(5,7).
IFN-free DAA treatment for HCV is associated with
high success rates of viral clearance and significantly improved
overall and disease-free survival following curative treatment in
patients with HCV-related HCC (8-10).
Previous studies reported that anti-HCV treatment with IFN-free
DAAs induced clinical remission of HCV-related indolent, low-grade
B-cell NHLs (11-18),
and concomitant or subsequent use of DAAs with chemotherapy
resulted in higher disease-free survival rates in patients with
HCV-related DLBCL compared with chemotherapy alone (13,19,20).
Thus, anti-HCV treatment with DAAs has been suggested as a possible
therapeutic intervention for patients with HCV-related
lymphoproliferative disorders, particularly in terms of improving
patient outcomes (17,19-21).
Although recent studies have reported late-onset B-cell NHLs
following HCV clearance with DAAs (22-26)
(Table I), the mechanisms
underlying this association have not been clarified. Accordingly,
the current study presents a case of a patient with DLBCL occurring
after HCV clearance with sofosbuvir-ledipasvir treatment and
discusses the possible underlying mechanisms by reviewing recent
publications.
 | Table IReported cases of late-onset B-cell
NHLs after HCV clearance with DAAs. |
Table I
Reported cases of late-onset B-cell
NHLs after HCV clearance with DAAs.
| Author, year | Age
(years)/sex | Liver status | Antiviral
agents | Time to onset of
NHLs after antiviral treatment | Histological
classification of NHLs | Gene mutation | Treatment for
NHLs | Outcome | (Refs.) |
|---|
| Lin et al,
2016 | 69 M | CH | SOF/RBV | 1 | Aggressive MCL | N/A | R-hyperCVAD | PR | (22) |
| Lin et al,
2016 | 61 M | CH | SOF/RBV | 1 | Aggressive MCL | p53 | R-hyperCVAD | SD | (22) |
| Ohzato et
al, 2017 | 81 F | CH | SOF/LDV | 7 | DLBCL | N/A | Tumor
resection | CR | (23) |
| Rodríguez de
Santiago et al, 2018 | 73 M | LC | SOF/LDV/RBV | 10 | MZL | N/A | R-B | PR | (24) |
| Rodríguez de
Santiago et al, 2018 | 62 F | LC | SOF/LDV | 19 | Indolent MCL | N/A | No treatment
(Follow up) | SD | (24) |
| Andrade et
al, 2018 | 55 M | LC | SOF/RBV | 12 | DLBCL | N/A | R-CHOP | N/A | (25) |
| Iwane et al,
2019 | 70 M | CH | SOF/LDV | 1 | DLBCL | N/A | R-DeVIC | CR | (26) |
| Current study | 77 M | CH | SOF/LDV | 2 | DLBCL | N/A | R-CHOP | CR | - |
Case report
A 61-year-old man was diagnosed with chronic
hepatitis C in April 2000 and treated by conventional IFN
monotherapy for 24 weeks from August 2000 to January 2001. However,
he did not achieve SVR and was subsequently treated with
ursodeoxycholic acid (UDCA) at a local clinic. He was followed up
every 3 months and serum alanine aminotransferase (ALT) levels
remained normal for 15 years. In February 2016, when the patient
was 77 years of age, he was admitted to Gifu University Hospital
(Gifu, Japan) to evaluate the indication of IFN-free DAA treatment.
His HCV was genotyped as 1b with an HCV RNA 6.5 log IU/ml and no
genetic alterations were observed in both non-structural protein 3
(NS3) and non-structural protein 5A (NS5A) regions. Imaging studies
with systemic dynamic computed tomography showed the appearance of
chronic liver damage, as demonstrated by blunting of the liver
edge; however, neither liver cirrhosis nor hepatocellular carcinoma
were observed. Moreover, no detectable lymph node swelling was
observed. Although serum ALT levels and platelet counts were
normal, the patient was categorized into the intermediate liver
cancer risk group owing to his advanced age. Therefore, according
to the treatment guidelines for chronic hepatitis C published by
The Japan Society of Hepatology (4), 12 weeks of sofosbuvir-ledipasvir
treatment were initiated in March 2016 to reduce the risk of liver
cancer in this patient. Serum HCV RNA levels rapidly decreased to
an undetectable level at week 4 of treatment and serum ALT levels
remained normal without UDCA. Thereafter, sofosbuvir-ledipasvir
treatment was completed as scheduled in May 2016 without any
adverse events.
