Introduction
Vaginal intraepithelial neoplasia (VAIN) is a rare
disease associated with human papillomavirus (HPV) infection. The
incidence of VAIN has been reported at approximately 0.2-0.3 per
100,000 women and is considered to be a precursor of vaginal
carcinoma (1). In the World Health
Organization (WHO) 2014 classification, VAIN lesions are graded as
vaginal low-grade and high-grade squamous intraepithelial lesions
(LSIL and HSIL, respectively) with LSIL including VAIN 1 and HSIL
including VAIN 2 and VAIN 3. VAIN 1 can also be considered a form
of productive HPV infection, which can be expectantly managed with
a spontaneous regression rate of over 50% (2). VAIN 2 and 3 are considered
precancerous lesions, so treatment is required for high-grade VAIN.
Especially, VAIN 3 owes to the high risk of progression to
carcinoma in approximately 11-13% of cases (3). However, current treatment
recommendations vary, and there is no universally accepted standard
of care as the best treatment modality (4).
High-grade VAIN is usually treated with either
excisional or ablative therapy, however recurrent VAIN lesions are
common and require repeat treatments which may be mutilating and
cause vaginal scarring (3).
Imiquimod is a toll-like receptor 7 (TLR7) agonist which modulates
an immune response by inducing secretion of interferon-α and
interferon-γ, and is used for the treatment of condylomas and other
HPV-related neoplasias including VAIN (5). Recently some studies have reported
that 5% imiquimod is an effective treatment for VAIN (5,6).
We here report a case of a woman who was diagnosed
with recurrent VAIN 3 and treated with 5% topical imiquimod cream
after surgical treatment and achieved a complete response. This
case thus provides further evidence for the clinical efficacy and
safety of 5% topical imiquimod in the treatment of recurrent
VAIN.
Case report
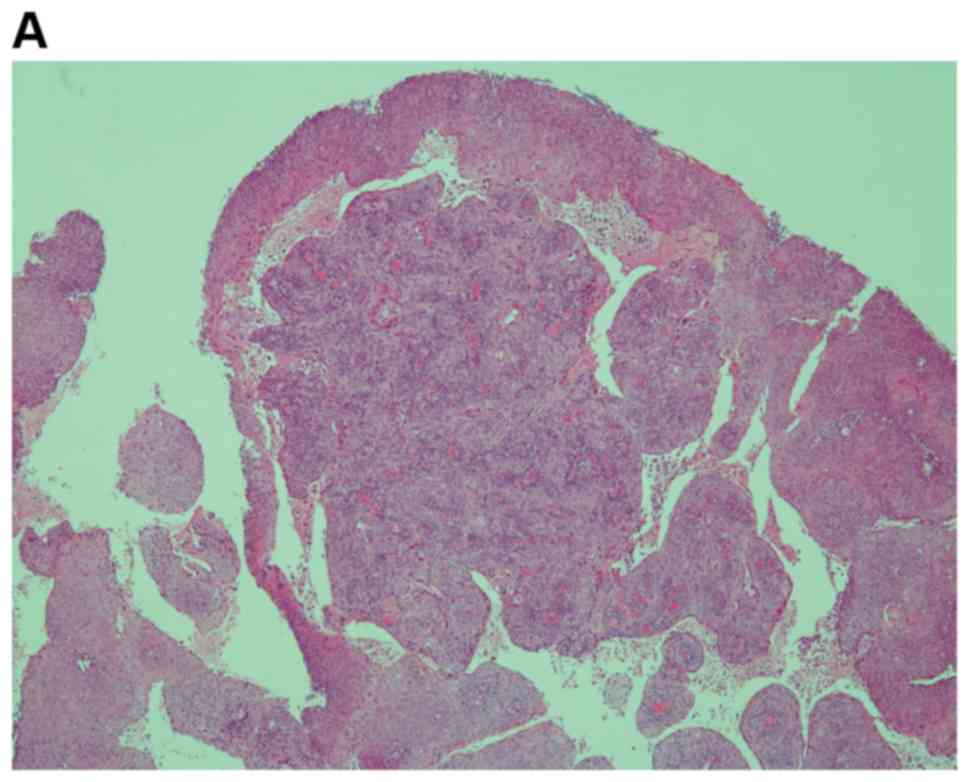
A 53-year-old, gravida 5, para 2 postmenopausal
woman with no past medical history was diagnosed with papillary
squamous cell carcinoma by biopsy (Fig.
