Introduction
Ras/phosphoinositide 3-kinase (PI3K)/AKT-associated
factors were one of the key molecules involved in the
epithelial-to-mesenchymal transition of gastric cancer cells
(1). Ras-like GTPases, RalA and
RalB, are members of the Ras superfamily of small GTPases. These
GTPases are aberrantly induced during tumorigenesis by oncogenic
Ras (2). RalA and RalB have been
reported as key cancer phenotypic markers and biomarkers of
cellular migration, invasion and metastasis (3,4). RalA
expression has been shown to be associated with aggressive
clinicopathological characteristics and progression in squamous
cell carcinoma (3,4). In gastric cancer, Ajani et al
(5) reported that Gal-3 induced
c-MYC expression through increased RalA activity and an enhanced
YAP1/RalA/RalBP complex to confer an aggressive phenotype.
Some IgG autoantibodies have been found to respond
to tumor-associated antigens in the sera of patients with cancer,
even at the early stages (6,7). Since
RalA is a tumor antigen, autoantibodies against RalA (s-RalA-Abs)
have been reported as potential biomarkers for hepatocellular
(8), esophageal (9), colorectal (10), breast (11) and ovarian (12) carcinoma. Although the role of other
autoantibodies has been investigated in patients with gastric
cancer (13), the significance of
the clinicopathological and prognostic impact of s-RalA-Abs has not
yet been demonstrated.
Therefore, the clinicopathological significance and
prognostic value of preoperative s-RalA-Abs levels were evaluated
in patients with gastric cancer who underwent radical surgery.
Patients and methods
Collection of sera
Pre-treatment serum samples were obtained from 291
patients with histologically proven gastric adenocarcinoma and from
73 healthy individuals. Double cancer was excluded. All patients
with gastric cancer were surgically treated (between July 2011 and
July 2013) at the Toho University Omori Hospital (n=76) and the
Chiba Cancer Center (n=215). Among these, 184 were diagnosed with
stage I, 28 with stage II, 29 with stage III, and 50 with stage IV
gastric cancer. The patients included 201 men and 90 women (mean
age, 67.5 years; range, 36-93 years). Written informed consent was
obtained from all patients. The samples were anonymized. Each serum
sample was centrifuged at 3,000 x g, at room temperature for 5 min,
and the resulting supernatant was stored at -80˚C until further
analysis. Due care was taken to avoid the repeated thawing and
freezing of samples. The present study was approved by the
institutional review boards at the Chiba Cancer Center (approval
no. #21-26) and the Toho University School of Medicine (approval
nos. #22-112 and #22-047).
Purification of recombinant RalA and
enzyme-linked immunosorbent assay (ELISA) to detect s-RalA-Abs
RalA construct inserted in pET28 plasmid and
expressing the N-terminal His-tagged protein was provided by Dr
Jian-Ying Zhang (The University of Texas, El Paso, TX). The details
of this procedure have been described previously (9). Sera from patients and healthy controls
were analyzed by the previously established ELISA (9). Briefly, purified recombinant proteins
were placed in 96-well microtiter plates (Nunc MaxiSorp; Thermo
Fisher Scientific, Inc.). RalA was diluted in phosphate-buffered
saline (PBS) to a final concentration of 1.0 µg/ml and added to the
plates (100 µl/well), which were then incubated overnight at 4˚C.
PBS was used as a control. After two washes with PBS, proteins were
blocked using 200 µl of PBS, containing 1% bovine serum albumin and
5% sucrose, at room temperature for 3 h. All human sera were
diluted (1:100) in PBS containing 0.15% Tween-20, 1% casein, and
0.2 mg/ml E. coli extract. Then, 100 µl diluted sera was added to
each RalA- or PBS-coated well and incubated at room temperature,
while agitating at 250 rpm for 60 min. After four washes with PBS
containing 0.05% Tween-20 (PBST), 100 µl horseradish
peroxidase-conjugated antihuman IgG (1:5,000; Medical &
Biological Laboratories Co., Ltd), diluted in 20 mM
2-[4-(2-hydroxyethyl)-1-piperazinyl] ethanesulfonic acid, 135 mM
NaCl, 1% Bovine serum albumin and 0.1% hydroxiphenylacetic acid,
was added to each well as a secondary antibody. The plates were
incubated at room temperature, while agitating at 250 rpm for 60
min. The wells were washed four times with PBST buffer, and
autoantibodies were detected by adding 100 µl of
3,3',5,5'-tetramethylbenzidine substrate. After incubation at room
temperature for 30 min, the reaction was terminated by adding 0.25
N H2SO4 (100 µl/well). Absorbance was
measured at 450 nm using the SUNRISE Microplate Reader (Tecan Japan
Co., Ltd). RalA signals were determined by calculating the
difference between the absorbance values for wells containing RalA
and PBS. Serum carcinoembryonic antigen (CEA) and carbohydrate
antigen 19-9 (CA19-9) markers were also evaluated as described
previously (14).
