Introduction
Colorectal cancer (CRC) is one of the most common
malignant cancers worldwide (1).
Currently, patients with CRC are diagnosed based on various
predictors, including performance status, clinicopathological
factors and TNM classification (2).
Moreover, systemic immune response may be a useful determinant of
the tumor stage (3). Certain
studies have confirmed that immune system factors are actively
involved in the development and invasion of CRC cells (4). These factors include the levels of
serum white blood cells, the number of neutrophils, lymphocytes and
platelets, and the expression levels of acute-phase proteins
(5). It has also been demonstrated
that high serum levels of acute-phase proteins, such as C-reactive
protein (CRP), are significantly correlated with poor survival of
patients with CRC (6). Rasic et
al (7) demonstrated that the
serum level of CRP was an independent predictor of the CRC stage.
Yamamoto et al (8) indicated
that the combination of pre- and postoperative CRP levels was
predictive of the prognosis of CRC patients who underwent surgery.
Recent studies suggested that the combination of the acute-phase
factors and systemic whole-blood parameters, such as
neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio
(PLR) and prognostic nutritional index may have prognostic
significance in the progression of CRC (9,10).
Zhou et al (11) confirmed
that patients with a higher postoperative NLR, neutrophil count and
monocyte-to-lymphocyte ratio, PLR and systemic immune inflammation
index, exhibited shorter progression-free survival. Moreover, it
has been shown that a high NLR is a prognostic factor for poor
survival in mismatch repair-proficient CRC subjects (12). In light of this evidence, neutrophil
count, lymphocyte count and NLR were assessed in blood samples
collected pre- and postoperatively from patients with CRC. These
indices were analyzed in association with disease-free survival
(DFS) and with specific clinicopathological variables.
Materials and methods
Patients
The medical records of 144 patients diagnosed with
CRC (56 women and 88 men; mean age, 63 years; range, 32-86 years)
were analyzed. The patients underwent surgery at the Department of
Oncological Surgery, in the Comprehensive Cancer Center of
Bialystok, between April 2014 and December 2016. All the patients
were subjected to routine diagnostic laboratory examinations, such
as electrocardiography, spirometry and arterial blood gas
measurements, as well as X-ray imaging and computerized tomography
of the chest. The clinical efficiency was assessed with the 5-point
scale of Zubroda (World Health Organization) (13). The clinical staging of CRC was
performed according to the TNM classification (2). The type of pre/postoperative therapy
was selected on the basis of the current recommendations for CRC
treatment. Patients diagnosed with neoplasms in the rectum (n=53)
received preoperative therapy; specifically, they received
radiotherapy (n=39), chemotherapy (n=7) and radiochemotherapy
(n=7). A radiation dose of 25 Gy to the pelvic area was
administered to the patients in fractions of 5 Gy over 1 week.
Patients with tumors localized in other areas received neither
inflammatory nor immunosuppressive therapy. The response to
preoperative therapy was estimated according to the Response
Evaluation Criteria in Solid Tumors v1.1(14). A total of 26 patients were diagnosed
with stable disease and 27 with partial response to treatment.
Histopathological examination of 4-µm sections stained with
hematoxylin and eosin (cat. no. 468802128; POCH S.A.) was
performed. The routine histopathological assessment of the sections
took into consideration the type of tumor growth, tumor size,
histological type and percentage of the mucinous component, grade
of malignancy and pTNM stage. In addition, the presence of venous,
lymphatic and perineural invasion was assessed, and specific
features of lymph node invasion were characterized, such as the
number of resected and invaded lymph nodes, the presence of micro-
and macrometastases, invasion of the lymph node pouch (passage of
cancer cells through the lymph node capsule and subsequent
infiltration of the local fat tissue) and the presence of distant
metastases. The methodology used was similar to that of our
previous study (15).
The present study was performed according to the
principles outlined in the Declaration of Helsinki for human
experimentation and the protocol was approved by the Bioethics
Committee of the Medical University of Bialystok (approval no.
