Introduction
Mycosis fungoides (MF) is a cutaneous malignant
lymphoma usually with CD4+ T cell phenotype (1) representing almost the 50% of all
primary cutaneous lymphomas and more than 70% of cutaneous T-cell
lymphomas (CTCLs) (2). This disease
typically begins affecting the skin with a sequential appearance of
patches followed by plaques and has tumors as final outcome. There
are several clinical variants of MF including bullous, follicular,
granulomatous, pustular, hyperkeratotic, hyperpigmented or
hypopigmented, adnexotropic, and purpuriform forms (2). Several authors currently consider
Sezary syndrome as an erythrodermic leukemic variant of MF, but in
the World Health Organization-European Organization for Research
and Treatment of Cancer (WHO-EORTC) classification of cutaneous
lymphomas, it is classified separately as an aggressive form of
CTCL (3). In the late stages, MF
may have a systemic dissemination with involvement of various
organs such us lymph node/peripheral blood, liver, spleen, lung,
bone marrow, gastrointestinal tract, pancreas, and kidney.
Gastrointestinal (GI) lesions have been reported in some MF
patients, although they are mentioned in the literature very rarely
(2). In most cases, GI lymphomas
are non-Hodgkin type and are commonly characterized by
proliferating B cells while infiltrating T cells are observed less