Statistical analyses
Mann-Whitney U test and Fisher's exact probability
test, were used to examine the differences between two groups, and
Kruskal-Wallis test and Steel-Dwass test were used to compare
multiple comparisons. Clinicopathological parameters associated
with overall survival were evaluated by univariate analyses using
log-rank test based on the Kaplan-Meier survival curves.
Multivariate analyses were performed using the Cox proportional
hazards model. All statistical analyses were performed using EZR
(Saitama Medical Centre, Jichi Medical University; Saitama, Japan)
(15), which is a graphical user
interface for R (The R Foundation for Statistical Computing;
version 2.13.0). P<0.05 was considered to indicate a
statistically significant difference.
Results
s-RalA-Ab titer
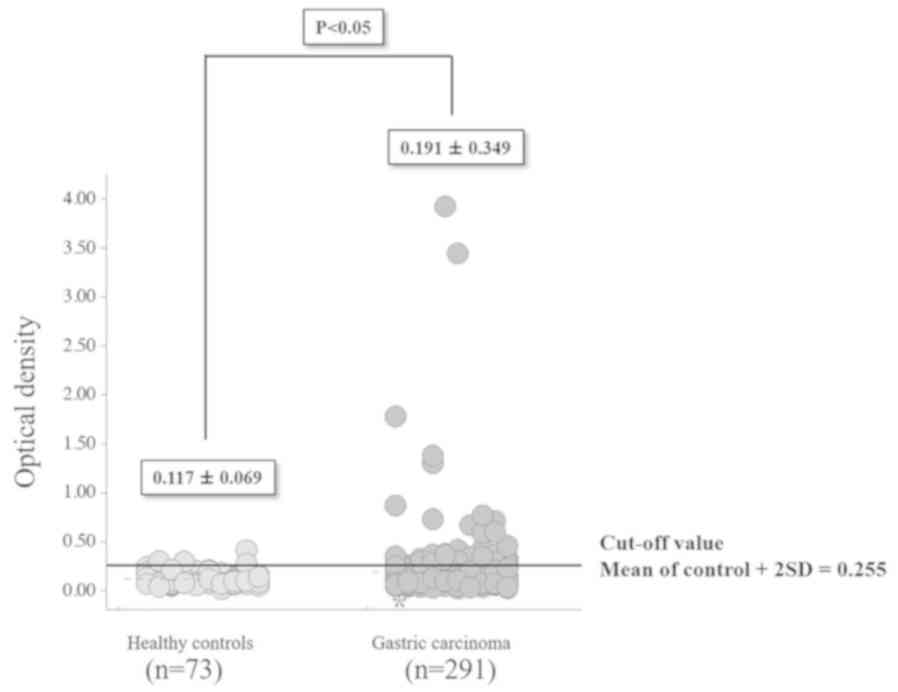
The optical density (mean ± standard deviation) of
s-RalA-Abs was significantly higher (P<0.05) for the 291
patients with gastric cancer (0.191±0.349) compared with 73 healthy
controls (0.117±0.069; Fig. 1).
s-RalA-Ab levels were divided into two groups: Normal optical
density values, below the cut-off level of 0.255 (calculated as
mean + 2 standard deviations of the values in healthy controls) and
abnormal or positive values that were 0.255 or higher. The overall
positivity rate for s-RalA-Abs was 15% (43 of 291 patients).