R-I-002/353/2016). Written informed consent was obtained from all
participants.
Blood samples
Blood samples were obtained within 3 days prior to
and following surgical treatment. Venous blood samples were also
obtained from 42 healthy control subjects (21 women and 21 men;
mean age, 45 years; range, 25-65 years). The differential white
blood cell count was analyzed using the Sysmex XN-1000 apparatus
(Sysmex Corporation) based on the manufacturer's protocol.
The absolute neutrophil and lymphocyte counts were
measured prior to and following surgery. The NLR was defined as the
absolute neutrophil count divided by the absolute lymphocyte count.
Receiver operating characteristic (ROC) curve analysis was used to
investigate the cut-off values of the pre- and postoperative
neutrophil count (preNEU/postNEU), lymphocyte count
(preLYMPH/postLYMPH) and NLR (preNLR/postNLR). The significance of
the correlations was evaluated by constructing ROC curves. The
scores of neutrophil count/lymphocyte count/NLR were defined as 1
or 2 when patients exhibited low or high levels of the analyzed
parameters, respectively, as determined in the blood samples
collected pre- and postoperatively.
Follow-up data
The patients were followed up during the last 2-3
years. They were monitored by physical examination, colonoscopy and
the measurement of the carcinoembryonic antigen (CEA) and
carbohydrate antigen (CA) 19-9 levels. In addition, radiological
imaging was performed, which included computerized tomography of
the chest, abdomen and pelvis, bone scan and positron emission
tomography scans. Local and distant recurrence was defined as
pathological evidence of tumor spread in the region of the
anastomosis (local recurrence) and/or the presence of cancer cells
outside of the primary tumor at other sites, including the lungs,
bones and brain (distant recurrence). These indices were confirmed
by the aforementioned techniques.
Statistical analysis
Statistical analysis was conducted using the