frequently. T-cell lymphomas are classified into enteropathy
associated T-cell lymphoma (EATL), nasal type NK cell lymphoma and
other types unassociated with enteropathy (WHO 2018 classification)
(4). Few cases of association
between GI lymphoma and MF are reported in literature. Mycosis
fungoides represents the most frequent CTCL and usually affects
middle-aged men (5) with a 2:1 male
to female ratio. This malignancy typically involves the skin,
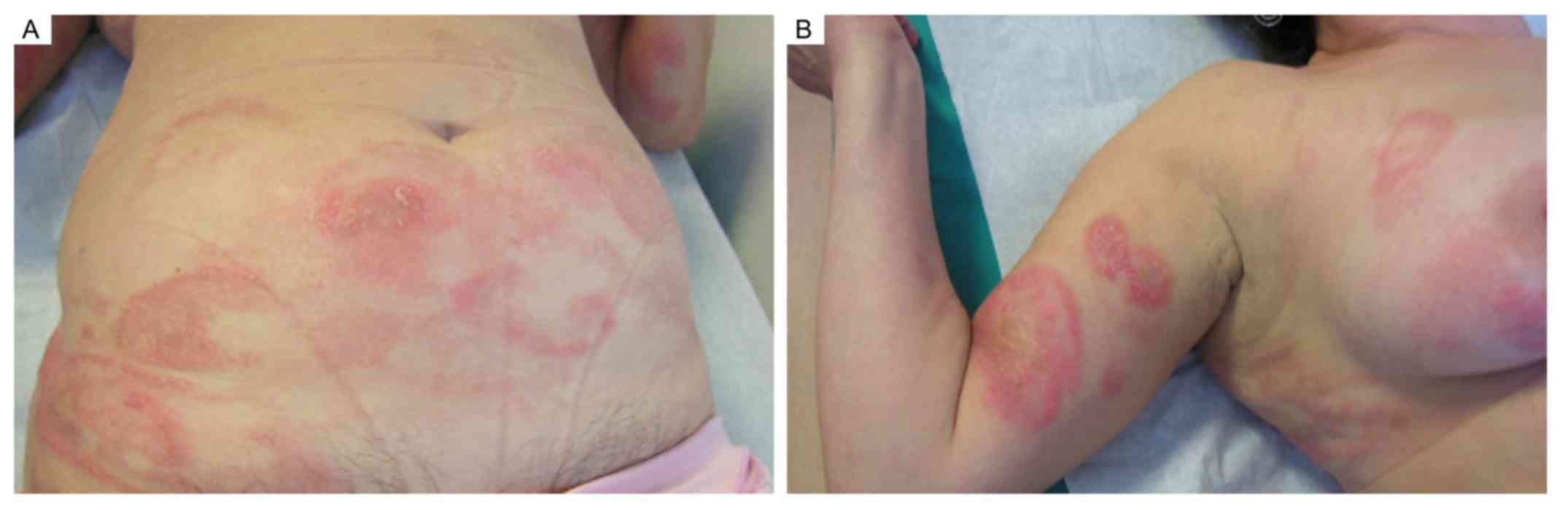
mainly in unexposed areas such as trunk, buttocks and thighs
(Fig. 1) even if, in the later
stages, lymph node and visceral involvement can be observed. The
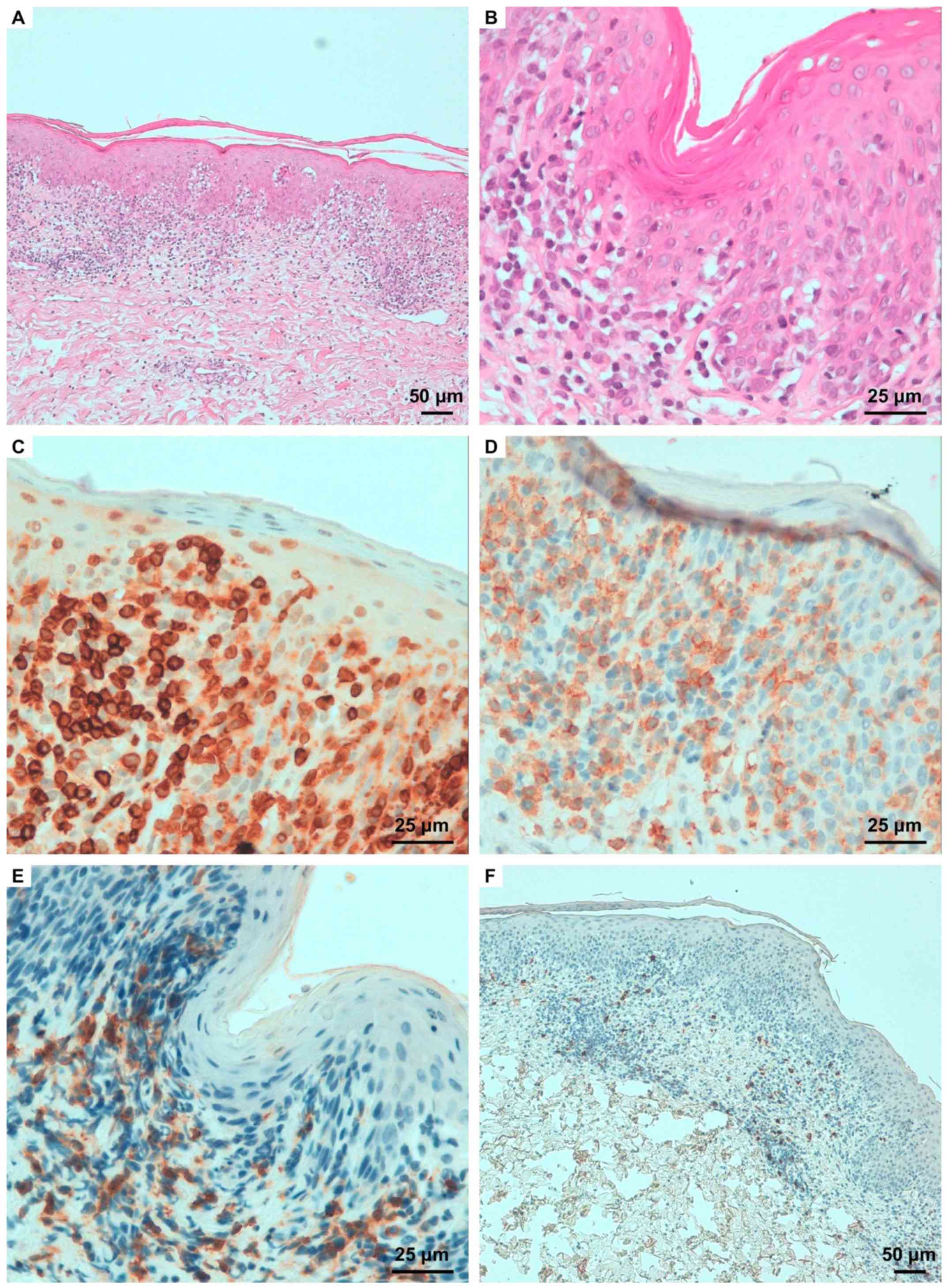
neoplastic infiltrate in MF is mainly represented by
CD4+ cells (Fig. 2) that
express the T-cell receptor-β and are incline to loss the
expression of surface markers such as CD2, CD3, CD5, CD7 and CD26
at variable extent. Notably, the loss of CD7 and CD5 is frequently
observed in MF and up to 20% of cases exhibit a CD8+
phenotype (6). Clinical and
immunophenotypic variants of MF include folliculotropic (follicular
mucinosis), bullous, hypopigmented, psoriasiform and palmoplantar
forms. The prognostic significance of these variants is still not
clear. The prognosis directly correlates with the extent of skin
involvement as well as to the presence of extracutaneous disease.