Clinicopathological characteristics,
conventional serum markers, and presence of s-RalA-Abs
No statistically significant differences were
observed between s-RalA-Abs-positive and s-RalA-Abs-negative
patients with respect to sex, age, Tumor-Node-Metastasis
classification (TNM) stage (16),
tumor depth and lymph node status (Table I). Moreover, no association of
s-RalA-Abs with other tumor markers (CEA and CA19-9) was observed
(Table I). Although the differences
were not statistically significant, lower positivity rates for
s-RalA-Abs was shown in T1/T2 tumors and node-positive tumors
compared with others. Venous invasion was significantly associated
with s-RalA-Abs (P<0.05). Lymphatic invasion and tumor size were
not associated with s-RalA-Abs.
 | Table IComparisons of clinicopathological
characteristics and conventional serum markers of patients with
serum RalA antibodies. |
Table I
Comparisons of clinicopathological
characteristics and conventional serum markers of patients with
serum RalA antibodies.
| Variables (n) | s-RalA-Abs-positive
(n=43) % | s-RalA-Abs-negative
(n=248) | P-values |
|---|
| Sex | | | 0.372 |
|
Male
(201) | 27(13) | 174 | |
|
Female
(90) | 16(18) | 74 | |
| Age, years | | | 0.089 |
|
<65(182) | 32(18) | 150 | |
|
≧65(109) | 11(10) | 98 | |
| TNM stage | | | 0.359 |
|
I+II
(212) | 34(16) | 178 | |
|
III+IV
(79) | 9(11) | 70 | |
| Depth | | | 0.774 |
|
T1+T2(195) | 28(14) | 167 | |
|
T3+T4(96) | 15(16) | 81 | |
| Lymph
nodea | | | 0.125 |
|
Negative
(185) | 32(17) | 153 | |
|
Positive
(105) | 11(10) | 94 | |
| CEAa | | | 1 |
|
Negative
(226) | 33(15) | 193 | |
|
Positive
(45) | 6(13) | 39 | |
| CA19-9a | | | 0.318 |
|
Negative
(231) | 36(16) | 195 | |
|
Positive
(36) | 3(8) | 33 | |
| Tumor
sizea | | | 0.327 |
|
<5 cm
(126) | 16(12) | 110 | |
|
≧5 cm
(67) | 12(17) | 55 | |
| Lya | | | 0.520 |
|
ly (-)
(102) | 16(15) | 86 | |
|
ly (+)
(96) | 12(12) | 84 | |
| va | | | 0.012 |
|
v (-)
(106) | 21(19) | 85 | |
|
v (+)
(93) | 7(7) | 86 | |
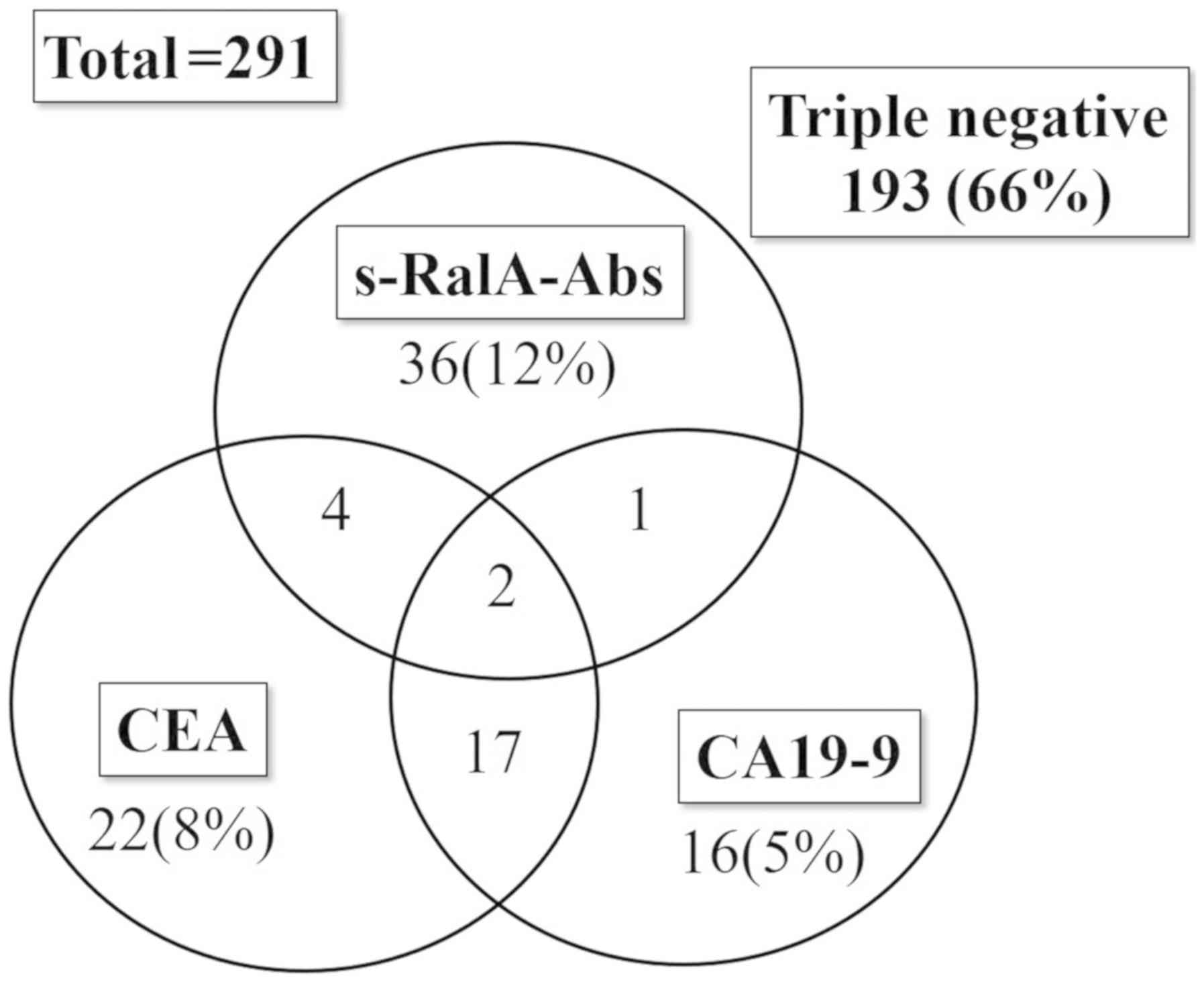
The positivity rates for various markers in patients