In July 2016, 2 months after the end of
sofosbuvir-ledipasvir treatment, the patient had occasionally found
swelling of the right cervical lymph nodes, although no subjective
symptoms such as fever, night sweats and body weight loss were
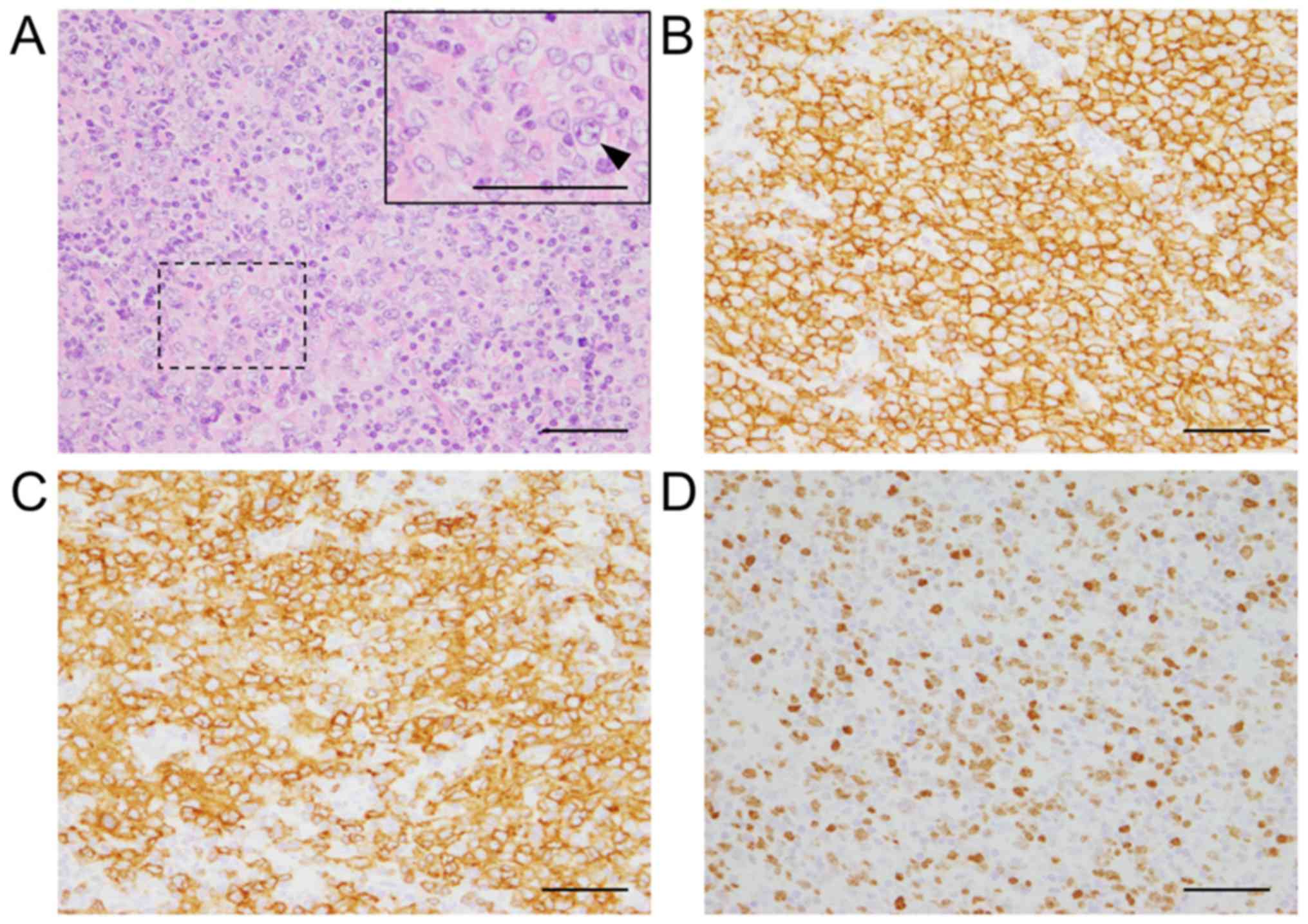
observed. Lymph node biopsy revealed diffuse proliferation of large
abnormal cells (Fig. 1A) and
immunostaining confirmed the expression of CD20 (Fig. 1B) and CD79α (Fig. 1C) in abnormal cells, leading to a
diagnosis of DLBCL. Additionally, the Ki-67 proliferation index of
the abnormal cells was ~80% (Fig.