Here we present a case of a 65 years old woman, affected by MF who
developed a gastric T-cell lymphoma. According to our current
knowledge this is the first case described in the scientific
literature.
Case report
A 65 years old woman came along to our observation
at Dermatology Department in January 2012, presenting a cutaneous
eruption characterized by the occurrence of multiple and extensive
inflammatory erythematous patches, slightly scaly. The main
diameter of patches ranged, in average, from 2 cm to more than 10
cm and lesions were primarily located on the buttocks, abdomen and
legs (Fig. 1). Some lesions had
annular appearance with an erythematous and infiltrative border.
The patient reported that skin clinical manifestations appeared
since ten years before the establishment of a diagnosis of
lymphomatoid contact dermatitis. Topical corticosteroid therapy was
recommended, but it led to very poor benefit. Because of the
referred worsening of cutaneous clinical manifestations, we decided
to perform new biopsies of skin lesions and immunohistochemical
analysis revealed an epidermotropic lymphocytic infiltrate. The
histological and immunohistochemical procedures were carried out
according to the previously described methods (4,7,8). The
immunohistochemical analyses, performed by using the antibodies
listed in Table I, revealed a
pattern with simultaneous presence of CD3+,
CD4+ and CD7− antigens, confirming the
diagnosis of MF (Fig. 2). A topical
corticosteroid treatment was prescribed while the patient was
undergoing to an attentive follow-up. Due to the appearance of GI
symptoms such as gastric pain, an esophagogastroduodenoscopy (EGD)
procedure was performed, revealing the presence of an ulcerative
nodule into the gastric mucosa. The histological analysis of this
tissue revealed a widespread proliferation of lymphoid elements
with small and medium size with destruction of gastric glandular
structures. Mitotic figures and apoptotic bodies were also evident.
The immunohistochemical studies characterized the lymphocytic
infiltrates CD3+, CD43+, CD4+,
CD20−, CD30− TdT−,
CD99−. These results were compatible with the
histological diagnosis of gastric T-cell lymphoma (Fig. 3). Several cycles of IFNα-based
therapy were carried out, administered according classic protocols
at a dose of 1.5 MU/day s.c. or i.m. during the first week and
increased up to 3 MU/day s.c. or i.m. in the second week (9), resulting in clinical benefit and
disease stabilization. However, clinical progression of gastric and
skin lesions was observed after one year of treatment. Skin lesions
further progressed to tumor stage generating large and diffused
nodules on the trunk. Patient was hospitalized for planning
alternative treatments but clinical conditions rapidly worsened and
the patient died within few weeks for metabolic acidosis and multi
organ failure.