1) and underwent conization, resulting in a pathological
diagnosis of grade 3 cervical intraepithelial neoplasia (CIN).
Total abdominal hysterectomy and bilateral salpingo-oophorectomy
were performed and a histological examination revealed CIN 3 with
free surgical margins.
Eight months after the hysterectomy, a vaginal smear
revealed atypical squamous cells, cannot exclude HSIL (ASC-H).
Because no abnormal mitosis was observed in the biopsy, p16 was
negative and Mib-1 index was 0.1-1%, atrophy or maturation arrest
of squamous epithelia was suspected. Cytology further revealed
ASC-H in succession with no apparent finding related to invasive
carcinoma; therefore, careful follow-up was decided with no further
treatment.
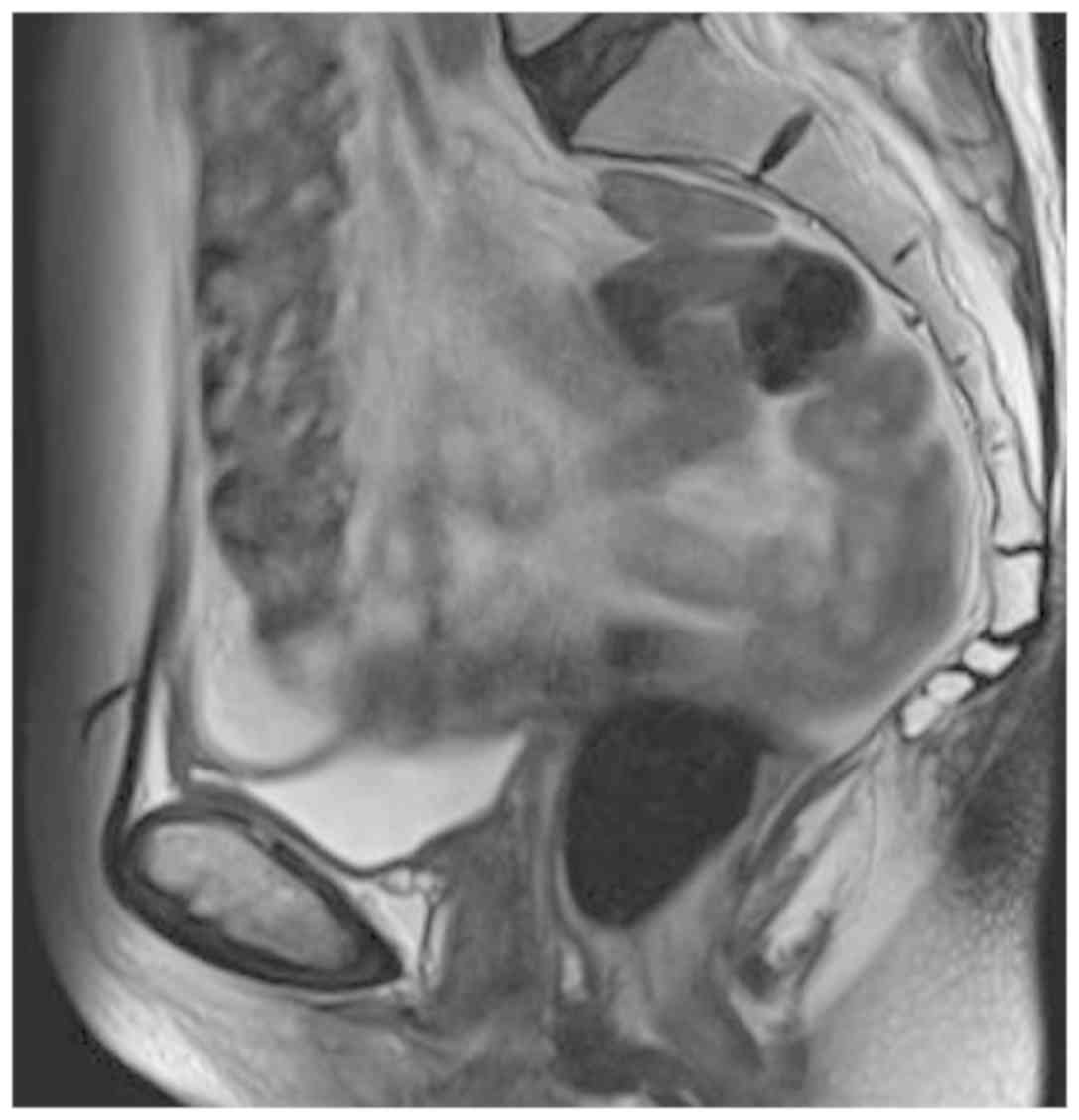
Three years after the hysterectomy, a follow-up
vaginal smear revealed ASC-H, and vaginoscopy after the application
of acetic acid showed increased vascularity; therefore, biopsy was
performed. On microscopic examination, atypia was observed in all
layers of the epithelium with no interstitial infiltration, leading