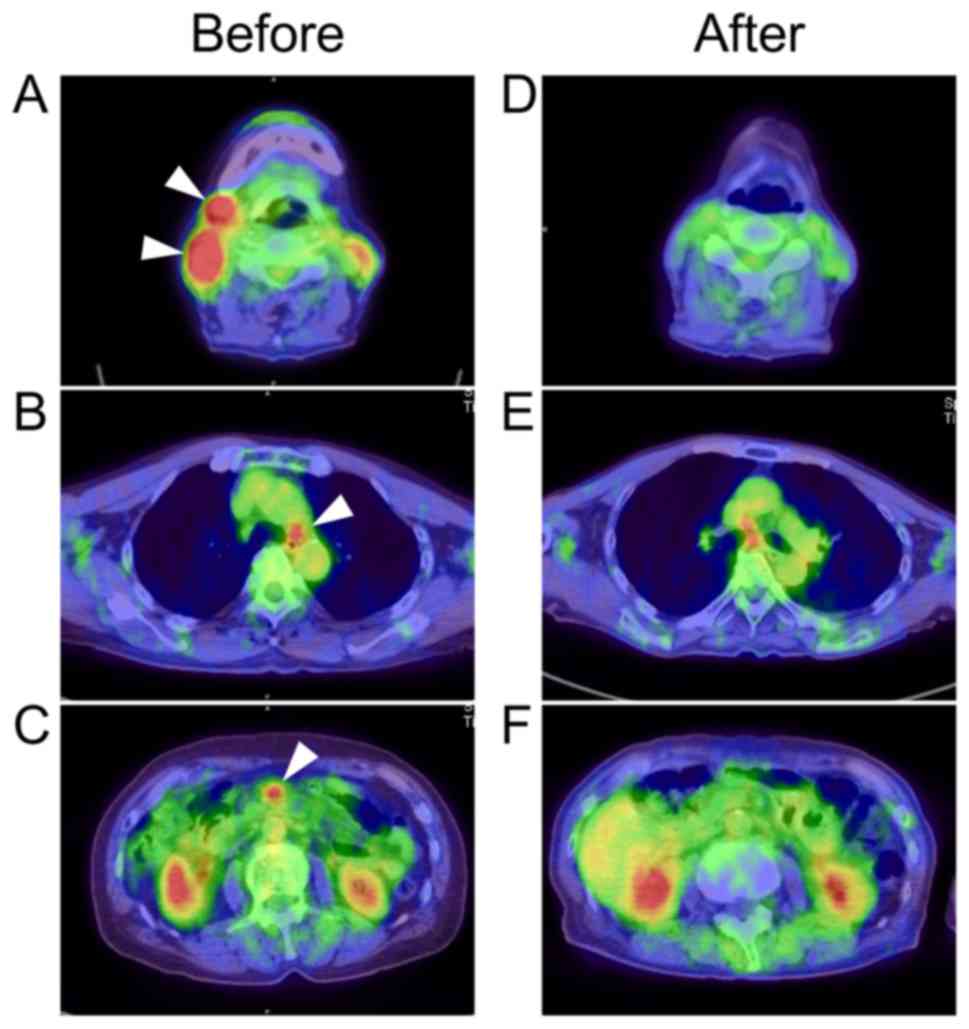
1D). Whole-body 18F-fluorodeoxyglucose positron
emission tomography with computed tomography (FDG-PET/CT) showed
increased FDG uptake in the right cervical, right submandibular
(Fig. 2A), mediastinal (Fig. 2B) and mesenteric (Fig. 2C) lymph nodes and maximum
standardized uptake values in these lesions were 18.83, 10.38, 4.75
and 4.89, respectively. In addition, no extranodal sites were
observed in the imaging study or bone marrow examinations.
Laboratory analyses revealed slight elevation of serum soluble
interleukin-2 receptor (sIL-2R); however, no abnormal values were
observed in peripheral blood or biochemistry, including lactate
dehydrogenase (Table II). Based on
the above aforementioned clinical data, his DLBCL was evaluated as
follows: Ann Arbor stage ⅢA; International Prognostic Index (IPI),
low-intermediate. Thereafter, he received six courses of R-CHOP
(rituximab, cyclophosphamide, doxorubicin, vincristine and
prednisolone) chemotherapy between September 2016 to December 2016
and achieved complete response for DLBCL in February 2017, as shown
by FDG-PET/CT after R-CHOP treatment (Fig. 2D-F). No recurrence of HCV RNA or
DLBCL was observed for at least 3 years after chemotherapy.
 | Table IILaboratory data at the time of DLBCL
diagnosis. |
Table II
Laboratory data at the time of DLBCL
diagnosis.