with gastric cancer were 15% (43/291) for s-RalA-Abs, 17% (45/271)
for CEA, and 14% (36/267) for CA19-9 (CEA and CA19-9 were not
measured in all cases). A total of 19 patients were positive for
both CEA and CA19-9, whereas only six were positive for both
s-RalA-Abs and CEA, and three were positive for both s-RalA-Abs and
CA19-9 (Fig. 2). Thus, the
positivity rates were significantly higher for the combination
assay with all the three markers [98 of 291 (34%)] compared with
the two conventional markers-CEA and/or CA19-9 [62 of 291 (21%)]
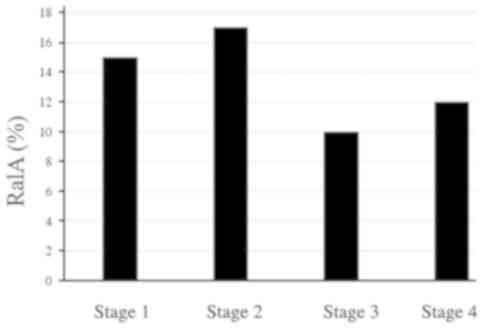
(P<0.01). Although the positivity rates for CEA and CA19-9
gradually increased with an increase in the tumor stage, the
positivity rates for s-RalA-Abs seemed to be similar at all stages
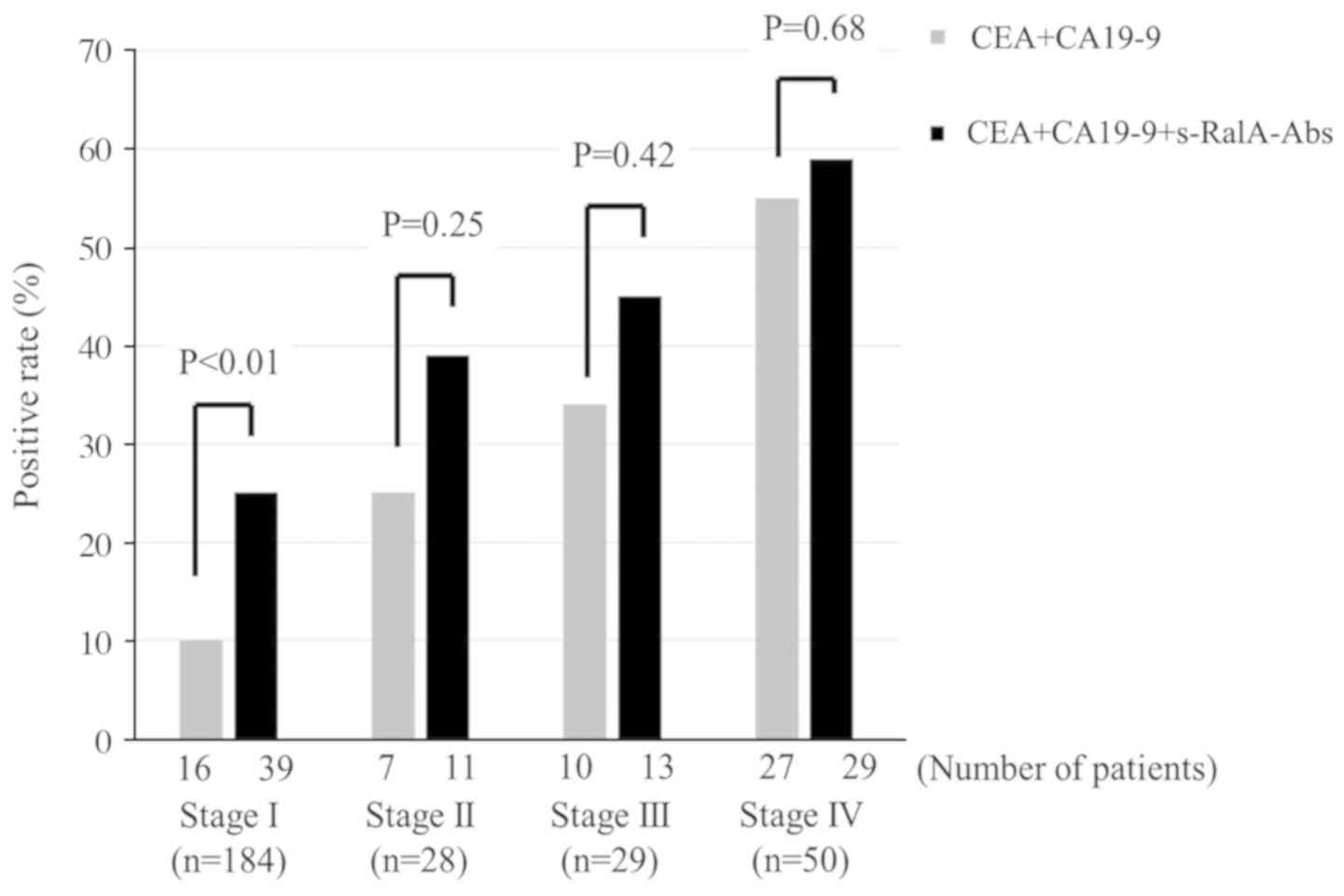
(Fig. 3). In addition, since
s-RalA-Abs was independent of CEA and CA19-9, the combination of
s-RalA-Abs with CEA and CA19-9 increased the detection rate for
gastric cancer at each tumor stage (Fig. 4).