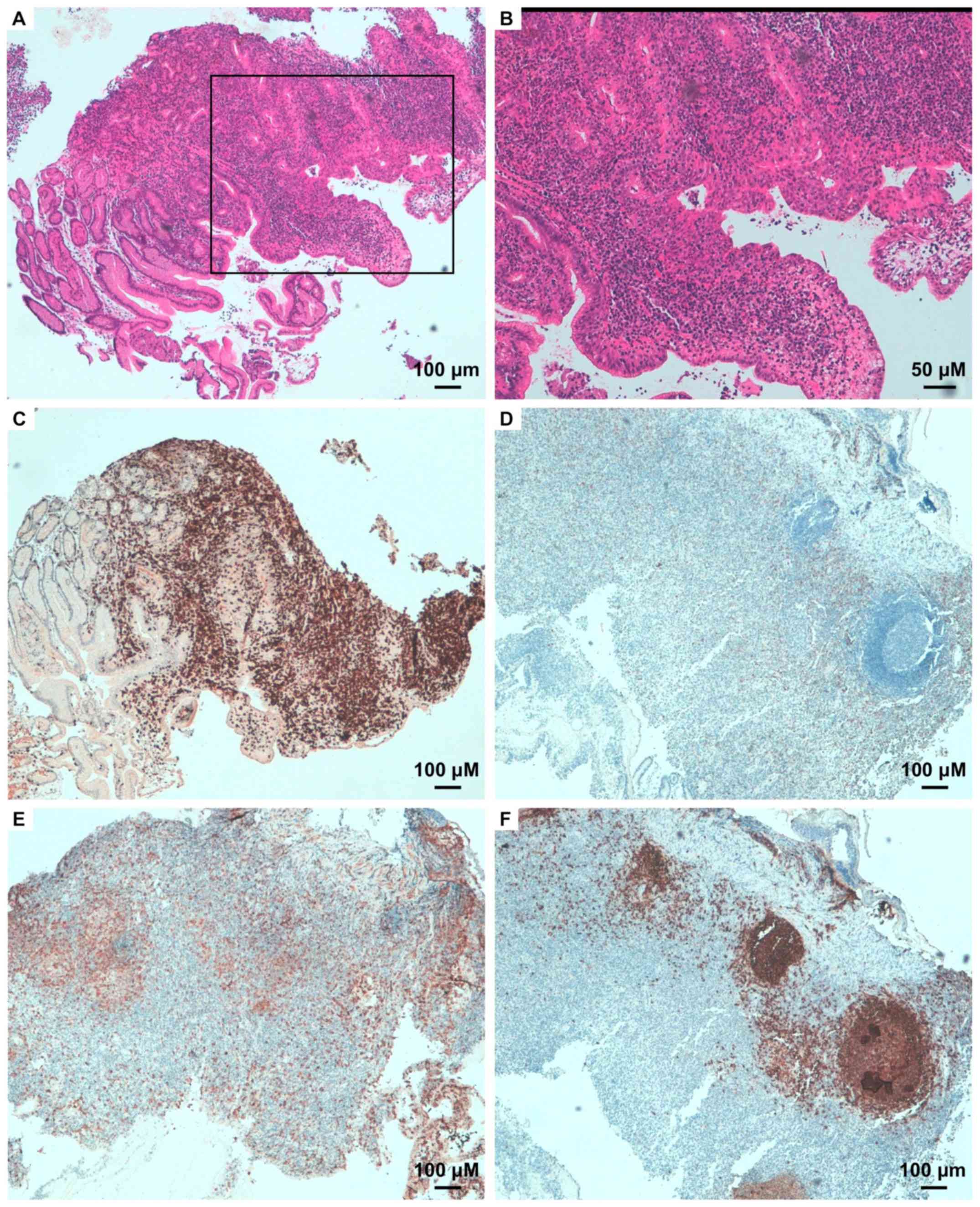 | Figure 3Small bowel biopsy. (A) High degrees
of lymphocytic infiltrate is present within the mucosa, as
evidenced via H&E staining (magnification, x40). The framed
section in the same panel is magnified in (B) (magnification,
x100). Immunophenotype is (C) CD3+ (magnification, x40),
(D) CD8− (magnification, x40), (E) CD4+
(magnification, x100), (F) CD20− (magnification, x100).