STATISTICA 12.0 program (Statsoft, Inc.). The Mann-Whitney U test
was used to compare the differences between the groups.
Correlations between the parameters were calculated by the
Spearman's correlation coefficient tests. DFS was calculated from
the date of diagnosis to the date of disease progression (local or
distant relapse). DFS was estimated using Kaplan-Meier analysis and
the survival curves were compared using log-rank tests. P<0.05
was considered to indicate a statistically significant
difference.
Results
Estimation of cut-off values of
neutrophil count, lymphocyte count and NLR
The preNEU and postNEU were significantly higher in
cancer patients compared with those in healthy subjects (P≤0.001).
Similarly, the preLYMPH/postLYMPH and preNLR/postNLR in the blood
samples were significantly higher in cancer patients compared with
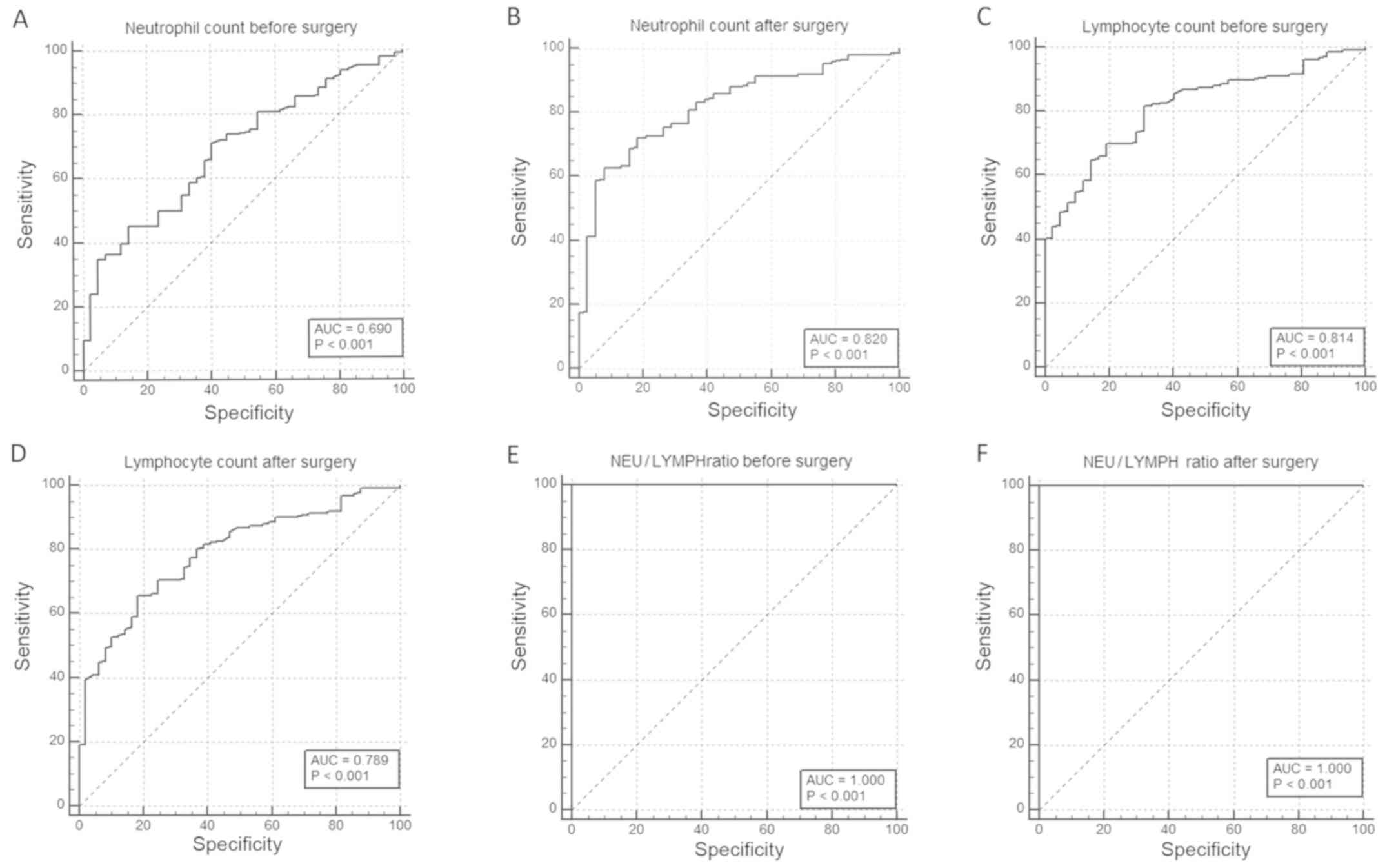
those in healthy volunteers (both P≤0.001; Table I). The cut-off values of the preNEU
and postNEU were 4.9 and 5.4, respectively (sensitivity and
specificity: preNEU, 70.5 and 59.5%; and postNEU, 62.67 and 92.11%,
respectively). The low preNEU group included 115 subjects, whereas
the high group included 29 subjects. The low postNEU group included
91 patients and the high postNEU group included 53 patients. The
cut-off values of the preLYMPH and postLYMPH were 1.9 and 1.6,
respectively, with a sensitivity and specificity of 81.76 and
69.05% (preLYMPH) and of 65.79 and 81.63% (postLYMPH),
respectively. The low preLYMPH group included 108 cases, whereas
the high group included 36 cases. A total of 70 cases with low
postLYMPH and 74 with high postLYMPH were observed. Moreover, the
cut-off values of preNLR and postNLR were 2.5 and 3.3,
respectively, with a sensitivity and specificity of 100% (Fig. 1A-F). The preNLR value was low in 99
cases and high in 45 cases. The low group of postNLR included 65
cases, whereas 79 cases were included in the high postNLR
group.