| Parameter | Data | N.R. |
|---|
| WBC (cells/µl) | 5,270 | 3,300-8,600 |
| RBC
(x106/µl) | 5.32 | 4.35-5.55 |
| Hb (g/dl) | 14.5 | 13.7-16.8 |
| Ht (%) | 44 | 40.7-50.1 |
| Plt
(x103/µl) | 167 | 158-348 |
| TP (g/dl) | 7.4 | 6.6-8.1 |
| Alb (g/dl) | 4.4 | 4.1-5.1 |
| AST (U/l) | 19 | 15-30 |
| ALT (U/l) | 11 | 10-42 |
| LDH (U/l) | 201 | 124-222 |
| ALP (U/l) | 286 | 106-322 |
| γ-GTP (U/l) | 13 | 13-64 |
| T.Bil (mg/dl) | 0.7 | 0.4-1.5 |
| UN (mg/dl) | 19.3 | 8.0-20.0 |
| Cr (mg/dl) | 0.88 | 0.65-1.07 |
| Na (mmol/l) | 140 | 134-145 |
| K (mmol/l) | 4.2 | 3.6-4.8 |
| Cl (mmol/l) | 107 | 101-108 |
| CRP (mg/dl) | 0.07 | <0.14 |
| FBS (mg/dl) | 94 | 73-109 |
| HbA1c (%) | 5.4 | 4.9-6.0 |
| PT (%) | 100 | 70-130 |
| FIB (mg/dl) | 357 | 200-400 |
| FDP (µg/ml) | 2.5 | <5.0 |
| D-dimer
(µg/ml) | 1.5a | <1.0 |
| IgG (mg/dl) | 1363 | 861-1,747 |
| IgA (mg/dl) | 266 | 93-393 |
| IgM (mg/dl) | 71 | 33-183 |
| β2-MG (mg/l) | 2.3a | 1.0-1.9 |
| sIL-2R (U/ml) | 899a | 122-496 |
| HCV-Ab | (+) | (-) |
| HCV-RNA (Log
IU/ml) | N.D. | N.D. |
| HBs-Ag | (-) | (-) |
| HBs-Ab | (-) | (-) |
| HBc-Ab | (-) | (-) |
| HIV-1/2 Ab | (-) | (-) |
| HTLV-1 Ab | (-) | (-) |
| AFP (ng/ml) | 2.3 | <10 |
Histological examination was performed on biopsied
lymph node specimens fixed with 10% formalin for 24 h at room
temperature. Paraffin-embedded tissues were cut into 4 µm-thick
sections and deparaffinized. These sections were stained with
hematoxylin and eosin or used for immunohistochemical analysis. For
hematoxylin and eosin staining, the sections were stained with 0.1%
hematoxylin solution for 4 min at room temperature and then stained
with 0.1% eosin Y (cat. no. 058-00062; Wako Pure Chemical
Industries, Ltd.) solution for 2 min at room temperature. For
immunohistochemistry, the deparaffinized sections were placed in a
citrate buffer solution (pH 6.0), and then autoclaved at 121˚C for
1 min for antigen retrieval. The sections were then rinsed and
blocked with 3% hydrogen peroxide in methanol for 10 min to remove
endogenous peroxidase activity. Non-specific binding sites were
blocked in 0.01 M phosphate-buffered saline containing 2% bovine
serum albumin (cat. no. 019-07494; Wako Pure Chemical Industries,
Ltd.) for 30 min. The sections were then incubated overnight at 4˚C
with the following antibodies in blocking buffer: Anti-CD20 [1:200,
mouse immunoglobulin G (IgG); cat. no. NCL-L-CD20-L26; Leica
Biosystems Inc.], anti-CD79α (1:200, mouse IgG; cat. no. IR621;
Dako; Agilent Technologies, Inc.), and anti-Ki-67 (1:200; mouse
IgG, cat. no. M7240; Dako; Agilent Technologies, Inc.). These
antibodies were detected using a biotinylated anti-mouse IgG
(1:300; cat. no. E0433; Dako; Agilent Technologies, Inc.) for 30
min at room temperature, followed by incubation with avidin-coupled
peroxidase (Vectastain ABC kit; Vector Laboratories, Inc.; Maravai
LifeSciences) for 30 min at room temperature. The peroxidase
binding sites were visualized by incubation with
3,3'-diaminobenzidine in 50 mM Tris-EDTA buffer and counterstained
with hematoxylin for a few seconds at room temperature. These
sections were then observed using a light microscope
(magnification, x400; Olympus BX53; Olympus Corporation).
