Introduction
According to epidemiological data gastrointestinal
cancer is one of the largest problems within the field of oncology
(1). In 2018, colorectal cancer
(CRC) was the cause of 881,000 deaths (2). However, treatment and management of
CRC have considerably changed over the last years.
Advances in systemic treatment, mainly targeted
therapy and, in limited cases immunotherapy, have significantly
changed the prognosis of patients with metastatic or recurrent CRC
(3,4). Second and subsequent lines of
treatment can slow the disease and, in effect, change it into a
chronic disease. However, the aim of modern oncology is to
definitively eliminate the danger of disease recurrence, which
might be achievable by individualization of the treatment.
Chronic inflammation is a cancer-promoting factor
that leads to genetic instability (5). Cyclooxygenase-2 (COX-2) is an enzyme
that catalyses the formation of prostaglandins and therefore
participates in the regulation of the consecutive stages of
inflammatory processes. In CRCs overexpression of COX-2 has been
shown to correlate with poor survival and metastasis (6).
Mucins are a family of glycoproteins form a
protective gel layer on the surface of the mucosa under normal
circumstances. Their expression varies in different areas of the
digestive tract and is altered in tumours. Mucins in cancer cells
are dysregulated on a genetic level and atypically glycosylated in
posttranscriptional setting (7,8). Their
quantitative and qualitative alterations impair their functions,
heavily affect the properties of the mucus layer and presumably
weaken the penetration of chemotherapeutic agents. Mucin 1 (MUC1)
is a structural membrane-bound mucin that is normally present only
on the apical borders of the secretory epithelium. In tumours, the
polarization of MUC1 is lost and the protein is overexpressed at
high levels over the entire cell surface (9). The positive correlation between
abundant expression of MUC1 and tumour invasiveness, metastasis and
poor prognosis has been reported in CRC (9,10).
The overexpression of COX-2 and the overexpression
of MUC1 might be related and this relationship has already been
studied in pancreatic cancer (11).
The authors concluded that the intracellular tail of MUC1
participates in the activation of proapoptotic genes and indirectly
upregulates COX-2 expression (11).
Hypothetically, this relationship may also apply to other
gastrointestinal cancers, including CRC. Perhaps this could be
easily observed as altered COX-2 and MUC1 expression. In addition,
MUC1 has been proposed as an alternative target for blocking COX-2
overexpression (11). In CRC, where
abundant expression of COX-2 occurs frequently, such a discovery
might be a substantial finding from the perspective of future
targeted therapy. The significance of the present study was to
confirm the presented hypotheses in CRC. The direct aim was to
investigate the correlation between COX-2 and MUC1 expression
patterns and clinical-pathological factors in CRC, with particular
attention to survival.
Materials and methods
Tissue samples
CRC samples were collected from the resources of the
Histopathology Department of the Independent Clinical Hospital No.
1 (SPSK 1) in Lublin (Poland) in the form of paraffin-embedded
tissue blocks. The studied material encompassed sections of
colorectal adenocarcinomas from 170 patients treated in the
Department of Surgical Oncology (Medical University of Lublin)
between August 2004 and January 2014. Only samples that contained
adenocarcinomas were included. Rectal tumour samples from patients
who had undergone neoadjuvant radiotherapy were excluded from
further analysis.
The clinicopathological data of patients were
collected from the Medical Records Department and Outpatient
Clinics of the Hospital. In the analysed population 102 (60%)
patients were male, the median age was 62 years, 126 (74%) of the
tumours were rectal cancers and the remaining 44 cases (26%) were
colon cancers. Among the disease stages there were: 8 (5%) cancers
in situ and 31 (18%) stage I, 51 (30%) stage II, 58 (34%)
stage III and 22 (13%) stage IV cancers. Detailed data concerning
the studied population are presented in Table I.
 | Table ICharacteristics of the study
population. |
Table I
Characteristics of the study
population.
| Characteristic | Value |
|---|
| Age, years |
|
≤55, n
(%) | 50 (29.41) |
|
56-69, n
(%) | 74 (43.53) |
|
≥70, n
(%) | 46 (27.06 |
|
M ± SD | 64.48±10.74 |
|
Me
(Q1-Q3) | 62 (54-70) |
|
Min-Max | 32-87 |
| Sex, n (%) |
|
Male | 102 (60.00) |
|
Female | 68 (40.00) |
| Localization, n
(%) |
|
Colon | 44 (25.88) |
|
Rectum | 126 (74.12) |
| Stage, n (%) |
|
0 | 8 (4.71) |
|
1 | 31 (18.23) |
|
2 | 51 (30.00) |
|
3 | 58 (34.12) |
|
4 | 22 (12.94) |
Antibodies
Immunostaining was performed using two antibodies
for both COX-2 and MUC1. These were: Anti-COX-2 rabbit monoclonal
antibody from Abcam (clone SP21, cat. no. ab16701) and anti-COX 2
mouse monoclonal antibody from Dako (clone CX-294); anti-Mucin
1C-term rabbit monoclonal antibody from antibodiesonline.com (clone G22-L, cat. no.
ABIN371859) and anti-MUC1 mouse monoclonal antibody from Dako
(EMA-human epithelial membrane antigen, clone E29, cat. no. M0613).