Prognostic role of s-RalA-Abs
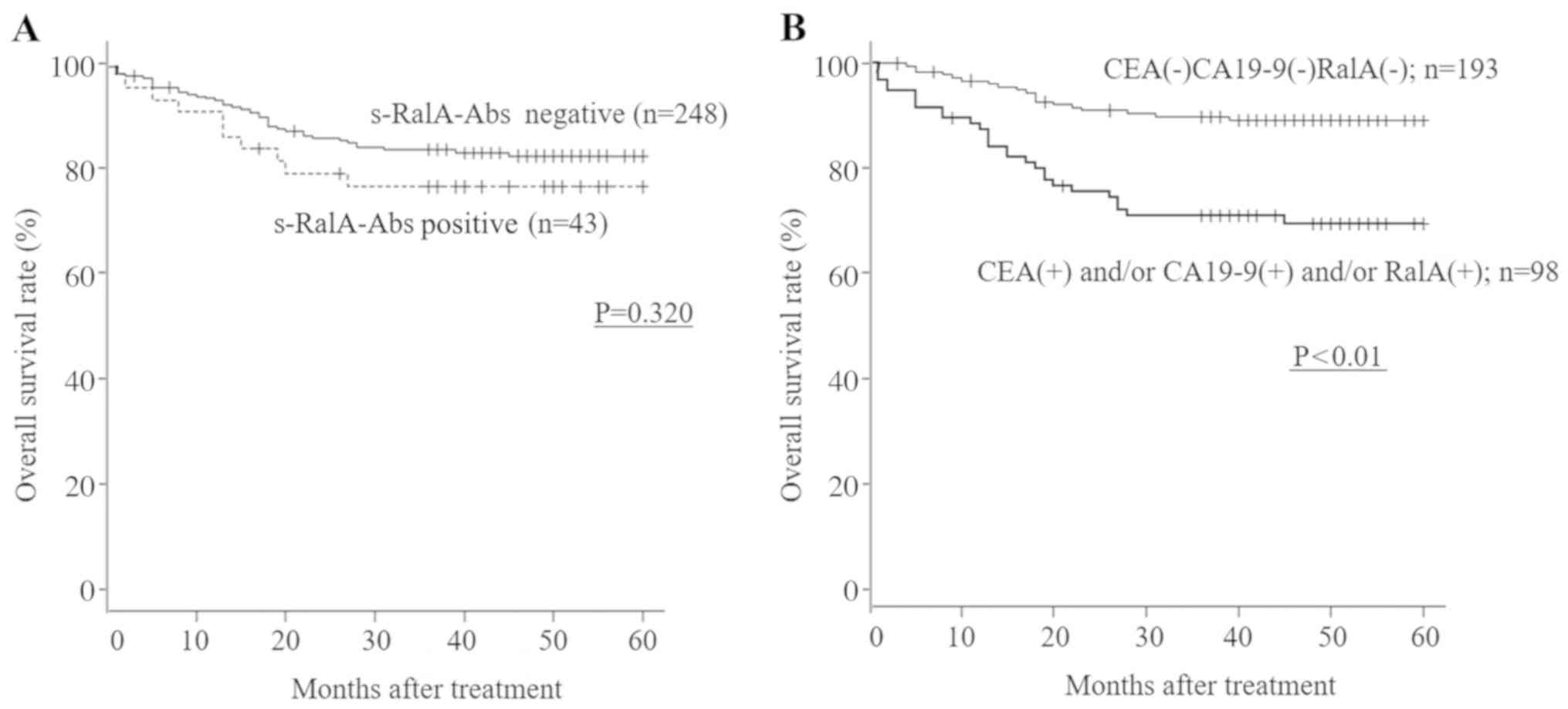
s-RalA-Abs-positive patients showed worse survival
compared with s-RalA-Abs-negative patients, although no
statistically significant differences with respect to overall
survival were observed between s-RalA-Abs-positive and
s-RalA-Abs-negative patients with gastric cancer (Table II; P=0.320; Fig. 5A). The patients who were
triple-negative for CEA, CA19-9 and s-RalA-Abs showed significantly
better overall survival compared with the other group (CEA- and/or
CA19-9- and/or RalA-positive individuals) (P<0.01; Fig. 5B). By multivariate analysis,
although tumor depth and lymph node status were independent risk
factors for patient survival, s-RalA-Abs were not found to be
independent risk factors for survival (Table II).
 | Table IIUnivariate and multivariate analysis
of risk factors for survival in patients with gastric cancer. |
Table II
Univariate and multivariate analysis
of risk factors for survival in patients with gastric cancer.
