Introduction
Gastric cancer is one of the most common
malignancies and is the leading cause of mortality both in Japan
and globally (1). Systemic
chemotherapies for the disease have progressed rapidly in recent
years, resulting in survival benefit to patients, although they
remain unsatisfactory. To move the treatment options beyond
systemic chemotherapy, novel modalities are urgently needed. To
this end, we have conducted adoptive immunotherapy (AIT) trials
using ex vivo-activated autologous lymphocytes-namely,
lymphokine-activated killer (LAK) cells, tumor-infiltrating
lymphocytes, in vitro tumor-sensitized lymphocytes, and
tumor antigen peptide-pulsed dendritic cell-activated killer
cells-although the tumor responses have remained poor (2,3).
However, other researchers have achieved survival benefits in
hepatocellular carcinoma patients using postoperative LAK cell
transfer (4) and in lung cancer
patients using LAK cell transfer in combination with
chemoradiotherapy (5), suggesting
that AIT may have a benefit in terms of survival rather than tumor
shrinkage per se.
Following on these results, we next established a
system for generating another type of effector lymphocytes,
zoledronate-activated killer (ZAK) cells, which consist of natural
killer (NK) cells and γδT cells (6). It has been reported that γδT cells
have the ability to kill a wide variety of tumor cells, and also
play an important role in the innate immune system (7). Moreover, γδT cells have been shown to
possess an antigen-presenting function (8). Other researchers have described the
safety and feasibility profiles of γδT cells for cancer treatment
(9,10). Since 2009, we have also conducted a
prospective observational study of AIT using ZAK cells for patients
with various types of incurable cancer.
In this study, we analyzed a series of cumulative
data from patients with advanced or metastatic gastric cancer and
demonstrated a possible survival benefit of ZAK cell AIT in
combination with chemotherapy. We also identified a candidate
biomarker for predicting a survival benefit from ZAK cell AIT.
Patients and methods
Study design
The data series from a prospective observational
study conducted at Kawasaki Medical School Hospital between May
2009 and July 2017 was analyzed. All participating patients had a
diagnosis of incurable gastric cancer with a performance status
that allowed them to visit our outpatient clinic. All patients
provided written informed consent. Exclusion criteria were as
follows: Consecutive use of steroids or immunosuppressants, the
presence of autoimmune diseases, a case that was too difficult to
manage at an outpatient clinic, and/or uncontrolled complications.
Participants were considered for the study until they were
deceased, they withdrew their consent, or follow-up contact was
lost. All aspects of patients’ treatments over time, including
specific chemotherapy agents and/or combinations, as well as the
dose, schedule, and duration of AIT, were determined by a physician
on a case-by-case basis. This prospective study was reviewed about
science and ethics and approved by the Research Ethics Committee of
Kawasaki Medical School and Hospital (approval no. 240,
UMIN000021797).
ZAK cell generation and transfer
ZAK cell generation has been described in detail
elsewhere (6). Briefly, PBMCs were
obtained from the heparinized venous blood of patients by
centrifugation, then stimulated with interleukin-2 plus zoledronate
and cultured for 10 to 14 days. ZAK cells were harvested by
centrifugation, washed twice, resuspended in 100 ml saline after
filtering through a 200-µm mesh, and administered intravenously for
30 min every 3-4 weeks in a chemotherapy-off period. At each
infusion, patients had blood drawn to prepare ZAK cells for the
next transfer. Bacterial, endotoxin, and mycoplasma examinations
were completed before each administration to make sure there was no
contamination.
Clinical efficacy
Survival data of the patients were collected from
patient records. If the prognosis was unknown, a letter was sent
requesting this information from the doctor in charge. Objective
tumor response was evaluated by computed tomographic examinations.
Data were collected at baseline (before ZAK cell AIT) and every 2
to 3 months. Complete response (CR), partial response (PR), stable
disease (SD), and progressive disease (PD) were determined by the
investigator according to the RECIST v1.1 criteria (11). As tumor markers, the levels of
carcinoembryonic antigen (CEA) and carbohydrate antigen (CA)19-9
were also measured every 2 to 3 months.
QOL analysis
Assessment of QOL was performed by Functional
Assessment of Cancer Therapy-Biologic Response Modifier (FACT-BRM)
analysis (12) before and after 5
administrations of ZAK cells. Documents were collected by research
coordinators and analyzed independently of the physicians.
Statistics
Statistical analysis was conducted using SPSS
software (IBM, Corp.). Survival curves were drawn by Kaplan-Meyer
analysis to estimate the median survival time. Relationships
between survival and hemato-chemical blood examination data were
analyzed in a univariate setting using the log-rank test, where
patients were divided into two groups, a higher group and a lower
group based on the median value of each clinical measurement, then
compared statistically. Multivariate analysis using the Cox
proportional hazard model was also performed. Data sets of tumor
markers and QOL were analyzed using the paired t-test. Values are
presented as means ± standard deviations and P<0.05 was
considered to indicate a statistically significant difference.