In (F), strong and localized immunoreactivity is mainly due to the
lymphatic follicles of mucose associated lymphoid tissue. |
 | Table IAntibodies employed for
immunohistochemistry. |
Table I
Antibodies employed for
immunohistochemistry.
| Antibody | Source/clonality | Dilution | Company |
|---|
| Anti-CD3 | Mouse/monoclonal | 1:500 | Leica Biosystem |
| Anti-CD4 | Mouse/monoclonal | 1:100 | Leica Biosystems |
| Anti-CD8 | Mouse/monoclonal | 1:50 | Leica Biosystems |
| Anti-CD20 | Mouse/monoclonal | 1:200 | Dako; Agilent
Technologies, Inc. |
Discussion
MF should be differentiated from benign and
malignant conditions that report similar clinicopathologic features
(10). Several T-cell lymphomas and
rarely B-cell lymphomas can display epidermotropic infiltrates, but
MF should be also distinguished from various benign inflammatory
conditions, including lymphomatoid drug eruptions, lichenoid
keratosis, lymphomatoid eczematous dermatitis and the inflammatory
stage of lichen sclerosus. Very rarely, other lymphoproliferative
disorders such as Castleman's disease or Castleman-like conditions
must be considered in the differential diagnosis of MF, especially
when multicentric or an anomalous pattern is manifested (11-14).
Primary GI lymphomas are not common diseases, occurring more
frequently in the 6th decade of age and in males (15,16).
There is evidence that tissue resident immune cells, also in CTCLs,
have the potential to strongly shape the tissue microenvironment
and influence behavior malignant lesions, both participating in
many inflammatory and immune reactions or regulating them (17,18).
These types of cancers can affect the whole GI tract, particularly
stomach and small intestine; esophagus, colon and rectum are
involved less frequently. Extracutaneous dissemination is reported
in less than 10% of patients with patch or plaque-accompanied
disease and in 30-40% of patients with tumors or generalized
erythrodermatous involvement (3).
In early stages, MF could be hardly differentiated from
inflammatory cutaneous conditions, even in hands of experienced
pathologists. Here because sometimes, this disease may follow its
indolent and silent course for many years (19). In the later stages the organs most
commonly affected are lymph nodes (60%), spleen (50%), lungs (43%),
liver (41%), bone (27%), kidney (27%), tongue and mucous membranes
(19%), heart (17%), pancreas (17%), and thyroid (14%) (2). Complications within the
gastrointestinal tract are rarely described in literature. Also,
the GI involvement can be primary or spread out from cutaneous
disease. The principal papers regarding this topic are listed in
Table II. MF can affect, albeit
rarely, every section of the GI tract from the mouth to the rectum,
but also the biliary tract and pancreas. The involvement of the GI
tract in most cases can run torpidly displaying non-specific
symptoms. However, some reports showed abrupt and fatal
complications such as small bowel obstruction (20) and massive haemorrhage from an
ulcerated tumor's nodule in the stomach (5). In our case MF had an indolent course
for several years until gastric involvement became apparent. The
immunohistochemical study of the neoplastic lymphocyte infiltrate
has provided consistent results for gastric and skin localization.
In fact, in both cases the T lymphocyte population was represented
almost exclusively by CD4+ elements with only rare
CD8+ lymphocytes. At that time, the disease became
aggressive with nodules arising on skin plaques until, after one
year of treatment, the patient died. Taking into account these data
and considering the small number of cases documented in the
literature, we feel like to affirm that gastric involvement in the
context of MF is underestimated and then could be not so rare.