to a pathological diagnosis of VAIN 3. No lesion was detected on
contrast magnetic resonance imaging (Fig. 2).
Partial vaginectomy with a loop electrosurgical
excision procedure (LEEP) was performed under spinal anesthesia.
However, it was difficult to perform the appropriate peeling
operation on the right and left sides of the stump, and a lesion at
the 3-o'clock position remained. The lesion was additionally
peeled. The resected specimen was 5.4x3.8 cm in size. Microscopic
examination demonstrated a VAIN 3 lesion measuring 3.5x2.5 cm
within the specimen. Neoplastic cells were confined to the
epithelium, but the surgical margin was positive. There were also
neoplastic cells in the additionally resected specimen. Neither
postoperative radiation therapy nor chemotherapy was planned,
opting instead for careful follow-up.
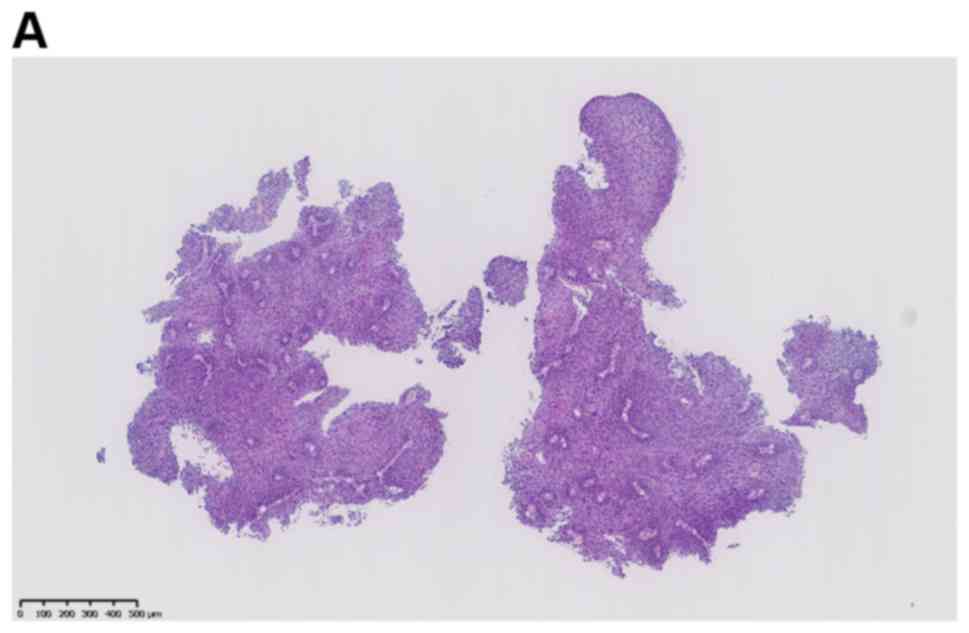
Four months after the vaginectomy, a vaginal smear
revealed HSIL. Vaginal biopsy was performed 1 month later on the
lesion at the 8-o'clock position during colposcopy, resulting in a
pathological diagnosis of VAIN 3 (Fig.