| Variables | Univariate
P-valuea | H.R.b | 95% CIc | Multivariate
P-valued |
|---|
| Sex | 0.705 | | | |
| Age | 0.364 | | | |
| Stage | <0.001 | | | |
| Tumor depth | <0.001 | 12.5 | 4.35-36.3 | <0.001 |
| Nodal status | <0.001 | 1.72 | 0.716-4.13 | 0.224 |
| s-RalA-Abs | 0.320 | | | |
| CEA | <0.001 | | | |
| CA19-9 | <0.001 | | | |
| CEA or RalA | <0.01 | 2.60 | 0.802-8.45 | 0.111 |
| CA19-9 or RalA | <0.001 | 5.24 | 1.55-17.6 | <0.01 |
| CEA or CA19-9 or
RalA | <0.001 | 1.82 | 1.02-3.24 | <0.05 |
Discussion
In the present study, the positivity rate for
s-RalA-Abs in patients with gastric cancer was 15%. The presence of
s-RalA-Abs did not show any direct association with tumor
progression. Since s-RalA-Abs were not associated with CEA or
CA19-9, the combination assay increased the positivity rate. There
was no significant difference in the overall survival between
s-RalA-Abs-positive and s-RalA-Abs-negative patients.
Among the various clinicopathological variables, age
and venous invasion seemed to be associated with the presence of
s-RalA-Abs. Younger patients were more likely to produce s-RalA-Abs
compared with elderly patients. This tendency was not the same as
that observed in case of other autoantibodies in patients with
gastric cancer (13). Since RalA
expression was reported to be associated with aggressive
clinicopathological characteristics and progression (3,4),
s-RalA-Abs could be predictive biomarkers for poor survival.
However, on univariate and multivariate analyses, s-RalA-Abs were
not found to be independent risk factors for the lower overall
survival. Such discrepancy can be partly explained by anti-tumoral
effects of autoantibodies or may be attributable to the small
sample size of patients in the present study.
The associations between TNM staging and positivity
rate of various autoantibodies have been reported in hepatocellular
carcinoma, colorectal cancer, breast cancer and gastric cancer
(8,10,11,13).
Generally, autoantibody-positive rate is relatively higher than the
positive rate for conventional serum markers at stage I/II of
esophageal squamous cell carcinoma (17). Among them, colorectal cancer
(10) and breast cancer (11) showed that the positive rates of
s-RalA-Abs were similar at all stages. A similar tendency was
demonstrated in the present study in patients with gastric
cancer.
A limitation of the present study was that the
results of immunohistochemical examination of resected specimens
were not used to evaluate the association of protein expression
with s-RalA-Abs reactions. Based on a previous report, which showed
the association between the presence of s-RalA-Abs and
immunoreactivity (9), s-RalA-Abs
may be associated with immunohistochemistry in the present study.
Furthermore, a larger study evaluating the association of protein
expression with s-RalA-Abs reactions may help clarify the
prognostic impact of s-RalA-Abs reactions.
In conclusion, in patients with gastric cancer, the
presence of s-RalA-Abs was found to be independent of other
conventional serum tumor markers. s-RalA-Abs may be useful serum
markers, in combination with CEA and CA19-9, for patients with
gastric cancer, particularly in patients with stage I/II/III
tumors. The presence of s-RalA-Abs, in combination with CEA and/or
CA19-9, was also associated with poor patients' survival in gastric
cancer.
Acknowledgements
The authors would like to thank Dr. Akiko Kuwajima
(Medical & Biological Laboratories Co., Ltd., Nagoya, Japan)
for preparing the RalA peptides.
Funding
This study was partly supported by Grant-in-Aid for
Scientific Research (grant nos. 16K10519 and 16K10520) from the
Ministry of Education, Culture, Sports, Science and Technology of
Japan. Research grants was obtained from the Medical &
Biological Laboratories Co., Ltd. (Nagoya, Japan).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TN, IH, MI and HS contributed to the study
conception and design. SY, YO, TS, YN, KF and FS contributed
analyze the data. TN and HS analyzed patients' data and drafted the
manuscript. IH revised the manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the institutional review
boards of the Chiba Cancer Center (approval no. #21-26) and the
Toho University School of Medicine (approval nos. #22-112 and
#22-047). Additionally, written informed consent was obtained from
all patients.
Patient consent for publication
No applicable.
Competing interests
Professor Hideaki Shimada received research grants
from the Medical & Biological Laboratories Co., Ltd. (Nagoya,
Japan). All other authors have no competing interests.
References
|
1
|
Tanaka S: Precision medicine based on
surgical oncology in the era of genome-scale analysis and genome
editing technology. Ann Gastroenterol Surg. 2:106–115.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Moghadam AR, Patrad E, Tafsiri E, Peng W,
Fangman B, Pluard TJ, Accurso A, Salacz M, Shah K, Ricke B, et al:
Ral signaling pathway in health and cancer. Cancer Med.