The antibodies were stepwise optimized and tested at dilutions from
1:100 to 1:800 and for one antibody (anti-mucin 1 C-term clone
G22-L, cat. no. ABIN371859 from antibodies online.com)
even at 1:3200. The staining was comparatively performed with DAB
and Bright-DAB and then the sections were counterstained with
haematoxylin.
In effect, two antibodies and two staining protocols
were chosen as the methods that provide the best sensitivity at the
lowest background noise: Anti-COX-2 from Abcam (clone SP21, cat.
no. ab16701), at a dilution of 1:200, incubated for 1 h and stained
with DAB and anti-Mucin 1 C-term from antibodies-online.com (clone G22-L, cat. no.
ABIN371859) diluted to 1:1,000, incubated overnight and stained
with Bright-DAB (Table II).
 | Table IIImmunohistochemistry methods. |
Table II
Immunohistochemistry methods.
| Antibody | Source | Dilution | Antigen
retrieval | Antibody
incubation | Chromogen |
|---|
| COX-2, clone SP21,
cat. no. ab16701 | Abcam | 1:200 | Sodium citrate buffer
(0,01 M/pH 6.0) 20 min at 100˚C | 1 h | DAB |
| MUC1 C-term, clone
G22-L, cat. no. ABIN371859 | Antibodies
Online | 1:1,000 | Sodium citrate buffer
(0,01 M/pH 6.0) 20 min at 100˚C | Overnight | Bright-DAB |
Immunohistochemistry
Preparation of microscope slides, immunostaining and
scoring were performed at University Medical Center Utrecht in the
Netherlands. Sections of tissues (4 µm) were first deparaffinized
and blocked for endogenous peroxidase activity by immersion in 0.3%
H2O2 in methanol for 20 min. Then, antigen
retrieval was performed in sodium citrate buffer (0.01 M/pH 6.0)
for 20 min at 100˚C. Nonspecific binding sites were blocked using
Protein Block Serum Free (DAKO, X0909) for 10 min, followed by
primary antibody incubation (anti-COX-2 at room temperature for 1 h
and anti-MUC1 at 4˚C overnight). Antibody binding was visualized
using the BrightVision+poly-HRP detection system (VWR
International, cat. no. VWRKDPVB110HRP), with 3,3-diamino-benzidine
as chromogen (DAB, Sigma D5637 for COX-2 and Bright-DAB, VWR
International VWRKBS04-110 for MUC1). Finally, the sections were
counterstained with haematoxylin, dehydrated and coverslipped using
Pertex (Table II).
Analysis of immunostaining intensity was conducted
at 20x original objective magnification in the area that
encompassed tumour cells with the strongest staining. Scoring
systems for COX-2 and MUC1 based on the references (12,13).
Scoring was performed independently by two researchers, the
discrepancies were reanalysed by the expert and scored in
accordance with his judgement.
The study was reviewed and approved by the Bioethics
Committee of Medical University of Lublin (number of approval
KE-0254/48/2015 from 26.02.2015).
Statistical analysis
The Statistica 9.1 software package was used for
statistical analysis. The analysed data concerned the level of
COX-2 and MUC1 expression with respect to age and sex of patients
as well as the localization and stage of the tumour. The
information regarding particular tests is included in the
description of the tables.
The analysis regarding the interplay between the
COX-2 and MUC1 expression levels was performed as a Spearman's rank
correlation. The results were verified by the Chi-square test
comparing the incidence of cases with high COX-2 expression and
high MUC1 expression, compared to the cases with low COX-2
expression and low MUC1 expression. An analogous analysis was
performed comparing the cases with high COX-2 expression and low
MUC1 expression compared to cases with low COX-2 expression and
high MUC1 expression. Survival analysis was performed for high and
low COX-2 expression and for high and low MUC1 expression, using
MedCalc software. The results are presented as Kaplan-Meier curves,
and the comparison of survival curves was performed using the
log-rank test. P-values <0.05 were considered significant.