 | Table IROC curve of pre- and postoperative
neutrophil count, lymphocyte count and NLR. |
Table I
ROC curve of pre- and postoperative
neutrophil count, lymphocyte count and NLR.
| | PreNEU | PostNEU | PreLYMPH | PostLYMPH | PreNLR | PostNLR |
|---|
| Cut-off value | 4.9 | 5.4 | 1.9 | 1.6 | 2.5 | 3.3 |
| AUC | 0.690 | 0.820 | 0.814 | 0.789 | 1.000 | 1.000 |
| Sensitivity (%) | 70.5 | 62.67 | 81.76 | 65.79 | 100 | 100 |
| Specificity (%) | 59.5 | 92.11 | 69.05 | 81.63 | 100 | 100 |
| Disease prevalence
(%) | 77.7 | 79.8 | 79.1 | 75.6 | 78.9 | 77.8 |
| P-value | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 | ≤0.001 |
Correlation between neutrophil count,
lymphocyte count, NLR and clinicopathological variables
PreNEU was correlated with tumor size, necrosis and
tumor budding (R=0.204, P=0.014; R=0.189, P=0.023; and R=-0.174,
P=0.036, respectively). Moreover, the postNEU value was
significantly associated only with histological type (R=0.174,
P=0.047). PreLYMPH was correlated with distant metastasis
(R=-0.153, P=0.046). PreNLR was correlated with age and lymphatic
invasion (R=-0.225, P=0.007; R=0.181, P=0.030; Table II). Moreover, postNLR was
associated with specific variables of tumor progression, such as
tumor growth, lymph node metastasis, number of invaded lymph nodes
and invasion of the node pouch (R=0.212, P=0.016; R=0.200, P=0.023;
R=0.175, P=0.047; and R=0.232, P=0.008, respectively; Table III). The results indicated no
correlation between the analyzed parameters and preoperative
treatment.
 | Table IICorrelation between neutrophil and
lymphocyte count and clinicopathological characteristics. |
Table II
Correlation between neutrophil and
lymphocyte count and clinicopathological characteristics.
| | Neutrophil
count | | Lymphocyte
count | |
|---|
| | Preoperative | | Postoperative | | Preoperative | | Postoperative | |
|---|
| Parameters | L | H | P-value | L | H | P-value | L | H | P-value | L | H | P-value |
|---|
| Age, years |
|
<60 | 24 | 14 | -0.127 | 16 | 18 | -0.163 | 27 | 11 | -0.043 | 13 | 22 | -0.095 |
|
>60 | 79 | 27 | 0.126 | 59 | 37 | 0.062 | 79 | 26 | 0.557 | 47 | 48 | 0.279 |
| Sex |
|
Female | 44 | 10 | 0.141 | 31 | 21 | 0.032 | 45 | 11 | 0.129 | 21 | 31 | -0.073 |
|
Male | 59 | 31 | 0.092 | 44 | 34 | 0.711 | 61 | 26 | 0.123 | 39 | 39 | 0.403 |
| Localization |
|
Right
colon | 12 | 5 | -0.047 | 10 | 7 | -0.123 | 13 | 3 | -0.053 | 7 | 10 | 0.106 |
|
Transverse