6:2998–3013. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Oxford G, Owens CR, Titus BJ, Foreman TL,
Herlevsen MC, Smith SC and Theodorescu D: RalA and RalB:
Antagonistic relatives in cancer cell migration. Cancer Res.
65:7111–7120. 2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Smith SC, Baras AS, Owens CR, Dancik G and
Theodorescu D: Transcriptional signatures of Ral GTPase are
associated with aggressive clinicopathologic characteristics in
human cancer. Cancer Res. 72:3480–3491. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ajani JA, Estrella JS, Chen Q, Correa AM,
Ma L, Scott AW, Jin J, Liu B, Xie M, Sudo K, et al: Galectin-3
expression is prognostic in diffuse type gastric adenocarcinoma,
confers aggressive phenotype, and can be targeted by YAP1/BET
inhibitors. Br J Cancer. 118:52–61. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Saito F, Shimada H, Ogata H, Otsuka T,
Nemoto T, Shibuya K and Kaneko H: Detection of the early phase of
esophageal cancer progression into lamina propria mucosae by the
serum p53 antibody. Esophagus. 14:366–369. 2017.
|
|
7
|
Shimada H, Nagata M, Cho A, Takiguchi N,
Kainuma O, Soda H, Ikeda A, Nabeya Y, Yajima S, Yamamoto H, et al:
Long-term monitoring of serum p53 antibody after neoadjuvant
chemotherapy and surgery for esophageal adenocarcinoma: Report of a
case. Surg Today. 44:1957–1961. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Okada R, Shimada H, Otsuka Y, Tsuchiya M,
Ishii J, Katagiri T, Maeda T, Kubota Y, Nemoto T and Kaneko H:
Profiling of Serum Autoantibodies in Japanese Patients with
Hepatocellular Carcinoma. Toho J Medicine. 3:84–92. 2017.
|
|
9
|
Nanami T, Shimada H, Yajima S, Oshima Y,
Matsushita K, Nomura F, Nagata M, Tagawa M, Otsuka S, Kuwajima A
and Kaneko H: Clinical significance of serum autoantibodies against
Ras-like GTPases, RalA, in patients with esophageal squamous cell
carcinoma. Esophagus. 13:167–172. 2016.
|
|
10
|
Ushigome M, Nabeya Y, Soda H, Takiguchi N,
Kuwajima A, Tagawa M, Matsushita K, Koike J, Funahashi K and
Shimada H: Multi-panel assay of serum autoantibodies in colorectal
cancer. Int J Clin Oncol. 23:917–923. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kubota Y, Ogata H, Otsuka S, Kuwajima A,
Saito F and Shimada H: Presence of autoantibodies against Ras-like
GTPases in Serum-in stage I/II breast cancer. Toho J Medicine.
3:125–130. 2017.
|
|
12
|
Sun H, Shi JX, Zhang HF, Xing MT, Li P,
Dai LP, Luo CL, Wang X, Wang P, Ye H, et al: Serum autoantibodies
against a panel of 15 tumor-associated antigens in the detection of
ovarian cancer. Tumour Biol. 39(1010428317699132)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hoshino I, Nagata M, Takiguchi N, Nabeya
Y, Ikeda A, Yokoi S, Kuwajima A, Tagawa M, Matsushita K, Yajima S
and Shimada H: Panel of autoantibodies against multiple
tumor-associated antigens for detecting gastric cancer. Cancer Sci.
108:308–315. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Suzuki T, Funahashi K, Ushigome M, Koike
J, Nemoto T and Shimada H: Diagnostic and prognostic impact of
serum p53 antibody titration in colorectal cancer. Toho J Med.
3:107–115. 2017.
|
|
15
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM Classification of Malignant Tumours. 8th edition.
John Wiley & Sons, Hoboken, NJ, 2017.
|
|
17
|
Shimada H: p53 molecular approach to
diagnosis and treatment of esophageal squamous cell carcinoma. Ann
Gastroenterol Surg. 2:266–273. 2018.PubMed/NCBI View Article : Google Scholar
|