Results
Characteristics of patients
Fifty-eight patients with gastric cancer were
treated with ZAK cell AIT but 3 patients in the postoperative
adjuvant setting were excluded from the analysis. The remaining 55
patients included 31 males and 24 females with a median age of 60,
ranging from 32 to 88 years. Metastatic organs included the
peritoneum, liver, lymph nodes, and bone; 48 patients had at least
one metastatic site and 7 had two or more metastatic organs.
Positive, negative, and unknown Her2 status were observed in 4, 11,
and 40 patients, respectively. First-line chemotherapy had already
failed in 51 (93%) patients. Concurrent anti-cancer chemotherapy
was administered in 43 patients (78%), and in most cases this
consisted of S-1, taxan or both, as shown in Table I.
 | Table IPatients enrolled in the ZAK cell AIT
trial. |
Table I
Patients enrolled in the ZAK cell AIT
trial.
| Variable | N (%) |
|---|
| Total no. | 55 |
| Male/female | 31/24 |
| Age (median,
range) | 60, 32-88 |
| Target and metastatic
organs | |
|
Peritoneum | 27(49) |
|
Liver | 16(29) |
|
Lymph
node | 14(25) |
|
Bone | 3(5) |
|
Others | 3(5) |
| Organs affected | |
|
1 | 48(87) |
|
≥2 | 7(13) |
| Her2 status | |
|
Positive | 4(7) |
|
Negative | 11(20) |
|
Uknown | 40(73) |
| Number of previous
regimens | |
|
0 | 4(7) |
|
≥1 | 51(93) |
| Concurrent
treatments | |
|
Chemotherapy | 43(78) |
|
S-1 | 10(18) |
|
S-1+CDDP | 9(16) |
|
PTX | 9(16) |
|
S1+PTX | 4(7) |
|
CPT-11 | 3(5) |
|
DTX | 2(4) |
|
Others | 6(11) |
|
None | 12(22) |
Feasibility of ZAK cell generation and
transfer
The generation of ZAK cells was carried out 412
times in total, and 393 cultures (95.4%) were uneventful (Table II). ZAK cells contained mainly
CD56+ NK cells and γδT cells at median values of 79 and 13%,
ranging from 69% to 87% and 3% to 52%, respectively. ZAK cells were
administered once to 4 times in 24 patients, 5 to 9 times in 16
patients, 10 to 19 times in 4 patients, 20 to 29 times in 3
patients, and 30 times or more in 3 patients; the median value was
4 times, including 5 patients who never received ZAK cell transfer
because of disease progression in 4 cases and no lymphocyte growth
in 1 case (Table II). The mean
number of total cells transferred was 6.5x108 cells
among all the treated patients and 12.3x108 cells among
those treated more than 5 times. No bacteria, endotoxin, or
mycoplasma was detected in any of the cultures.
 | Table IIFeasibility, quality and outcome of
the transfer of ZAK cell adoptive immunotherapy. |
Table II
Feasibility, quality and outcome of
the transfer of ZAK cell adoptive immunotherapy.
| Variable | Value |
|---|
| Total culture
no. | 412 |
| Success of culture, n
(%) | 393 (95.4%) |
| ZAK cell phenotype,
mean, range | |
|
CD3 | 37, 20-68 |