Discussion
The present case report presented a case of a
patient with DLBCL occurring early after HCV clearance with
sofosbuvir-ledipasvir treatment. Previous publications reported
that HCV clearance with DAAs improved outcomes in patients with
HCV-related B-cell NHLs (11-20),
whereas late-onset B-cell NHLs after HCV clearance with DAAs have
occasionally been reported (22-26).
Thus far, only seven cases have been reported since 2016 (22-26)
(Table I) and the underlying
mechanisms remain to be elucidated.
Lymphoproliferative disorders are important
extrahepatic manifestations associated with chronic HCV infection
(6). A recent meta-analysis of
epidemiological studies showed that HCV-seropositive patients have
an estimated 5- to 10-fold increased risk for B-cell NHLs compared
with the general population (27,28).
Thus, patients with chronic HCV infection are at higher risk of
B-cell NHLs.
Currently, the pathogenesis of HCV-related
lymphomagenesis remains unclear. However, the following three
general theories have been proposed to explain the association
(5). First, B-cell receptors are
continuously stimulated by external HCV viral antigens, leading to
consecutive B-cell proliferation. Second, HCV replication inside
B-cells produces HCV-derived viral proteins that induce genetic
damage in the B-cells. Third, permanent genetic B-cell damage can
be caused by transient intracellular HCV infection. Indeed, several
basic studies have shown that the HCV envelope protein E3 binds to
CD81, a surface protein on B-lymphocytes, and forms a costimulatory
complex with CD19 and CD21, which in turn stimulates intracellular
proliferative signals (29,30). Moreover, acute or chronic HCV
infections are known to be associated with increased frequencies of
BCL-6 and p53 gene mutations in B-cells in
vitro; mutations in these genes have been linked to DLBCL
(31). Accordingly, HCV-related
lymphomagenesis may be attributed to either chronic viral antigen
stimulation or genetic mutations that lead to the clonal expansion
and malignant transformation of B-cells, as previously reported
(7,5,25).
During the past few years, several clinical studies
have evaluated extrahepatic malignancies after DAA treatment for
chronic HCV infection. A retrospective cohort study showed that
three of 431 patients who received DAA treatment were diagnosed
with DLBCL after HCV eradication; the prevalence in this cohort was
696 per 100,000, which was ~30 times higher compared with the
general population (32). In
addition, El-Serag et al (1)
also reported that successful DAA treatment resulting in SVR was
not associated with reduction in NHL risk. Moreover, in a
comparative study of the risk of hematologic malignancies following
IFN-induced SVR and DAA-induced SVR, researchers showed that
IFN-induced SVR significantly reduced the risk of hematologic
malignancies, including lymphoma and myeloma (adjusted hazard
ratio, 0.67; 95% confidence interval, 0.53-0.84), whereas
DAA-induced SVR was not associated with a reduction in the risk of
hematologic malignancies (adjusted hazard ratio, 1.08; 95%
confidence interval, 0.66-1.78) (33). Thus, these results suggested that
the development of malignant lymphomas may occur after HCV
clearance with DAAs, supporting recent case reports (22-26),
including the present case (Table
I).
As shown in Table I,
only seven cases of late-onset B-cell NHLs after HCV clearance with
DAAs have been reported since 2016 (22-26).
Currently, the underlying mechanisms remain unclear. However,
several possible mechanisms have been proposed in recent
publications. Andrade et al (25) suggested that HCV-induced genetic
damage produced a survival signal in B-cells, which may lead to
late transformation, even years after successful HCV therapy.
Indeed, genetic mutations derived from either acute or chronic HCV
infection are known to persist, even after HCV clearance (31). Notably, unlike conventional IFN
therapy, DAAs lack the ability to either directly treat a
subclinical malignancy or enhance an immune response to malignancy
(33). In particular, in terms of
immune response, HCV clearance with DAAs was reported to be
associated with the persistence of CD4 regulatory T cells (34), which inhibit cytotoxic
CD8+ T cells exposed to B-cell NHLs (35). Moreover, Reig et al (36) suggested that DAA-induced SVR
promotes a hyporesponsive state of memory helper T cells to tumor
antigen, which may leave patients who overcame HCV infection
vulnerable to the development of malignancies. Thus, it is
plausible that premalignant B-cells with genetic mutations may
survive by escaping immune surveillance after HCV clearance with
DAAs, leading to transformation of these B-cells into malignant
clones, such as DLBCL.