3). Eight months after the vaginectomy, CO2 laser
vaporization was performed under spinal anesthesia. A small mass at
the 7 o'clock position of the vaginal wall was resected using a
scalpel. Microscopic examination demonstrated atypia in almost all
layers of the epithelium, and the pathological diagnosis was VAIN 3
with a positive margin.
Three months after CO2 laser excision,
the vaginal smear revealed HSIL with suspected recurrence.
Imiquimod treatment was then initiated by placing a sachet of 5%
imiquimod cream (0.25 g) in the entire vagina three times weekly.
The institutional review board approved the use of imiquimod for
VAIN (SGHIRB#2019049). As imiquimod is not a standard treatment for
VAIN, follow-up vaginal smear was conducted monthly after the start
of imiquimod treatment to evaluate the efficacy of the treatment.
At 53 days and 74 days after the treatment, the vaginal smear
revealed ASC-H and was negative for intraepithelial lesion or
malignancy (NILM). A vaginal smear 1 month later further confirmed
the NILM status (Table I). The
treatment was continued for 14 weeks with no apparent complication.
As of 3 years after the treatment, there has been no
recurrence.
 | Table IResults of pathological
examination. |
Table I
Results of pathological
examination.
| Months
post-operation |
Intervention/specimen | Result |
|---|
| 0 | TAH+BSO | CIN 3, cut end
negative |
| 2 | Cytology | NILM |
| 4 | Cytology | ASC-US |
| 6 | Cytology | NILM |
| 8 | Cytology | ASC-H |
| 10 | Vaginal biopsy | Atypical squamous
epitheliaa |
| 12,15,18 | Cytology | ASC-H |
| 22 | Cytology | NILM |
| 25,29,33 | Cytology | ASC-H |
| 36 | Biopsy | VAIN 3 |
| 39 | Partial vaginectomy
by LEEP | VAIN 3, cut end
positive |
| 40 | Cytology | NILM |
| 41 | Cytology | SCC |
| 43 | Cytology | HSIL |
| 45 | Vaginal biopsy | VAIN 3 |
| 47 | CO2 laser
vaporization, resection | VAIN 3, cut end
positive |
| 48 | Cytology | NILM |
| 48 | Cytology | ASC-H |
| 50 | Cytology | HSIL |
| 51 | Administration of 5%
imiquimod cream |
| 51 | Cytology | ASC-H |
| 52-90 | Cytology | NILM |
Discussion
Surgical treatment modalities for high-grade VAIN
include partial vaginectomy, wide local excision, a LEEP, and
CO2 laser vaporization. High-grade VAIN is typically
treated with either excisional or ablative therapy; however, the
VAIN lesions often recur, requiring repeated treatments that may
cause severe damage to the tissue and vaginal scarring (3). Moreover, surgical treatments require
anesthesia and can cause complications such as bleeding, infection,
scarring, and injury of neighboring anatomical structures as well
as high recurrence rates (4).
Therefore, alternative treatments are desired.
Non-surgical treatment modalities include imiquimod,
5-fluorouracil, and vaginal estrogens as well as radiotherapy
(4). Imiquimod is a TLR7 agonist,
which is used for the treatment of condylomas and other HPV-related
neoplasias, including VAIN. Imiquimod triggers the innate immune
response via TLR7-Myd88-dependent signaling by interacting with
TLR7 that is expressed by myeloid dendritic cells (DCs),
plasmacytoid DCs, monocytes, and macrophages. Subsequently, TLR7
activation induces the secretion of various pro-inflammatory
cytokines, such as interferon-α and interferon-γ, to in turn induce
a Th1 response leading to the apoptosis of cancer cells (4,7).