|
γδT | 13, 3-52 |
|
CD56 | 79, 69-87 |
| No. of
administrations | |
|
0 | 5a |
|
1-4 | 24 |
|
5-9 | 16 |
|
10-19 | 4 |
|
20-29 | 3 |
|
≥30 | 3 |
|
Median
(range) | 4 (0-44) |
|
Mean ±
SD | 7.0±9.3 |
| Total cell no.
administered, mean | |
|
All patients
treated |
6.5x108 |
|
Patients
treated ≥5 times |
12.3x108 |
| Contamination
detected | 0 |
| Endotoxin >4.0
pg/ml | 0 |
Survival analysis
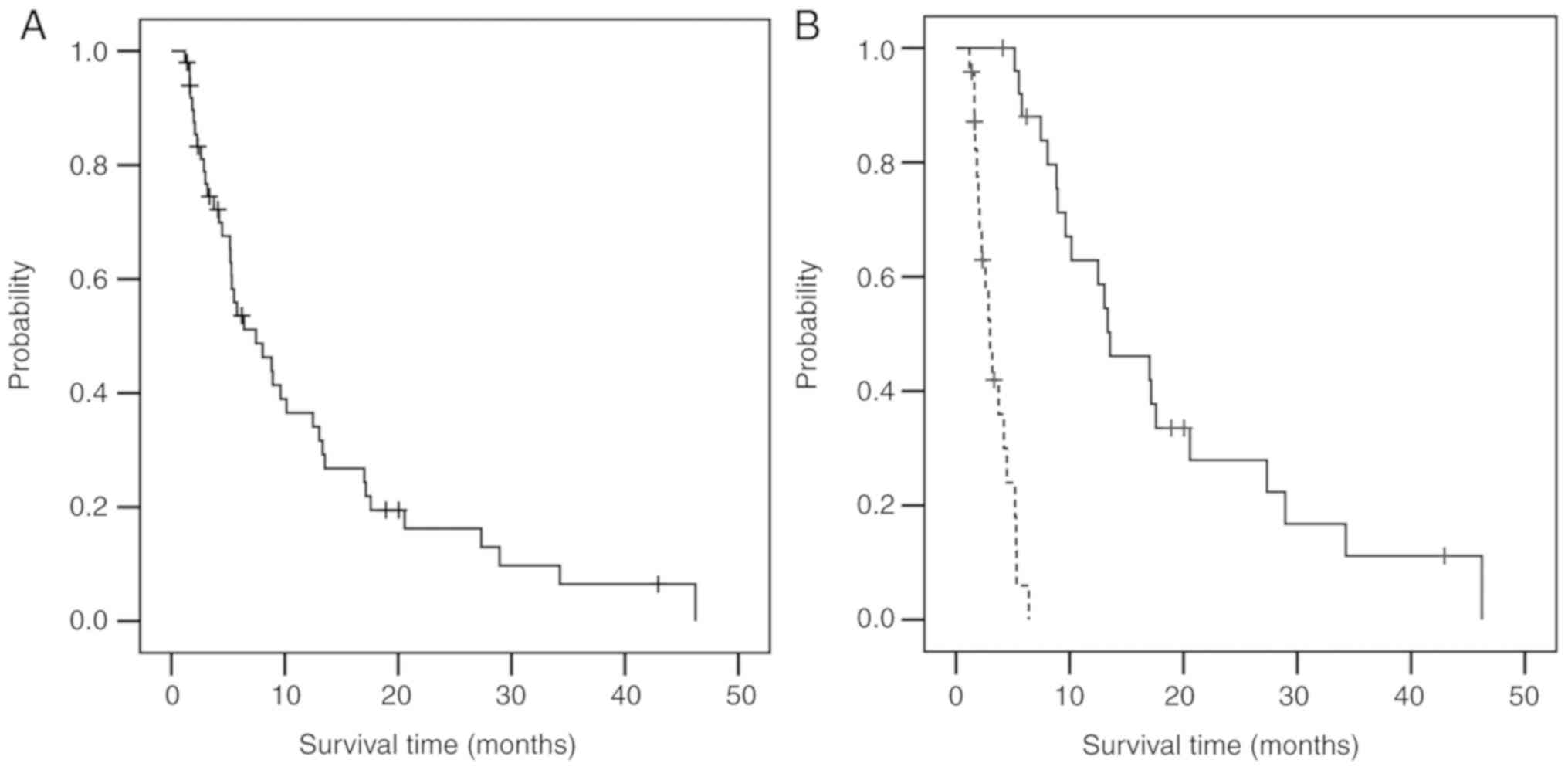
The overall survival (OS) of all the patients
treated is displayed in Fig. 1A and
B, while the survival analysis is
summarized in Table III. Five
patients who received no administration of ZAK cells were excluded
from the analysis. With a median follow-up time of 12.3 months
(range: 1.2-55.1), the median OS was 7.5 months (95% confidence
interval (CI): 3.9-11.0) for all patients treated (Fig. 1A); this value increased to 13.5
months (95% CI: 8.1-18.9) when limited to patients receiving more
than 5 ZAK cell AITs (Fig. 1B,
solid curve), but decreased to 3.0 months (95% CI: 2.2-3.8) in the
group of patients receiving less than 4 administrations (Fig. 1B, dotted curve; Table III). With respect to the
combination chemotherapy, the OS times of 1st line and 2nd line or
later chemotherapy plus 5 or more administrations of ZAK cell AIT
were 27.3 months (95% CI: 7.7-45.0) and 13.3 months (95% CI:
9.4-17.3), respectively, compared to an OS of 8.0 months in
patients who had already finished the 1st line chemotherapy and
received 5 or more administrations of ZAK cell AIT as monotherapy
(Table III).
 | Table IIIOS of ZAK cell adoptive
immunotherapy. |
Table III
OS of ZAK cell adoptive
immunotherapy.
| Therapy | No. of
patients | MST (months) | 95% CI
(months) |
|---|
| All patients | 50 | 7.5 | 3.4-11.5 |
|
≤4
times | 24 | 3.0 | 2.2-3.8 |
|
≥5
times | 26 | 13.5 | 8.1-18.9 |
| Combination | 40 | 9.6 | 2.9-16.3 |
|
≤4
times | 18 | 4.2 | 2.6-5.8 |
|
≥5
times | 22 | 17.0 | 11.3-22.7 |
| 1st line | 11 | 17.2 | 0.5-33.8 |
|
≤4
times | 3 | 4.5 | - |
|
≥5
times | 8 | 27.3 | 7.7-45.0 |
| ≥2nd line | 29 | 7.5 | 2.2-12.7 |
|
≤4
times | 15 | 3.7 | 2.6-4.9 |
|
≥5
times | 14 | 13.3 | 9.4-17.3 |
| ZAK alone | 10 | 2.3 | 1.5-3.1 |
|
≤4
times | 6 | 2.0 | 1.7-2.2 |
|
≥5
times | 4 | 8.0 | 4.4-11.7 |
Tumor response
Tumor response is shown in Table IV. Of all the patients treated, 28
were evaluable for objective tumor responses. No CR or PR was
observed. Nineteen patients (67.9%) showed SD status, and thus the
disease control rate was estimated as 67.9%. Changes in tumor
markers were analyzed in 26 patients who received 5 or more
administrations of ZAK cell AIT. Although the values of CEA and
CA19-9 decreased in 9 and 7 patients, respectively, the mean values
of each marker increased with no significant difference (Table V).