Results
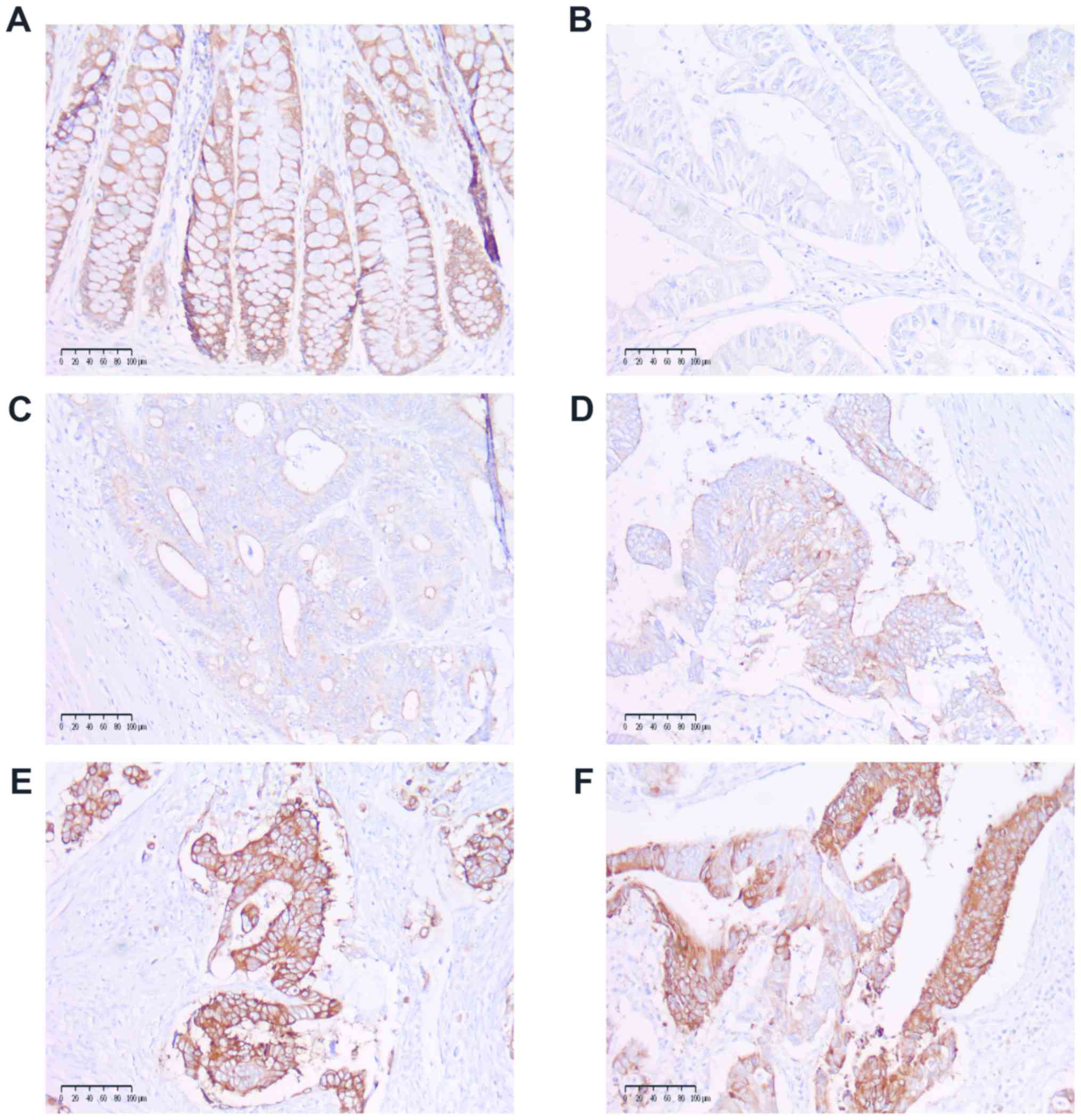
COX-2 and MUC1 expression. COX-2 expression
was categorized as follows: 0, no staining; 1, weak diffuse
cytoplasmic staining; 2, moderate to strong granular cytoplasmic
staining in 10-50% of cancer cells; and 3, >50% of tumour cells
stained with strong intensity (Fig.
1). Samples with scores of 0 and 1 were further categorized as
low COX-2 expression, and those with scores of 2 and 3 were
categorized as high COX-2 expression. The cases of patchy,
heterogenic COX-2 staining were distinguished and included as
stained with strong intensity. The expression level of COX-2 and
its statistical correlation with: Age, sex, localization and
disease stage are presented in Table
III.
 | Table IIICOX-2 expression and
clinicopathological features of patients. |
Table III
COX-2 expression and
clinicopathological features of patients.
| | COX-2 expression | |
|---|
| Characteristic | Low, n (%) | High, n (%) | Statistics |
|---|
| Age, years | | | H (2.170)=1.109;
P=0.574 |
|
≤55 | 10 (20.0) | 40 (80.0) | R=0.415; P=0.678 |
|
56-69 | 14 (18.9) | 60 (81.1) | |
|
≥70 | 11 (23.9) | 35 (76.1) | |
| Sex | | | Z=0.068; P=0.945 |
|
Male | 22 (21.6) | 80 (78.4) | |
|
Female | 13 (19.1) | 55 (80.9) | |
| Localization | | | Z=0.006; P=0.995 |
|
Colon | 7 (15.9) | 37 (84.1) | |
|
Rectum | 28 (22.2) | 98 (77.8) | |
| Stage | | | H (4.170)=1.203;
P=0.878 |
|
0 | 2 (25.0) | 6 (75.0) | R=0.084;
P=0.276 |
|
1 | 8 (25.8) | 23 (74.2) | |