Although it is still unclear whether DAAs have
direct effects on tumor development, a previous clinical study
revealed an association between DAA treatment and serum vascular
endothelial growth factor (VEGF) levels (37). The levels of serum VEGF during DAA
treatment in patients with HCV were approximately 4-fold higher
than those during pretreatment, and serum VEGF levels remained
elevated through the end of treatment (37). Notably, increased VEGF expression is
also observed in serum or tissues in hematologic malignancies,
thereby accelerating tumor growth by promoting angiogenesis and
vasopermeability (38). In
particular, DLBCL frequently expresses VEGF and its receptors
VEGFR-1 and VEGFR-2, both of which are correlated with the
development of DLBCL via autocrine and paracrine mechanisms
(39). Based on these findings,
extrinsic VEGF induced by DAA treatment may also be associated with
the development of DLBCL.
In all cases presented in Table I, the patients were treated with
antiviral regimens, including sofosbuvir, a new class of specific
nucleotide analog inhibitors of HCV NS5B polymerase (22). To date, sofosbuvir has been reported
to be effective in treating HCV-related MZL (17,18);
however, to the best of our knowledge, this agent has not been
reported to induce lymphomagenesis as an adverse effect. Further
studies are needed to investigate whether sofosbuvir indeed induces
lymphomagenesis. In addition, one case presented in Table I included a p53 gene mutation in the
NHL of the patient (22); however,
any common genetic mutations in the previously reported cases and
the current case are unknown due to the lack of information
available from the previous reports (22-26).
Currently, to the best of our knowledge, there are no reports
demonstrating an association between p53 gene mutation in B-cells
and sofosbuvir treatment.
Several possible mechanisms of late-onset B-cell NHL
after successful DAA treatment have been reported in the recent
publications described above; however, the detailed mechanisms
remain unclear owing to the small number of reported cases and the
small sample size in each study. More clinical studies are needed
to elucidate the relationships between DAA treatment and late-onset
B-cell NHLs after successful antiviral therapy.
In conclusion, the present study reported the case
of a patient with DLBCL that occurred early after HCV clearance
with sofosbuvir-ledipasvir treatment. The development of lymphoid
malignancies may occur, even after successful HCV treatment with
DAAs. Therefore, clinicians should be aware of such risks during
and after antiviral treatment with DAAs.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
HS and MShim collaborated in the conception and
design of the study. HS, TM, YI, TH, NN, KI, JK and YS performed
the case study and acquired the data and images for the case. HS
performed data analysis and interpretation and wrote the
manuscript. HS, KI, NK, AS, KT, MShir, and MShim revised the
manuscript and MShim supervised manuscript preparation. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was provided by the patient
for publication of this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
El-Serag HB, Christie IC, Puenpatom A,
Castillo D, Kanwal F and Kramer JR: The effects of sustained
virological response to direct-acting anti-viral therapy on the
risk of extrahepatic manifestations of hepatitis C infection.
Aliment Pharmacol Ther. 49:1442–1447. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fattovich G, Stroffolini T, Zagni I and
Donato F: Hepatocellular carcinoma in cirrhosis: Incidence and risk
factors. Gastroenterology. 127 (5 Suppl 1):S35–S50. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pawlotsky JM, Feld JJ, Zeuzem S and
Hoofnagle JH: From non-A, non-B hepatitis to hepatitis C virus
cure. J Hepatol. 62 (Suppl 1):S87–S99. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Drafting Committee for Hepatitis
Management Guidelines the Japan Society of Hepatology. JSH
Guidelines for the management of hepatitis C virus infection: A
2014 update for genotype 1. Hepatol Res. 44 (Suppl S1):S59–S70.
2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Peveling-Oberhag J, Arcaini L, Hansmann ML
and Zeuzem S: Hepatitis C-associated B-cell non-Hodgkin lymphomas.
Epidemiology, molecular signature and clinical management. J
Hepatol. 59:169–177. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
de Sanjose S, Benavente Y, Vajdic CM,
Engels EA, Morton LM, Bracci PM, Spinelli JJ, Zheng T, Zhang Y,
Franceschi S, et al: Hepatitis C and non-Hodgkin lymphoma among
4784 cases and 6269 controls from the International Lymphoma
Epidemiology Consortium. Clin Gastroenterol Hepatol. 6:451–458.
2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Carloni G, Fioretti D, Rinaldi M and
Ponzetto A: Heterogeneity and coexistence of oncogenic mechanisms
involved in HCV-associated B-cell lymphomas. Crit Rev Oncol
Hematol. 138:156–171. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dang H, Yeo YH, Yasuda S, Huang CF, Iio E,
Landis C, Jun DW, Enomoto M, Ogawa E, Tsai PC, et al: Cure with
interferon free DAA is associated with increased survival in
patients with HCV related HCC from both East and West. Hepatology.