 | Table IVObjective responses of patients
treated with ZAK cell adoptive immunotherapy. |
Table IV
Objective responses of patients
treated with ZAK cell adoptive immunotherapy.
| Response | No. of patients
(%) |
|---|
| CR | 0 (0) |
| PR | 0 (0) |
| SD | 19 (67.9) |
| PD | 9 (32.1) |
| DCR (CR+PR+SD) | 19 (67.9) |
 | Table VChanges of tumor markers in patients
treated with ZAK cell adoptive immunotherapy. |
Table V
Changes of tumor markers in patients
treated with ZAK cell adoptive immunotherapy.
| Tumor marker,
no. | Value (mean ±
SD) | P-value |
|---|
| CEA,
23a | | |
|
Baseline | 18.6±41.9 | 0.1001 |
|
After
ZAK | 50.8±96.5 | |
| CA19-9,
23b | | |
|
Baseline |
1,557.9±4,192.3 | 0.6107 |
|
After
ZAK |
2,560.5±10,051.4 | |
Adverse events
Of 50 patients treated, 1 showed temporary low-grade
fatigue (grade 1) after ZAK cell transfer. No other adverse events
higher than grade 2 related to ZAK cell administration were
experienced in any of the patients treated.
QOL analysis
The results of QOL analysis of 24 assessable data
sets are shown in Table VI. The
score of functional well-being was significantly improved after ZAK
cell transfer (P=0.024). Moreover, the total FACT-General and
FACT-BRM scores showed trends of improvement after ZAK cell AIT,
although these improvements were not statistically significant
(P=0.057 and 0.073, respectively).
 | Table VIQOL analysis. |
Table VI
QOL analysis.
| Subscale | QOL points at
baseline (mean ± SD) | QOL points after
AIT (mean ± SD) | Improvement
(points) | 95% CI of
improvement | P-value |
|---|
| Physical
well-being | 20.6±4.8 | 20.9±4.9 | 0.3 | -2.96-3.62 | 0.828 |
| Social
well-being | 20.0±5.9 | 21.1±5.2 | 1.0 | -2.48-4.56 | 0.528 |
| Emotional
well-being | 13.7±5.8 | 16.5±5.2 | 2.8 | -0.84-6.51 | 0.118 |
| Functional
well-being | 15.3±6.3 | 19.3±6.3 | 4.0 | 0.65-7.35 | 0.024 |
| BRM physical | 19.7±4.0 | 20.8±2.6 | 1.2 | -1.62-3.93 | 0.379 |
| BRM
cognitive/emotional | 14.1±5.7 | 16.6±5.2 | 2.5 | -0.56-5.56 | 0.100 |
| FACT-BRM TOI | 69.6±18.1 | 77.6±16.2 | 8.0 | -2.56-18.53 | 0.124 |
| FACT-general total
score | 69.5±18.6 | 77.7±17.0 | 8.2 | -0.29-16.71 | 0.057 |
| FACT-BRM total
score | 103.3±25.4 | 115.2±23.5 | 11.9 | -1.32-25.04 | 0.073 |
Analysis of patients with survival
benefit
The relationships between the survival and clinical
measurements at baseline were analyzed in an attempt to identify