|
2 | 10 (19.6) | 41 (80.4) | |
|
3 | 12 (20.7) | 46 (79.3) | |
|
4 | 3 (13.6) | 19 (86.3) | |
| Total | 35 (20.6) | 135 (79.4) | - |
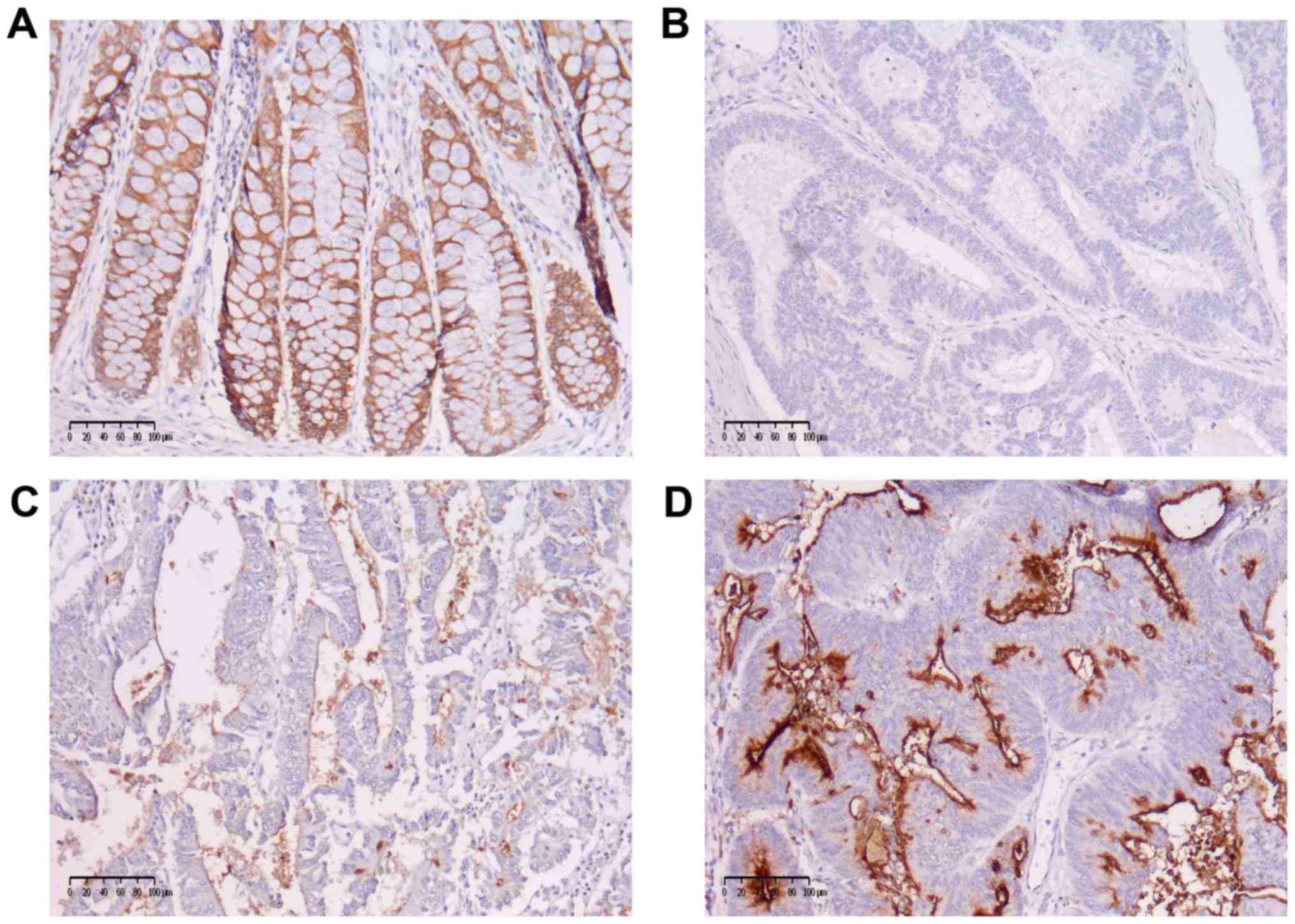
MUC1 staining was categorized as: 0, no staining; 1,
weak staining; 2, strong staining and further categorized as low
MUC1 expression (scores of 0 and 1) or high MUC1 expression (score
2) (Fig. 2). The correlations with
clinical data are presented in Tables
III and IV.
 | Table IVMUC1 expression and
clinicopathological features of patients. |
Table IV
MUC1 expression and
clinicopathological features of patients.
| | MUC1
expression | |
|---|
| Characteristic | Low (0-1), n
(%) | High (2), n
(%) | Statistics |
|---|
| Age, years | | | H (2.170)=1.965;
P=0.375 |
|
≤55 | 26 (52.0) | 24 (48.0) | R=0.107;
P=0.164 |
|
56-69 | 32 (43.2) | 42 (56.8) | |
|
≥70 | 18 (39.1) | 28 (60.9) | |
| Sex | | | Z=0.658;
P=0.510 |
|
Male | 44 (43.1) | 58 (56.9) | |
|
Female | 32 (47.1) | 36 (52.9) | |
| Localization | | | Z=-0.554;
P=0.580 |
|
Colon | 22 (50.0) | 22 (50.0) | |
|
Rectum | 54 (42.9) | 72 (57.1) | |
| Stage | | | H (4.170)=2.630;
P=0.622 |
|
0 | 3 (37.5) | 5 (62.5) | R=-0.048;
P=0.535 |
|
1 | 16 (51.6) | 15 (48.4) | |
|
2 | 19 (37.2) | 32 (62.7) | |
|
3 | 28 (48.3) | 30 (51.7) | |
|
4 | 10 (45.4) | 12 (54.6) | |
| Total | 76 (44.7) | 94 (55.3) | - |
Neither the COX-2 expression level nor the MUC1
expression level correlated with any of the clinicopathological
features.