Imiquimod has been shown to promote histological clearance of basal
cell carcinoma (BCC) in clinical studies and is approved for the
treatment of BCC by Food and Drug Administration (8,9).
Moreover, imiquimod has been used in combination with chemotherapy
to treat breast cancer patients in a phase II clinical trial
(10). Several studies reported
promising results with the use of imiquimod for the treatment of
patients with vulvar intra-epithelial neoplasia (VIN), CIN, and
VAIN (5,6,11-14).
A meta-analysis including 94 patients with VAIN treated with 5%
imiquimod showed a complete response, HPV clearance, and
non-recurrence in 76.5, 52.5, and 94.3% of patients, respectively
(4).
Imiquimod has emerged as a potentially useful
treatment for CIN, VIN, and VAIN; however, further clinical data
are required to evaluate the safety profile. Some physicians
reported systemic and local adverse effects after imiquimod
treatment for CIN, including headache, fever, fatigue, myalgia,
vaginal discharge, vaginal bleeding, vulvar pruritus, vulvar pain,
and vaginal edema (15). Wouters
et al (16) reported serious
adverse events in three patients who received imiquimod
intravaginally for CIN: Two of the patients were hospitalized with
headache, nausea, diarrhea, malaise, leukopenia, and hyponatremia,
and the other patient exhibited systemic symptoms and erosion of
the cornea. Although none of these adverse effects was directly
proven to be caused by imiquimod, all of the symptoms disappeared
after discontinuation of imiquimod. Such systemic adverse effects
could be caused by the imiquimod-induced peripheral Th1 immune
response (15). Tainio et al
(3) reported that all 10 patients
with VAIN, who received imiquimod in a randomized control study
developed side effects such as flu-like symptoms, local irritation
in the vagina and vulva, and lower abdominal pain. In one patient,
the dosage was reduced by half, but no patient required
discontinuation of the treatment. Moreover, one study concluded
that non-steroidal anti-inflammatory drugs can safely be used to
reduce side effects in usual type VIN (17). The same could apply to VAIN.
Overall, the side effects of imiquimod are considered to be
well-tolerated, and patients can be treated through outpatient
management. However, clinicians prescribing this treatment should
monitor patients closely for potential severe side effects.
In the present case, a patient with recurrence of
VAIN after surgical treatments received imiquimod with no apparent
adverse events, and the lesion ultimately disappeared. This case
therefore provides further support that topical imiquimod with
careful follow-up is effective and well-tolerated for the treatment
of recurrent VAIN. Nevertheless, more studies should be accumulated
on the clinical experience with imiquimod in patients with VAIN. In
conclusion, imiquimod can be considered as one of the options for
treatment of refractory VAIN.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the author on reasonable request.
Authors' contributions
NS, KK and YI interpreted the case and reviewed the
patient's clinical information, and drafted the manuscript and
revised it critically for important intellectual content. YS and KI
were responsible for the patient's treatment and management
decisions. JA, KY, RG, AK, MY and YY revised the manuscript
critically for important intellectual content. MS and KA were
responsible for the pathological diagnosis. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The Institutional Review Board of Shizuoka General
Hospital approved the use of imiquimod for VAIN
(SGHIRB#2019049).
Patient consent for publication
Informed consent for publication was obtained from
the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
De Vuyst H, Clifford GM, Nascimento MC,
Madeleine MM and Franceschi S: Prevalence and type distribution of
human papillomavirus in carcinoma and intraepithelial neoplasia of
the vulva, vagina and anus: A meta-analysis. Int J Cancer.
124:1626–1636. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Aho M, Vesterinen E, Meyer B, Purola E and
Paavonen J: Natural history of vaginal intraepithelial neoplasia.