biomarkers of ZAK cell AIT benefit in patients with incurable
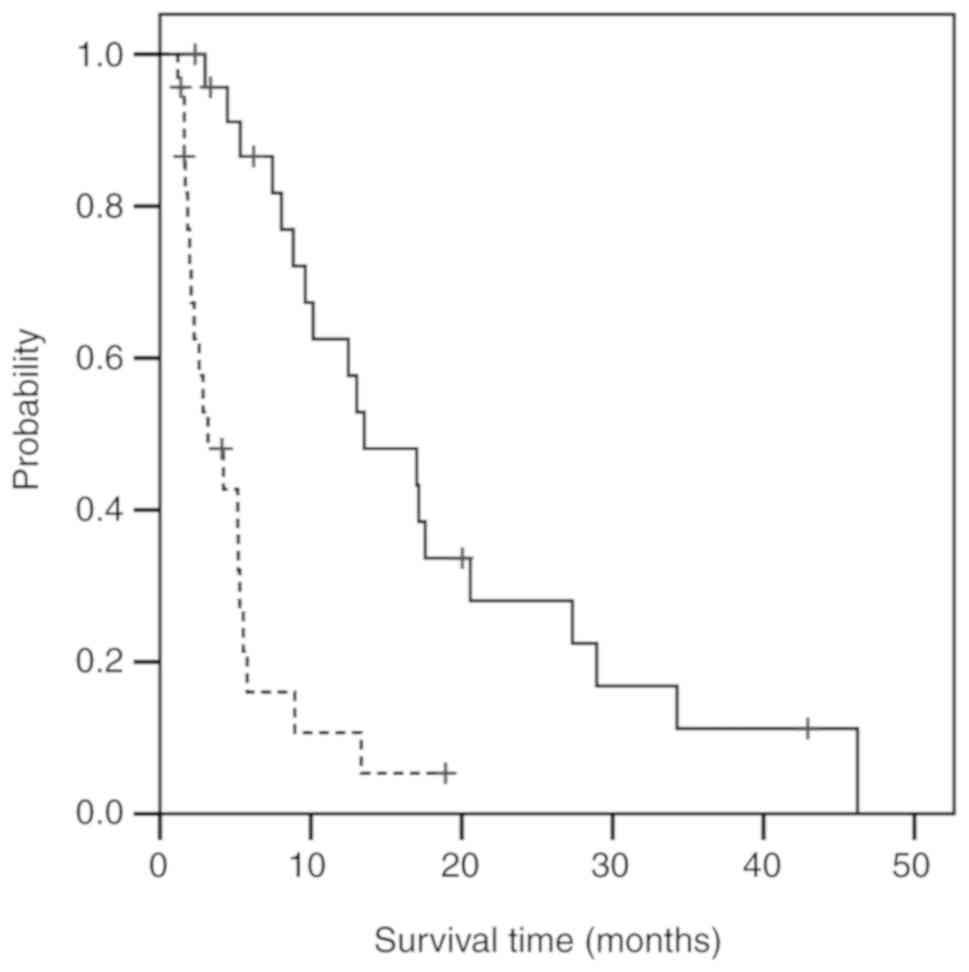
gastric cancer. In the univariate analysis, there was a significant
difference in several clinical measurements: Better survival was
observed in the groups with a lymphocyte percentage ≥28 in the
white blood cell count (P<0.001; Fig. 2, solid curve), serum albumin ≥3.6
(P=0.001), serum C-reactive protein (CRP) <0.17 (P=0.006), serum
carbohydrate antigen (CA)19-9 <40.2 (P=0.010), and
neutrophil-lymphocyte ratio (NLR) <2.3 (P<0.001), and in the
GPS0 (P<0.001) and GPS1 (P=0.042) groups compared with the GPS2
group (Table VII). A
representative comparison of the Kaplan-Meier curves of the
baseline lymphocyte percentage is shown in Fig. 2. In the multivariate analysis, GPS
was found to be associated with survival: Significantly better
survival was observed in the low GPS group with an HR of 3.201 (95%
CI, 1.165-8.791; P=0.024; Table
VIII).
 | Table VIIUnivariate survival analysis using
log-rank test on baseline biochemical measures in ZAK cell adoptive
immunotherapy. |
Table VII
Univariate survival analysis using
log-rank test on baseline biochemical measures in ZAK cell adoptive
immunotherapy.
| Parameter | n | MST | 95% CI | P-value |
|---|
| Lymphocyte (%) |
|
<28 | 23 | 3.2 | 0.9-5.5 | <0.001 |
|
≥28 | 24 | 13.5 | 6.8-20.3 | |
| Lymphocyte count
(/µl) |
|
<1,345 | 23 | 5.2 | 1.8-8.6 | 0.093 |
|
≥1,345 | 23 | 8.9 | 5.8-12.1 | |
| Albumin (g/dl) |
|
<3.6 | 22 | 4.2 | 1.4-7.0 | 0.001 |
|
≥3.6 | 25 | 12.5 | 6.7-18.3 | |
| CRP (mg/dl) |
|
<0.17 | 14 | 17.2 | 10.4-23.9 | 0.006 |
|
≥0.17 | 15 | 5.3 | 3.6-7.0 | |
| CEA (ng/ml) |
|
<6.8 | 22 | 12.5 | 6.8-18.1 | 0.093 |
|
≥6.8 | 23 | 5.2 | 3.3-7.0 | |
| CA19-9 (U/ml) |
|
<40.2 | 22 | 13.3 | 11.8-14.9 | 0.010 |
|
≥40.2 | 23 | 5.2 | 3.6-6.7 | |
| NLR |
|
<2.3 | 23 | 17.0 | 11.1-23.0 | <0.001 |
|
≥2.3 | 23 | 3.2 | 0.9-5.5 | |
| GPS |
|
0 | 17 | 13.0 | 1.0-25.1 | 0.065 for GPS (1),
<0.001 for GPS (2) |
|
1 | 9 | 5.8 | 3.7-7.8 | 0.042 for GPS
(2) |
|
2 | 3 | 2.0 | - | |
 | Table VIIIMultivariable Cox regression analysis
between the survival and biochemical measurement at baseline of ZAK
cell adoptive immunotherapy. |
Table VIII
Multivariable Cox regression analysis
between the survival and biochemical measurement at baseline of ZAK
cell adoptive immunotherapy.