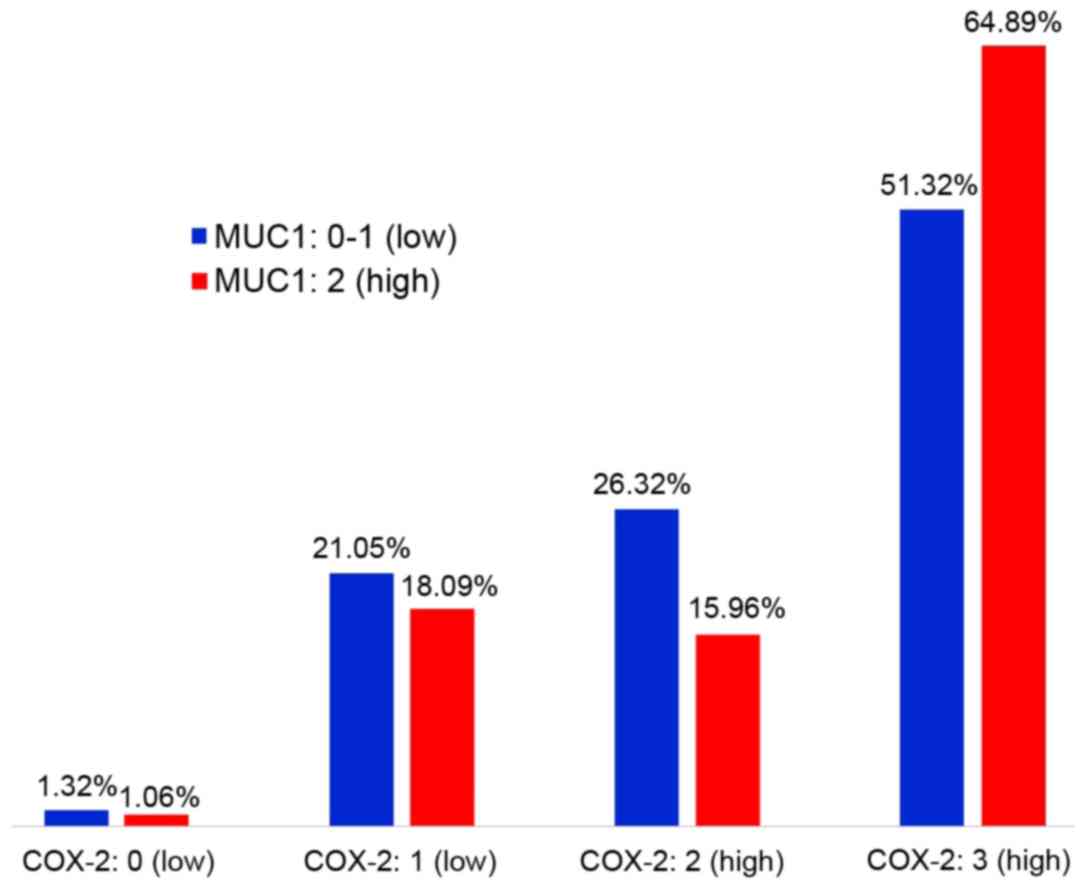
The ratio of COX-2 to MUC1 expression is shown in
Fig. 3. There was no linear
correlation between the expression levels of these proteins
(P=0.117). However, there were significantly more cases with the
simultaneous occurrence of high COX-2 and MUC1 expression compared
to those in which the level of COX-2 and MUC1 expression was low
(P<0.001). There were also significantly more cases with high
expression of COX-2 and low expression of MUC1 than cases with
simultaneous low expression of COX-2 and high expression of MUC1
(P<0.001) (Table V).
 | Table VRatio of COX-2 to MUC1
expression. |
Table V
Ratio of COX-2 to MUC1
expression.