Cancer. 68:195–197. 1991.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tainio K, Jakobsson M, Louvanto K,
Kalliala I, Paavonen J, Nieminen P and Riska A: Randomised trial on
treatment of vaginal intraepithelial neoplasia-Imiquimod, laser
vaporisation and expectant management. Int J Cancer. 139:2353–2358.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tranoulis A, Laios A, Mitsopoulos V,
Lutchman-Singh K and Thomakos N: Efficacy of 5% imiquimod for the
treatment of Vaginal intraepithelial neoplasia-A systematic review
of the literature and a meta-analysis. Eur J Obstet Gynecol Reprod
Biol. 218:129–136. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
De Witte CJ, Van De Sande AJM, Van
Beekhuizen HJ, Koeneman MM, Kruse AJ and Gerestein CG: Imiquimod in
cervical, vaginal and vulvar intraepithelial neoplasia: A review.
Gynecol Oncol. 139:377–384. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Policiano ACF, Lopes JPM, Barata SAM,
Colaço AM and Calhaz-Jorge C: Topical therapy with imiquimod for
vaginal intraepithelial neoplasia: A Case Series. J Low Genit Tract
Dis. 20:e34–e36. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chi H, Li C, Zhao FS, Zhang L, Ng TB, Jin
G and Sha O: Anti-tumor activity of toll-like receptor 7 agonists.
Front Pharmacol. 8(304)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Geisse J, Caro I, Lindholm J, Golitz L,
Stampone P and Owens M: Imiquimod 5% cream for the treatment of
superficial basal cell carcinoma: Results from two phase III,
randomized, vehicle-controlled studies. J Am Acad Dermatol.
50:722–733. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hanna E, Abadi R and Abbas O: Imiquimod in
dermatology: An overview. Int J Dermatol. 55:831–44.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Salazar LG, Lu H, Reichow JL, Childs JS,
Coveler AL, Higgins DM, Waisman J, Allison KH, Dang Y and Disis ML:
Topical imiquimod plus nab-paclitaxel for breast cancer cutaneous
metastases. JAMA Oncol. 3(969)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Terlou A, van Seters M, Ewing PC, Aaronson
NK, Gundy CM, Heijmans-Antonissen C, Quint WGV, Blok LJ, van
Beurden M and Helmerhorst TJM: Treatment of vulvar intraepithelial
neoplasia with topical imiquimod: Seven years median follow-up of a
randomized clinical trial. Gynecol Oncol. 121:157–162.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Buck HW and Guth KJ: Treatment of vaginal
intraepithelial neoplasia (primarily low grade) with imiquimod 5%
cream. J Low Genit Tract Dis. 7:290–293. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Diaz-Arrastia C, Arany I, Robazetti SC,
Dinh TV, Gatalica Z, Tyring SK and Hannigan E: Clinical and
molecular responses in high-grade intraepithelial neoplasia treated
with topical imiquimod 5%. Clin Cancer Res. 7:3031–3033.
2001.PubMed/NCBI
|
|
14
|
Haidopoulos D, Diakomanolis E, Rodolakis
A, Voulgaris Z, Vlachos G and Intsaklis A: Can local application of
imiquimod cream be an alternative mode of therapy for patients with
high-grade intraepithelial lesions of the vagina? Int J Gynecol
Cancer. 15:898–902. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Koeneman MM, van de Sande AJ, van
Beekhuizen HJ, Gerestein KG, van de Laar R, Kruitwagen RF and Kruse
AJ: Physicians' awareness, attitudes, and experiences regarding
imiquimod treatment of vaginal and cervical intraepithelial
neoplasia. J Low Genit Tract Dis. 20:75–79. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wouters T, Hendriks N, Koeneman M, Kruse
AJ, van de Sande A, van Beekhuizen HJ, Gerestein KG, Bekkers RLM
and Piek JMJ: Systemic adverse events in imiquimod use for cervical
intraepithelial neoplasia - A case series. Case Reports Women's
Heal. 21(e00105)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Terlou A, Kleinjan A, Beckmann I,
Heijmans-Antonissen C, van Seters M, Santegoets LAM, van Beurden M,
Helmerhorst TJM and Blok LJ: Nonsteroidal anti-inflammatory drugs
do not interfere with imiquimod treatment for usual type vulvar
intraepithelial neoplasia. Int J Cancer. 128:2463–2469.
2011.PubMed/NCBI View Article : Google Scholar
|