| Parameter | HR | 95% CI | P-value |
|---|
| Lymphocyte (%) | 1.021 | 0.889-1.172 | 0.772 |
| Lymphocyte count
(µl) | 1.000 | 0.998-1.002 | 0.862 |
| Albumin
(mg/dl) | 1.821 | 0.050-66.162 | 0.744 |
| CRP (mg/dl) | 1.387 | 0.666-2.891 | 0.382 |
| CEA (ng/ml) | 1.000 | 1.000-1.001 | 0.198 |
| CA19-9 (lU/ml) | 1.000 | 1.000-1.000 | 0.104 |
| NLR | 0.867 | 0.643-1.169 | 0.350 |
| GPS | 3.201 | 1.165-8.791 | 0.024 |
Discussion
We have been conducting an observational study of
AIT using ZAK cells for the treatment of patients with incurable
cancer since 2009. In this series, we have already reported a
possible survival benefit of ZAK cell AIT in combination with
chemotherapy in patients with pancreatic cancer (13). In this paper, we analyzed patients
with gastric cancer. Our analysis showed that, over approximately
400 ZAK cell generations in gastric cancer patients, 95% of
cultures were uneventful with no contamination, indicating that our
system had good feasibility for the preparation of ZAK cells for
gastric cancer patients, just as it was previously shown to have
good feasibility for the preparation of ZAK cells for pancreatic
cancer patients (13). ZAK cells
from gastric cancer patients also showed a heterogenous phenotype
consisting of NK cells and γδT cells in our ZAK cell generation
system, although γδT cells accounted for a lower percentage of
total ZAK cells in gastric cancer patients compared to pancreatic
cancer patients (13% vs. 45%, respectively) (13), suggesting that the ability to
generate ZAK cells may differ among cancer types.
The survival analysis showed that although the
median OS was 7.5 months in all patients after our ZAK cell AIT,
the OS was prolonged to 13.5 months in patients who received ZAK
cell AIT 5 times or more, while the OS in those who received ZAK
cell AIT fewer than 4 times was very poor. The OS times were
extended further, to 27.3 and 13.3 months, when ZAK cell AIT was
performed 5 times or more in combination with 1st line and 2nd line
or later chemotherapy, respectively. It was reported that the
median OS was 13.0 months in patients assigned to S-1 plus
cisplatin treatment in the SPIRITS study, which is a pivotal phase
III trial of 1st line chemotherapy for advanced or refractory
gastric cancer patients (14). The
START study also reported a similar OS of 12.5 months in gastric
cancer patients treated with docetaxel plus S-1(15). Moreover, the RAINBOW study, which is
a randomized, placebo-controlled, double-blind, phase 3 trial for
advanced gastric cancer in a 2nd line setting, showed an OS of 9.6
months in the ramucirumab plus paclitaxel group, which was
significantly longer than that of 7.4 months in the placebo plus
paclitaxel group (16). Qiao et
al (17), have indicated the
benefit of the combination of dendritic cell-cytokine-induced
killer (DC-CIK) cell immunotherapy over S-1 plus cisplatin
chemotherapy in advanced gastric cancer, and they reported that the
DC-CIK infusions demonstrated a preferable disease control rate
(CR+PR+SD) of 76.9% in the DC-CIK combined with the S-1 plus
cisplatin group compared with that of 47.1% in the S-1 plus
cisplatin group. Therefor, our OS times of 13.5, 27.3 and 13.3
months in patients who received ZAK cell AIT 5 times or more and
our disease control rate of 67.9% would seem to constitute a
favorable result, although ZAK cell AIT alone had only a marginal
effect. ZAK cell AIT was well tolerated with no serious adverse
events, and the FACT-BRM analysis revealed a possible improvement
of QOL after ZAK cell AIT. Taken together, these results suggest
that our ZAK cell AIT in combination with chemotherapy might be a
promising treatment option for patients with incurable gastric
cancer, as well as for patients with pancreatic cancer as shown in
our previous study (13).
What is a mechanism by which ZAK cell AIT extends
the benefits of chemotherapy? Kono et al (18), performed AIT with tumor-associated
lymphocytes in patients with stage IV gastric or colon cancer and
indicated that the expression of TCR zeta chains, which were made
up of T-cell receptor-CD3-associated signal transducing molecules,
was further down-regulated in correspondence with disease
progression in the individual patients, and that AIT could induce
increased or stable TCR zeta expression, indicating the
significance of the addition of AIT in treating gastric cancer.
More recently, an anti-programmed death-1 (-PD-1) antibody,
nivolumab, showed a clear survival benefit for patients with
previously treated gastric cancer (19). This indicates that the host immune
system does respond to cancer cells and that approaches which
involve the host immune system are important in the treatment of
gastric cancer. Interestingly, Iwasaki et al (20) demonstrated the possible involvement
of PD-1-PD-ligand 1 (PD-L1) interaction in the negative regulation
of γδT cells for cytokine production and cytotoxic activity. Zhao
et al (21), developed
chimeric antigen receptor-modified T (CAR-T) cells bi-specific for
tumor antigen Trop2 and PD-L1 and showed that Trop2/PD-L1 CAR-T
cells were able to target Trop2/PD-L1 and checkpoint blockade, and
also had a killing effect on gastric cancer, resulting in an
improvement of the killing effect of CAR-T cells. These findings
suggest the exciting possibility of using ZAK cell AIT combined
with acti-PD-1/PD-L1 antibody for the treatment of gastric
cancer.