71:1910–1922. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ikeda K, Kawamura Y, Kobayashi M, Kominami
Y, Fujiyama S, Sezaki H, Hosaka T, Akuta N, Saitoh S, Suzuki F, et
al: Direct-acting antivirals decreased tumor recurrence after
initial treatment of hepatitis C virus-related hepatocellular
carcinoma. Dig Dis Sci. 62:2932–2942. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Imai K, Takai K, Hanai T, Suetsugu A,
Shiraki M and Shimizu M: Sustained virological response by
direct-acting antivirals reduces the recurrence risk of hepatitis
C-related hepatocellular carcinoma after curative treatment. Mol
Clin Oncol. 12:111–116. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Michot JM, Canioni D, Driss H, Alric L,
Cacoub P, Suarez F, Sibon D, Thieblemont C, Dupuis J, Terrier B, et
al: Antiviral therapy is associated with a better survival in
patients with hepatitis C virus and B-cell non-Hodgkin lymphomas,
ANRS HC-13 lympho-C study. Am J Hematol. 90:197–203.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Arcaini L, Besson C, Frigeni M, Fontaine
H, Goldaniga M, Casato M, Visentini M, Torres HA, Loustaud-Ratti V,
Peveling-Oberhag J, et al: Interferon-free antiviral treatment in
B-cell lymphoproliferative disorders associated with hepatitis C
virus infection. Blood. 128:2527–2532. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nicolini LA, Zappulo E, Viscoli C and
Mikulska M: Management of chronic viral hepatitis in the
hematological patient. Expert Rev Anti Infect Ther. 16:227–241.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rossotti R, Travi G, Pazzi A, Baiguera C,
Morra E and Puoti M: Rapid clearance of HCV-related splenic
marginal zone lymphoma under an interferon-free, NS3/NS4A
inhibitor-based treatment. A case report. J Hepatol. 62:234–237.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Merli M, Carli G, Arcaini L and Visco C:
Antiviral therapy of hepatitis C as curative treatment of indolent
B-cell lymphoma. World J Gastroenterol. 22:8447–8458.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Arcaini L, Rossi D and Paulli M: Splenic
marginal zone lymphoma: From genetics to management. Blood.
127:2072–2081. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hirose S, Yamaji Y, Tsuruya K, Ogawa Y,
Miyaoka M and Kagawa T: Rapid regression of B-cell non-Hodgkin's
lymphoma after eradication of hepatitis C virus by direct antiviral
agents. Case Rep Gastroenterol. 13:336–341. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Carrier P, Jaccard A, Jacques J, Tabouret
T, Debette-Gratien M, Abraham J, Mesturoux L, Marquet P, Alain S,
Sautereau D, et al: HCV-associated B-cell non-Hodgkin lymphomas and
new direct antiviral agents. Liver Int. 35:2222–2227.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Persico M, Aglitti A, Caruso R, De Renzo
A, Selleri C, Califano C, Abenavoli L, Federico A and Masarone M:
Efficacy and safety of new direct antiviral agents in hepatitis C
virus-infected patients with diffuse large B-cell non-Hodgkin's
lymphoma. Hepatology. 67:48–55. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Merli M, Frigeni M, Gotti M, Grossi P,
Bruno R, Passamonti F and Arcaini L: Direct-acting antivirals
during or after immunochemotherapy in hepatitis C virus-positive
diffuse large B-cell lymphomas. Hepatology. 66:1341–1343.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zignego AL, Ramos-Casals M, Ferri C,
Saadoun D, Arcaini L, Roccatello D, Antonelli A, Desbois AC,
Comarmond C, Gragnani L, et al: International therapeutic
guidelines for patients with HCV-related extrahepatic disorders A
multidisciplinary expert statement. Autoimmun Rev. 16:523–541.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lin RJ, Moskovits T, Diefenbach CS and
Hymes KB: Development of highly aggressive mantle cell lymphoma
after sofosbuvir treatment of hepatitis C. Blood Cancer J.
6(e402)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ohzato Y, Murakami M, Shimizu J, Koga C,
Marukawa D, Yoshida Y, Yasuyama A, Matsumura T, Takada A, Kameda C,
et al: A case report of inguinal malignant lymphoma after surgery
for hepatocellular carcinoma. Gan To Kagaku Ryoho. 44:1638–1640.