Therefore, from clues we can glean that are skin manifestations
getting worse, the appearance of gastric pain or other
gastrointestinal symptoms it follows that MF must be carefully
evaluated because the picture could be indicative of an actual
systemic T-cell lymphoma and probably carrying back a poor
prognosis.
 | Table IIA collection of reports on GI tract
localization in MF. |
Table II
A collection of reports on GI tract
localization in MF.
| GI involvement | Location, (no of
cases) | Authors | (Refs.) |
|---|
| P | Small-bowell (1) | Camisa and Goldstein,
1981 | (21) |
| P | Esophagus (1) | Redleaf et al,
1993 | (22) |
| S | Stomach (7)
Small-bowell (13) | Epstein et al,
1972 | (23) |
| S | Oesophagus (4)
Stomach (4) Small-bowell (6) Pancreas (12) | Rappaport and Thomas,
1974 | (24) |
| S | Oesophagus (1) | Kim et al,
1990 | (25) |
| S | Small-bowell (1) | Velagapudi et
al, 2011 | (20) |
| S | Biliary tract
(1) | Madsen et al,
1999 | (26) |
| S | Pancreas (1) | Gottlieb et
al, 2008 | (27) |
| S | Duodenal papilla
(1) | Gómez-Venegas and
Vargas-Rubio, 2016 | (7) |
| S | Rectum (1) | Tan et al,
2016 | (28) |
| S | Oral cavity (1)
Small-bowell (1) | Emge et al,
2016 | (29) |
| S | Small-bowell
(1) | Chen et al,
1998 | (30) |
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Author's contributions
ES, GD and SPN conceived the current study. ES, GD,
IP, AD, GS, SD, MR and NM drafted the manuscript. IP, AD, GS, SD,
NM, MR and CM reviewed and edited the manuscript. NM, MR, GS and SD
collected the data. AD performed immunohistochemistry analysis. CM
and IP constructed the figures. GD and ES supervised the current
study. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval was obtained from of the local
regional Ethics Committee (‘Comitato Etico Regione Calabria, Area
Centro, Azienda Ospedaliero Universitaria, Mater Domini,
Catanzaro’) for all procedures.
Patient consent for publication
Written informed consent was obtained from the
patient at the start of observation proposing the possibility of
publication in anonymous form.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cerroni L: Lymphoproliferative lesions of
the skin. J Clin Pathol. 59:813–826. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Burg G: Systemic involvement in mycosis
fungoides. Clin Dermatol. 33:563–571. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zinzani PL, Ferreri AJM and Cerroni L:
Mycosis fungoides. Crit Rev Oncol Hematol. 65:172–182.
2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cerroni L: Mycosis fungoides-clinical and
histopathologic features, differential diagnosis, and treatment.
Semin Cutan Med Surg. 37:11–17. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Eder J, Rogojanu R, Jerney W, Erhart F,
Dohnal A, Kitzwögerer M, Steiner G, Moser J and Trautinger F: Mast
cells are abundant in primary cutaneous T-cell lymphomas: Results
from a computer-aided quantitative immunohistological study. PLoS
One. 11(e0163661)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Scali E, Mignogna C, Di Vito A, Presta I,
Camastra C, Donato G and Bottoni U: Inflammation and macrophage
polarization in cutaneous melanoma: Histopathological and
immunohistochemical study. Int J Immunopathol Pharmacol.
29:715–719. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gómez-Venegas ÁA and Vargas-Rubio RD:
Unusual involvement in mycosis fungoides: Duodenal papilla. Rev Esp
Enfermedades Dig. 108:513–516. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Furue M and Kadono T: New aspects of the
clinicopathological features and treatment of mycosis fungoides and
Sézary syndrome. J Dermatol. 42:941–944. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Perrotta I, Bruno L, Maltese L, Russo E,
Donato A and Donato G: Immunohistochemical analysis of the
ubiquitin-conjugating enzyme UbcH10 in lung cancer: A useful tool
for diagnosis and therapy. J Histochem Cytochem. 60:359–365.