In contrast to the possible survival benefits, the
objective tumor response to the treatment with ZAK cell AIT and
chemotherapy was minimal. One possible explanation for this is that
about 80% of the patients in this observational study underwent
treatment in a 2nd line or later chemotherapy setting. Another
possible explanation is that the poor tumor response was due to an
inherent property of immunotherapy. In a vaccine trial of
sipuleucel-T, only 1 of more than 300 patients with prostatic
cancer showed an objective tumor response (22), suggesting that immunotherapy may
provide a survival benefit without inducing an objective tumor
response. More attention should be paid to this property of cancer
immunotherapy.
We also sought adequate biomarkers to identify
gastric cancer patients suitable for ZAK cell transfers. We
initially had an interest in the Her2 status, which may influence
the efficacy of ZAK cell AIT. However, in the end we decided to
remove Her2 status from the analysis, because only limited data
were available and there was no clear difference in survival
between Her2-positive and -negative patients (data not shown).
Univariate analysis showed significantly longer survival in
patients having a baseline lymphocyte percentage in white blood
cells of ≥28%, serum albumin≥3.6, CRP<0.17, CA19-9<40.2, NLR
value<2.2, or a GPS of 0 or 1. However, only the GPS value was
shown to be a significant survival marker in this trial when
analyzed using a multivariate Cox proportional hazards model.
Hirahara et al demonstrated that the NLR-platelet-lymphocyte
ratio might be a promising marker for predicting tumor response and
prognosis in the chemotherapy of patients with advanced gastric
cancer (23). Yuan et al
indicated that CA19-9, palliative gastrectomy, first-line
chemotherapy, and GPS are the prognostic factors that predict OS
when treating patients with advanced gastric cancer (24). Taken together, these results suggest
that the lymphocyte percentage in white blood cells, serum albumin,
CRP, CA19-9, NLR, and GPS levels at baseline may be possible
biomarkers not only for chemotherapy but also for ZAK cell AIT in
patients with incurable gastric cancer. A large-scale prospective
study is necessary to fully investigate this possibility.
This study has some limitations: There may have been
selection biases in the observational study, different numbers of
patients were analyzed in the individual analyses, and the data
sets were incomplete for some of the analyses. Any of these could
have led to a misinterpretation of the study results. We plan to
clear up these limitations by conducting a next phase II trial for
ZAK cell AIT, plans for which are already underway.
In summary, although it is too early for a
definitive conclusion, ZAK cell AIT in combination with
chemotherapy is safe and feasible and might be a promising
treatment option for patients with incurable gastric cancer. The
baseline value of GPS is a candidate biomarker for this
chemo-immunotherapy.
Acknowledgements
The authors would like to thank Mrs. Yukari Minobe,
Miss Naoko Okada, Mrs. Sonoko Sakuma, Mrs. Tomomi Yoshimitsu, Miss
Akiyo Tamura, Miss Yumi Nishiwaki and Mr. Akihiro Nyuuya
(Department of Clinical Oncology, Kawasaki Medical School) for
their invaluable assistance with the lymphocyte culture and
immunological analysis. The authors; would also like to acknowledge
Mrs. Kikue Tokuda (Department of Clinical Oncology, Kawasaki
Medical School) for her excellent management of the clinical
data.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY, YK and MO designed the current study and
performed ZAK cell AIT. FS monitored the quality of ZAK cells and
study progress. HT, MY and TN analyzed clinical data. YY wrote the
manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Research Ethics Committee of Kawasaki Medical School and Hospital
(approval no. 240, UMIN000021797). Written informed consent was
obtained from all participants.
Patient consent for publication
Patient consent for publication was obtained from
all participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yamaguchi Y, Ohshita A, Kawabuchi Y, Ohta
K, Shimizu K, Minami K, Hihara J, Miyahara E and Toge T: Adoptive
immunotherapy of cancer using activated autologous
lymphocytes-current status and new strategies. Hum Cell.
16:183–189. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yamaguchi Y, Ohta K, Kawabuchi Y, Ohshita
A, Okita R, Okawaki M, Hironaka K, Matsuura K and Toge T:
Feasibility study of adoptive immunotherapy for metastatic lung
tumors using peptide-pulsed dendritic cell-activated killer (PDAK)
cells. Anticancer Res. 25:2407–2415. 2005.PubMed/NCBI
|
|
4
|
Takayama T, Sekine T, Makuuchi M, Yamasaki
S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi
Y and Kakizoe T: Adoptive immunotherapy to lower postsurgical
recurrence rates of hepatocellular carcinoma: A randomised trial.
Lancet. 356:802–807. 2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kimura H and Yamaguchi Y: A phase III
randomized study of interleukin-2 lymphokine-activated killer cell
immunotherapy combined with chemotherapy or radiotherapy after
curative or noncurative resection of primary lung carcinoma.