| | MUC1 | |
|---|
| COX-2 | Low, n (%) | High, n (%) | Total |
|---|
| Low | 17
(10.0)a | 18
(10.6)b | 35 (20.6) |
| High | 59
(34.7)c | 76
(44.7)d | 135 (79.4) |
| Total | 76 (44.7) | 94 (55.3) | 170 (100.0) |
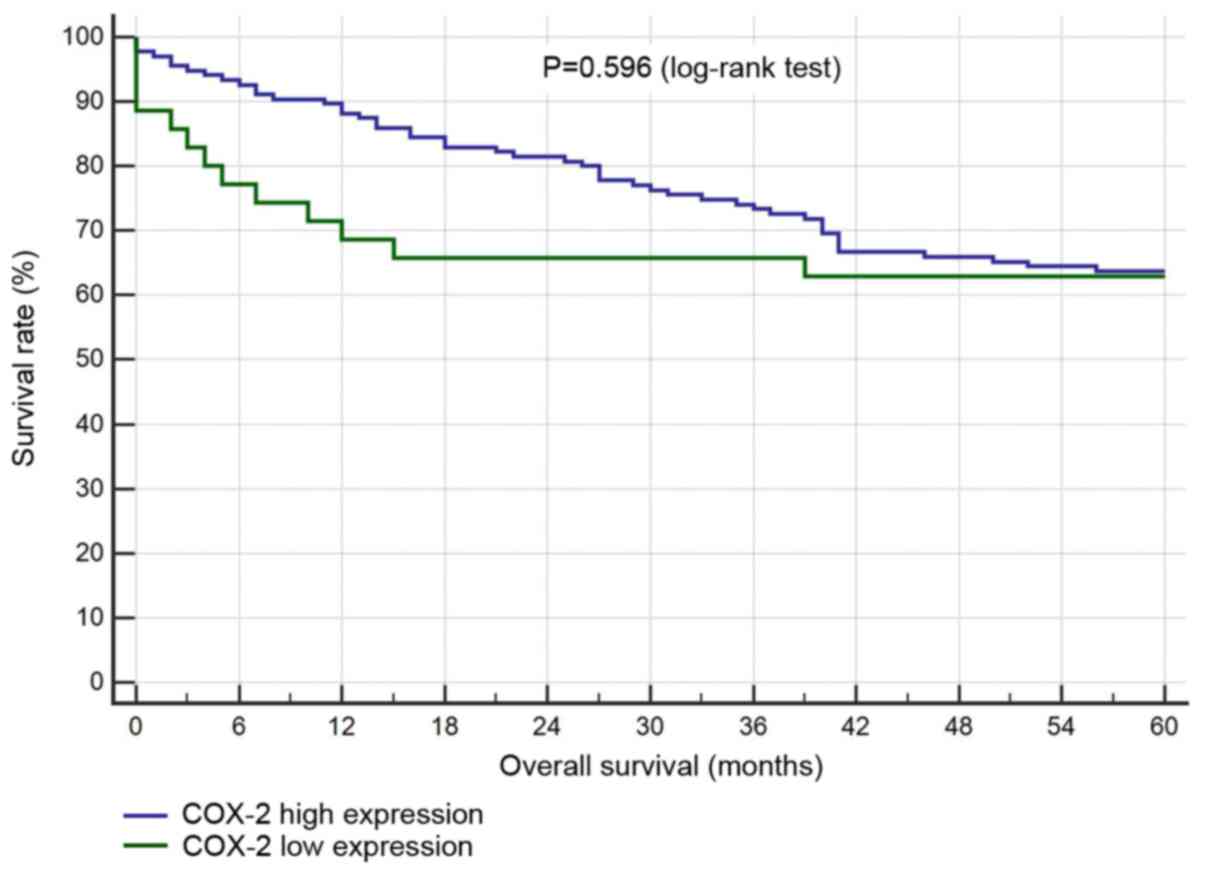
Survival analysis
There was no statistical correlation between
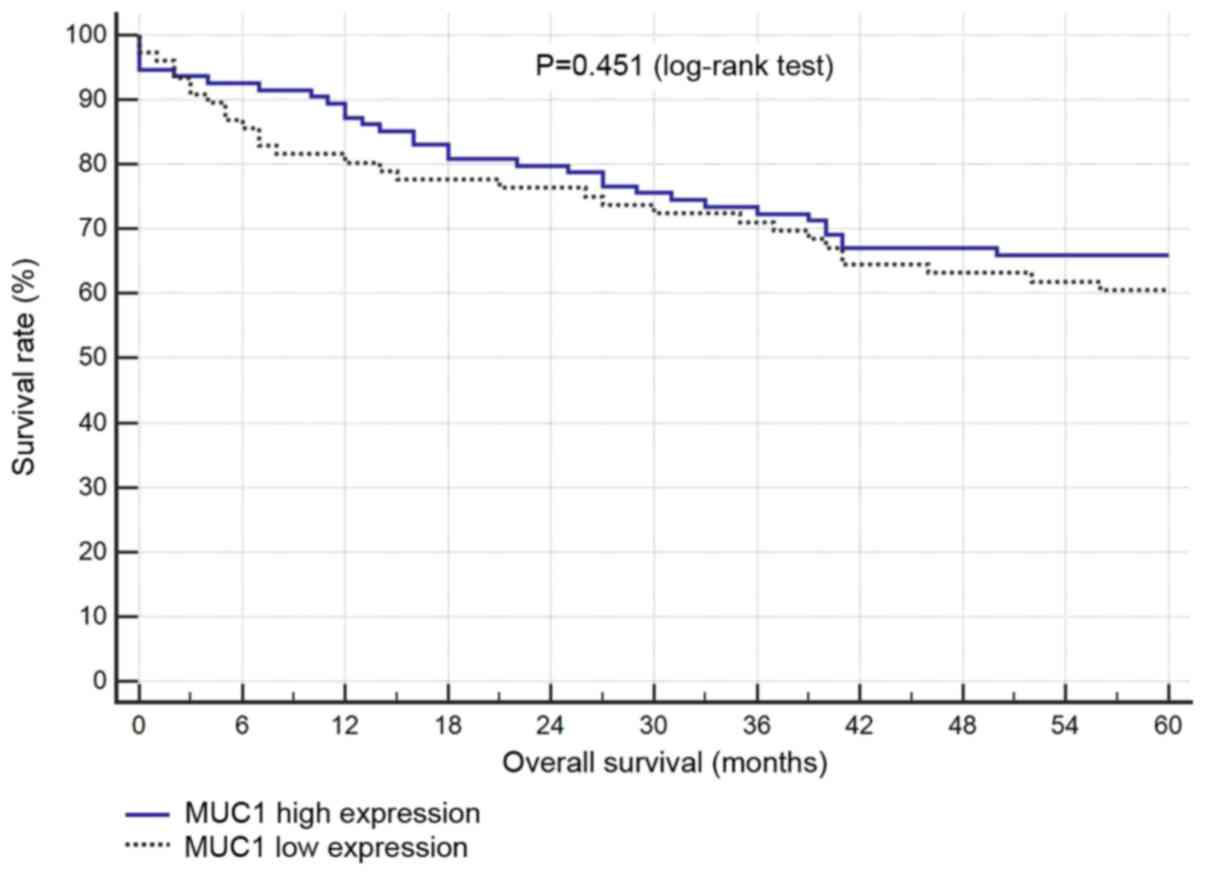
survival and the levels of COX-2 or MUC1 expression (Figs. 4 and 5).