2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Donato G, Conforti F and Allegra E: A rare
case of primary nodalhemangioendothelioma. Oncol Lett. 6:1759–1761.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mignogna C, Staropoli N, Botta C, De Marco
C, Rizzuto A, Morelli M, Di Cello A, Franco R, Camastra C, Presta
I, et al: Aurora Kinase A expression predicts platinum-resistance
and adverse outcome in high-grade serous ovarian carcinoma
patients. J Ovarian Res. 9(31)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Whittaker S, Hoppe R and Prince HM: How I
treat mycosis fungoides and Sézary syndrome. Blood. 127:3142–3153.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ahmed B, Tschen JA, Cohen PR, Zaki MH,
Rady PL, Tyring SK, Corringham RE and Kurzrock R: Cutaneous
castleman's disease responds to anti-interleukin-6 treatment. Mol
Cancer Ther. 6:2386–2390. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Montroni I, Sabattini E, Ramadori E,
Koprivica V, Ugolini G, Locatelli F, Ghignone F, Rosati G,
Taffurelli M and Pileri S: Small bowel obstruction caused by a
large B-cell lymphoma in a patient with multicentric Castleman's
disease: An unusual occurrence. Int J Surg Pathol. 22:434–437.
2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Donato G, Ferraro G, Signorelli F, Iofrida
G, Lavano A, Amorosi A, Maltese L, Perrotta I, Tripepi S,
Pardatscher K and Signorelli CD: Chordoid meningioma: Case report
and literature review. Ultrastruct Pathol. 30:309–314.
2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Di Vito A, Mignogna C and Donato G: The
mysterious pathways of cardiac myxomas: A review of histogenesis,
pathogenesis and pathology. Histopathology. 66:321–332.
2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shirsat HS and Vaiphei K: Primary
gastrointestinal lymphomas-A study of 81 cases from a Tertiary
Healthcare Centre. Indian J Cancer. 51:290–292. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Peng JC, Zhong L and Ran ZH: Primary
lymphomas in the gastrointestinal tract. J Dig Dis. 16:169–176.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jawed SI, Myskowski PL, Horwitz S,
Moskowitz A and Querfeld C: Primary cutaneous T-cell lymphoma
(mycosis fungoides and Sézary syndrome): Part II. Prognosis,
management, and future directions. J Am Acad Dermatol.
70:223.e1–e17. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Velagapudi P, Turagam M, Uzoaru I and
Graham D: Small bowel obstruction due to mycosis fungoides: An
unusual presentation. Am J Med Sci. 341:508–509. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Camisa C and Goldstein A: Mycosis
fungoides: Small-bowel involvement complicated by perforation and
peritonitis. Arch Dermatol. 117:234–237. 1981.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Redleaf MI, Moran WJ and Gruber B: Mycosis
fungoides involving the cervical esophagus. Arch Otolaryngol Neck
Surg. 119:690–693. 1993.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Epstein EH Jr, Levin DL, Croft JD Jr and
Lutzner MA: Mycosis fungoides: Survival, prognostic features,
response to therapy, and autopsy findings. Medicine (Baltimore).
51:61–72. 1972.PubMed/NCBI
|
|
24
|
Rappaport H and Thomas LB: Mycosis
fungoides: The pathology of extracutaneous involvement. Cancer.
34:1198–1229. 1974.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kim OD, Cantave I and Schlesinger PK:
Esophageal involvement by cutaneous T-cell lymphoma, mycosis
fungoides type: Diagnosis by endoscopic biopsy. J Clin
Gastroenterol. 12:178–182. 1990.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Madsen JA, Tallini G, Glusac EJ, Salem RR,
Braverman I and Robert ME: Biliary tract obstruction secondary to
mycosis fungoides: A case report. J Clin Gastroenterol. 28:56–60.
1999.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gottlieb K, Anders K and Kaya H:
Obstructive jaundice in a patient with mycosis fungoides metastatic
to the pancreas. EUS findings. J Pancreas. 9:719–724.
2008.PubMed/NCBI
|
|
28
|
Tan E, Shao H and Friedman M: Mycosis
Fungoides of the Rectum: Case Report and Review of the Literature.
J Gastrointest Cancer. 47:417–419. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Emge DA, Bassuner J, Lewis DJ and Duvic M:
A rare case of mycosis fungoides in the oral cavity and small
intestine complicated by perforation. Case Rep Dermatol. 8:294–302.
2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chen KR, Tanaka M and Miyakawa S:
Granulomatous mycosis fungoides with small intestinal involvement
and a fatal outcome. Br J Dermatol. 138:522–525. 1998.PubMed/NCBI View Article : Google Scholar
|