Cancer. 80:42–49. 1997.PubMed/NCBI
|
|
6
|
Nagamine I, Yamaguchi Y, Ohara M, Ikeda T
and Okada M: Induction of gamma delta T cells using zoledronate
plus interleukin-2 in patients with metastatic cancer. Hiroshima J
Med Sci. 58:37–44. 2009.PubMed/NCBI
|
|
7
|
Braza MS and Klein B: Anti-tumour
immunotherapy with Vγ9Vδ2 T lymphocytes: From the bench to the
bedside. Br J Haematol. 160:123–132. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Brandes M, Willimann K and Moser B:
Professional antigen-presentation function by human gammadelta T
cells. Science. 309:264–268. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sakamoto M, Nakajima J, Murakawa T, Fukami
T, Yoshida Y, Murayama T, Takamoto S, Matsushita H and Kakimi K:
Adoptive immunotherapy for advanced non-small cell lung cancer
using zoledronate-expanded γδTcells: A phase I clinical study. J
Immunother. 34:202–211. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Noguchi A, Kaneko T, Kamigaki T, Fujimoto
K, Ozawa M, Saito M, Ariyoshi N and Goto S: Zoledronate-activated
Vγ9γδ T cell-based immunotherapy is feasible and restores the
impairment of γδ T cells in patients with solid tumors.
Cytotherapy. 13:92–97. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bacik J, Mazumdar M, Murphy BA, Fairclough
DL, Eremenco S, Mariani T, Motzer RJ and Cella D: The functional
assessment of cancer therapy-BRM (FACT-BRM): A new tool for the
assessment of quality of life in patients treated with biologic
response modifiers. Qual Life Res. 13:137–154. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yamaguchi Y, Katata Y, Okawaki M, Sawaki A
and Yamamura M: A prospective observational study of adoptive
immunotherapy for cancer using zoledronate-activated killer (ZAK)
cells-an analysis for patients with incurable pancreatic cancer.
Anticancer Res. 36:2307–2313. 2016.PubMed/NCBI
|
|
14
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, et al: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): A phase III
trial. Lancet Oncol. 9:215–221. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Koizumi W, Kim YH, Fujii M, Kim HK,
Imamura H, Lee KH, Hara T, Chung HC, Satoh T, Cho JY, et al:
Addition of docetaxel to S-1 without platinum prolongs survival of
patients with advanced gastric cancer: A randomized study (START).
J Cancer Res Clin Oncol. 140:319–328. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wilke H, Muro K, Van Cutsem E, Oh SC,
Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et
al: Ramucirumab plus paclitaxel versus placebo plus paclitaxel in
patients with previously treated advanced gastric or
gastro-oesophageal junction adenocarcinoma (RAINBOW): A
double-blind, randomised phase 3 trial. Lancet Oncol. 15:1224–1235.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Qiao G, Wang X, Zhou L, Zhou X, Song Y,
Wang S, Zhao L, Morse MA, Hobeika A, Song J, et al: Autologous
dendritic cell-cytokine induced killer cell immunotherapy combined
with S-1 plus cisplatin in patients with advanced gastric cancer: A
prospective study. Clin Cancer Res. 25:1494–1504. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kono K, Ichihara F, Iizuka H, Sekikawa T
and Matsumoto Y: Expression of signal transducing T-cell receptor
zeta molecules after adoptive immunotherapy in patients with
gastric and colon cancer. Int J Cancer. 78:301–305. 1998.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y,
Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al: Nivolumab in
patients with advanced gastric or gastro-oesophageal junction
cancer refractory to, or intolerant of, at least two previous
chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomized,
double-blind, placebo-controlled, phase 3 trial. Lancet.
390:2461–2471. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Iwasaki M, Tanaka Y, Kobayashi H,
Murata-Hirai K, Miyabe H, Sugie T, Toi M and Minato N: Expression
and function of PD-1 in human γδ T cells that recognize
phosphoantigens. Eur J Immunol. 41:345–355. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhao W, Jia L, Zhang M, Huang X, Qian P,
Tang Q, Zhu J and Feng Z: The killing effect of novel bi-specific
Trop2/PD-L1 CAR-T cell targeted gastric cancer. Am J Cancer Res.
9:1846–1856. 2019.PubMed/NCBI
|
|
22
|
Kantoff PW, Higano CS, Shore ND, Berger
ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims
RB, et al: Sipuleucel-T immunotherapy for castration-resistant
prostate cancer. N Engl J Med. 363:411–422. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hirahara T, Arigami T, Yanagita S,
Matsushita D, Uchikado Y, Kita Y, Mori S, Sasaki K, Omoto I,
Kurahara H, et al: Combined neutrophil-lymphocyte ratio and
platelet-lymphocyte ratio predicts chemotherapy response and
prognosis in patients with advanced gastric cancer. BMC Cancer.
19(672)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yuan SQ, Nie RC, Chen YM, Qiu HB, Li XP,
Chen XJ, Xu LP, Yang LF, Sun XW, Li YF, et al: Glasgow prognostic
score is superior to ECOG PS as a prognostic factor in patients
with gastric cancer with peritoneal seeding. Oncol Lett.
15:4193–4200. 2018.PubMed/NCBI View Article : Google Scholar
|