Discussion
There are many reports of the importance of COX-2
overexpression in patients with CRC. Studies carried out so fa, on
populations of different races, have shown that COX-2
overexpression slightly worsens overall survival (6). In a recently published paper, Kim
et al presented that in a group of Korean patients elevated
COX-2 expression was not a prognostic factor, but COX-2 expression
might have been an independent predictive marker of late recurrence
for patients with stage I to III CRC (14). Our studies showed no correlation
between the expression of COX-2 and any studied clinical variables,
or prognosis. The conclusions of Kim et al (14). seem to be consistent with ours,
however, our analysis was narrower, because it did not relate to
recurrence rates and it was also carried out among Caucasian
patients so the results cannot be directly compared.
Our research did not confirm the correlation between
the expression of MUC1 and clinical variables, including stage of
the disease and survival. In a similar study performed
independently at the same time as ours, there was no correlation
between MUC1 expression and clinicopathological variables of the
patients, but there was a significant increase in MUC1 mRNA
expression in CRC compared to healthy tissues (15). This could mean that in tumours, the
level of MUC1 changes with the progression of the disease. In the
cited study MUC1 expression was more often detected in patients
with CRC with synchronic lymph node metastases, than in those
without them (15). Duncan et
al, in a study on a population of 462 patients showed that MUC1
expression can be considered an independent marker of poor
prognosis, which is in contrast to our results. However, they did
not confirm the correlation of MUC1 with any of the
clinicopathological variables including tumour grade and stage,
vascular invasion and tumour type, which coincides with our results
(16). Betge et al also
showed a correlation of MUC1 expression with various
clinicopathological variables as well as disease progression and
lymph node metastasis. However, their study did not confirm a
correlation between MUC1 expression and survival in patients with
CRC (17). It is interesting that
all these cited studies concerned similar populations, i.e.,
Caucasian patients were recruited consecutively for CRC surgery.
MUC1 overexpression occurs in CRC with lymph node invasion
(18). Therefore, hypothetically,
the negative results of our and other authors' work may result from
a small number of patients with lymph node metastases.
Nevertheless, the quality assessment of the MUC1
expression level based only on immunohistochemistry is limited in
credibility. This is because mucins are alternatively glycosylated
in tumours (7). Evaluation of the
MUC1 expression level may be understated due to the specificity of
the chosen antibody. In one of the larger earlier studies, MUC1 was
detected only in 32.5% of CRC specimens (19). Therefore, we take into account that
in our study, COX-2 level assessment is more reliable than MUC1.
The expression of mucins and associated O-glycans differ in
colorectal polyp subtypes (20).
Most likely, it also applies to pathological subtypes of CRC. The
distribution of goblet cells, which produce mucins, increases along
the entire length of the digestive tract (21). These observations might explain the
discrepancies in our and other cited studies, because all of them
differed in the number of pathological subtypes of CRC and the
number of tumours from different localizations of the colon. In
most of the papers about MUC1 in CRC, parts of the colon were not
distinguished. In our study, there was a similar number of MUC1-low
and MUC1-high expression in the colon, but there were definitely
more cases of MUC1-high expression in the rectum. The latter result
is more reliable because most of the examined tissues were cancers
of the rectum.
The present study does not confirm a direct
relationship between the intensity of expression of COX-2 and MUC1
or between the expression of either of them and the
clinicopathological characteristics of patients with CRC. In
addition, neither protein had prognostic value for survival, which
contradicts some previous reports. This issue needs further
investigation based on larger sample analysis or stratified
analysis.
Acknowledgements
Not applicable.
Funding
The present study was supported by internal grants
from the Medical University of Lublin (grant no. DS201/2018) and an
educational grant for young researchers (grant no. M. Szlendak,
MNsd228/2018).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MS, RS and RM concieved and designed the current
study. RM, RS, FM, MS, MB and JM acquired the data. MS, JM, GJAO
and WPP analyzed and interpreted the data. MS and RS drafted the
manuscript. All authors critically revised the manuscript, with
major contributions from GJAO and WPP. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The board of the Bioethics Committee of the Medical
University of Lublin approved the present study protocol (approval
no. KE-0254/48/2015 from February 26, 2015). Informed consent was
obtained at the time of original tissue collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Stewart BW and Wild CP: World Cancer
Report 2014. Lyon, France, IARC Press, 2014.
|
|
2
|
Rawla P, Sunkara T and Barsouk A:
Epidemiology of colorectal cancer: Incidence, mortality, survival,
and risk factors. Prz Gastroenterol. 14:89–103. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ohhara Y, Fukuda N, Takeuchi S, Honma R,
Shimizu Y, Kinoshita I and Dosaka-Akita H: Role of targeted therapy
in metastatic colorectal cancer. World J Gastrointest Oncol.