2017.PubMed/NCBI(In Japanese).
|
|
24
|
Rodríguez de Santiago E, Velázquez Kennedy
K, García González M, Gea Rodríguez F, Téllez Villajos L, Tavío
Hernández E and Albillos A: HCV-positive lymphoma after sustained
virological response with direct-acting antiviral agents: The game
is not over after HCV eradication. J Viral Hepat. 25:614–615.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Andrade XA, Paz LH, Nassar M, Oramas DM,
Fuentes HE, Kovarik P, Mishra S and Singh A: Primary liver diffuse
large B-cell lymphoma following complete response for hepatitis C
infection after direct antiviral therapy. Acta Haematol. 139:77–80.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Iwane K, Kayahara T, Takabatake H,
Morimoto Y, Iseki A, Mizuno M and Notohara K: Recurrence of
malignant lymphoma immediately after treatment for hepatitis C
virus using direct-acting antivirals. Nihon Shokakibyo Gakkai
Zasshi. 116:177–183. 2019.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
27
|
Gisbert JP, García-Buey L, Pajares JM and
Moreno-Otero R: Prevalence of hepatitis C virus infection in B-cell
non-Hodgkin's lymphoma: Systematic review and meta-analysis.
Gastroenterology. 125:1723–1732. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Matsuo K, Kusano A, Sugumar A, Nakamura S,
Tajima K and Mueller NE: Effect of hepatitis C virus infection on
the risk of non-Hodgkin's lymphoma: A meta-analysis of
epidemiological studies. Cancer Sci. 95:745–752. 2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rosa D, Saletti G, De Gregorio E, Zorat F,
Comar C, D'Oro U, Nuti S, Houghton M, Barnaba V, Pozzato G and
Abrignani S: Activation of naive B lymphocytes via CD81, a
pathogenetic mechanism for hepatitis C virus-associated B
lymphocyte disorders. Proc Natl Acad Sci USA. 102:18544–18549.
2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Carter RH and Fearon DT: CD19: Lowering
the threshold for antigen receptor stimulation of B lymphocytes.
Science. 256:105–107. 1992.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Machida K, Cheng KT, Sung VM, Shimodaira
S, Lindsay KL, Levine AM, Lai MY and Lai MM: Hepatitis C virus
induces a mutator phenotype: Enhanced mutations of immunoglobulin
and protooncogenes. Proc Natl Acad Sci USA. 101:4262–4267.
2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Khoury J, Nassar G, Kramsky R and Saadi T:
Extrahepatic malignancies after treatment with direct antiviral
agents for chronic HCV infection. J Gastrointest Cancer.
51:584–590. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ioannou GN, Green PK, Berry K and Graf SA:
Eradication of hepatitis C virus is associated with reduction in
hematologic malignancies: Major differences between interferon and
direct-acting antivirals. Hepatol Commun. 3:1124–1136.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Langhans B, Nischalke HD, Krämer B, Hausen
A, Dold L, van Heteren P, Hüneburg R, Nattermann J, Strassburg CP
and Spengler U: Increased peripheral CD4+ regulatory T
cells persist after successful direct-acting antiviral treatment of
chronic hepatitis C. J Hepatol. 66:888–896. 2017.PubMed/NCBI View Article : Google Scholar : (In Albanian,
English).
|
|
35
|
Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE
and Ansell SM: Attenuation of CD8(+) T-cell function by
CD4(+)CD25(+) regulatory T cells in B-cell non-Hodgkin's lymphoma.
Cancer Res. 66:10145–10152. 2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Reig M, Boix L, Mariño Z, Torres F, Forns
X and Bruix J: Liver cancer emergence associated with antiviral
treatment: An immune surveillance failure? Semin Liver Dis.
37:109–118. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Villani R, Facciorusso A, Bellanti F,
Tamborra R, Piscazzi A, Landriscina M, Vendemiale G and Serviddio
G: DAAs rapidly reduce inflammation but increase serum VEGF level:
A rationale for tumor risk during anti-HCV treatment. PLoS One.
11(e0167934)2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Podar K and Anderson KC: The
pathophysiologic role of VEGF in hematologic malignancies:
Therapeutic implications. Blood. 105:1383–1395. 2005.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gratzinger D, Zhao S, Marinelli RJ, Kapp
AV, Tibshirani RJ, Hammer AS, Hamilton-Dutoit S and Natkunam Y:
Microvessel density and expression of vascular endothelial growth
factor and its receptors in diffuse large B-cell lymphoma subtypes.
Am J Pathol. 170:1362–1369. 2007.PubMed/NCBI View Article : Google Scholar
|