8:642–655. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kalyan A, Kircher S, Shah H, Mulcahy M and
Benson A: Updates on immunotherapy for colorectal cancer. J
Gastrointest Oncol. 9:160–169. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Colotta F, Allavena P, Sica A, Garlanda C
and Mantovani A: Cancer-related inflammation, the seventh hallmark
of cancer : Links to genetic instability. Carcinogenesis.
30:1073–1081. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Peng L, Zhou Y, Wang Y, Mou H and Zhao Q:
Prognostic significance of COX-2 immunohistochemical expression in
colorectal cancer: A meta-analysis of the literature. PLoS One.
8(e58891)2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Skierucha M, Milne AN, Offerhaus GJ,
Polkowski WP, Maciejewski R and Sitarz R: Molecular alterations in
gastric cancer with special reference to the early-onset subtype.
World J Gastroenterol. 22:2460–2474. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Terada T: An immunohistochemical study of
primary signet-ring cell carcinoma of the stomach and colorectum:
II Expression of MUC1, MUC2, MUC5AC, and MUC6 in normal mucosa and
in 42 cases. Int J Clin Exp Pathol. 6:613–621. 2013.PubMed/NCBI
|
|
9
|
Niv Y: MUC1 and colorectal cancer
pathophysiology considerations. World J Gastroenterol.
14:2139–2141. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zeng Y, Zhang Q, Zhang Y, Lu M, Liu Y,
Zheng T, Feng S, Hao M and Shi H: MC1 predicts colorectal cancer
metastasis: A systematic review and meta-analysis of case
controlled studies. PLoS One. 10(e0138049)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nath S, Roy LD, Grover P, Rao S and
Mukherjee P: Mucin 1 regulates Cox-2 gene in pancreatic cancer.
Pancreas. 44:909–917. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sitarz R, Leguit RJ, de Leng WW, Polak M,
Morsink FM, Bakker O, Maciejewski R, Offerhaus GJ and Milne AN: The
COX-2 promoter polymorphism-765 G>C is associated with
early-onset, conventional and stump gastric cancers. Mod Pathol.
21:685–690. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Eminaga O, Wei W, Hawley SJ, Auman H,
Newcomb LF, Simko J, Hurtado-Coll A, Troyer DA, Carroll PR, Gleave
ME, et al: MUC1 Expression by immunohistochemistry is associated
with adverse pathologic features in prostate cancer: A
multi-institutional study. PLoS One. 11(e0165236)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kim SH, Ahn BK, Paik SS and Lee KH:
Cyclooxygenase-2 expression is a predictive marker for late
recurrence in colorectal cancer. Gastroenterol Res Pract.
2018(7968149)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kasprzak A, Siodla E, Andrzejewska M,
Szmeja J, Seraszek-Jaros A, Cofta S and Szaflarski W: Differential
expression of mucin 1 and mucin 2 in colorectal cancer. World J
Gastroenterol. 24:4164–4177. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Duncan TJ, Watson NF, Al-Attar AH,
Scholefield JH and Durrant LG: The role of MUC1 and MUC3 in the
biology and prognosis of colorectal cancer. World J Surg Oncol.
5(31)2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Betge J, Schneider NI, Harbaum L,
Pollheimer MJ, Lindtner RA, Kornprat P, Ebert MP and Langner C:
MUC1, MUC2, MUC5AC, and MUC6 in colorectal cancer: Expression
profiles and clinical significance. Virchows Arch. 469:255–265.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xu F, Liu F, Zhao H, An G and Feng G:
Prognostic significance of mucin antigen MUC1 in various human
epithelial cancers: A meta-analysis. Medicine (Baltimore).
94(e2286)2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Baldus SE, Monig SP, Hanisch FG, Zirbes
TK, Flucke U, Oelert S, Zilkens G, Madejczik B, Thiele J, Schneider
PM, et al: Comparative evaluation of the prognostic value of MUC1,
MUC2, sialyl-Lewis(a) and sialyl-Lewis(x) antigens in colorectal
adenocarcinoma. Histopathology. 40:440–449. 2002.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Krishn SR, Kaur S, Sheinin YM, Smith LM,
Gautam SK, Patel A, Jain M, Juvvigunta V, Pai P, Lazenby AJ, et al:
Mucins and associated O-glycans based immunoprofile for
stratification of colorectal polyps: Clinical implication for
improved colon surveillance. Oncotarget. 8:7025–7038.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kim JJ and Khan WL: Goblet cells and
mucins: Role in innate defense in enteric infections. Pathogens.
2:55–70. 2013.PubMed/NCBI View Article : Google Scholar
|