colon | 3 | 2 | 0.587 | 3 | 1 | 0.175 | 4 | 3 | 0.539 | 2 | 2 | 0.598 |
|
Left
colon | 12 | 2 | | 10 | 3 | | 12 | 0 | | 8 | 5 | |
|
Sigmoid
colon | 15 | 6 | | 12 | 8 | | 16 | 6 | | 9 | 11 | |
|
Rectum | 55 | 19 | | 36 | 31 | | 54 | 22 | | 30 | 37 | |
| Tumor growth |
|
Expanding | 82 | 33 | -0.087 | 61 | 46 | -0.030 | 87 | 31 | 0.037 | 52 | 56 | 0.131 |
|
Infiltrative | 21 | 5 | 0.297 | 14 | 9 | 0.731 | 19 | 6 | 0.654 | 8 | 14 | 0.134 |
| Tumor size, cm |
|
<2.5 | 20 | 4 | 0.204 | 13 | 8 | 0.011 | 17 | 7 | -0.026 | 12 | 9 | 0.094 |
|
2.5-5.0 | 71 | 23 | 0.014 | 50 | 39 | 0.894 | 71 | 24 | 0.756 | 40 | 49 | 0.283 |
|
>5.0 | 12 | 11 | | 12 | 8 | | 18 | 6 | | 8 | 12 | |
| TNM stage |
|
1 | 15 | 9 | -0.086 | 21 | 12 | -0.037 | 29 | 9 | -0.084 | 13 | 19 | -0.091 |
|
2 | 55 | 12 | 0.303 | 13 | 14 | 0.629 | 20 | 8 | 0.313 | 14 | 14 | 0.299 |
|
3 | 20 | 4 | | 29 | 24 | | 31 | 19 | | 22 | 30 | |
|
4 | 7 | 13 | | 12 | 5 | | 26 | 1 | | 11 | 6 | |
| Adenocarcinoma
type |
|
Mucinous
component | 22 | 32 | -0.057 | 10 | 15 | 0.174 | 24 | 5 | -0.107 | 9 | 16 | 0.085 |
|
Non-mucinous | 81 | 6 | 0.496 | 65 | 40 | 0.047 | 82 | 32 | 0.200 | 51 | 54 | 0.331 |
| Grade of
malignancy |
|
2 | 97 | 36 | 0.021 | 70 | 52 | -0.030 | 100 | 35 | 0.021 | 55 | 67 | -0.125 |
|
3 | 6 | 2 | 0.798 | 5 | 3 | 0.726 | 6 | 2 | 0.796 | 5 | 3 | 0.153 |
| pT stage |
|
1 | 2 | 1 | 0.052 | 1 | 2 | -0.042 | 2 | 1 | -0.034 | 2 | 1 | -0.049 |
|
2 | 42 | 13 | 0.529 | 29 | 20 | 0.629 | 41 | 13 | 0.683 | 21 | 27 | 0.575 |
|
3 | 57 | 23 | | 43 | 33 | | 60 | 23 | | 35 | 42 | |
|
4 | 2 | 1 | | 2 | 0 | | 3 | 0 | | 2 | 0 | |
| Lymphatic
invasion |
|
Absent | 78 | 25 | 0.092 | 55 | 41 | -0.014 | 80 | 25 | 0.058 | 45 | 51 | 0.010 |
|
Present | 25 | 13 | 0.272 | 20 | 14 | 0.871 | 26 | 12 | 0.486 | 15 | 19 | 0.904 |
| Lymph node
metastasis |
|
Absent | 58 | 25 | -0.022 | 41 | 35 | 0.025 | 63 | 20 | 0.184 | 34 | 42 | 0.052 |
|
Present | 45 | 13 | 0.541 | 34 | 20 | 0.775 | 43 | 17 | 0.827 | 26 | 28 | 0.554 |
| Number of
metastatic lymph nodes |
|
<5 | 30 | 9 | -0.052 | 24 | 15 | -0.041 | 31 | 10 | 0.031 | 19 | 20 | 0.014 |
|
>5 | 15 | 4 | 0.536 | 10 | 5 | 0.642 | 12 | 7 | 0.708 | 7 | 8 | 0.870 |
| Lymph node pouch
invasion |
|
Absent | | | -0.048 | 48 | 38 | -0.053 | 71 | 23 | 0.023 | 40 | 46 | -0.005 |
|
Present | | | 0.568 | 23 | 17 | 0.541 | 35 | 14 | 0.782 | 20 | 24 | 0.948 |
| Distant
metastasis |
|
Absent | 91 | 35 | -0.075 | 65 | 51 | -0.066 | 92 | 36 | -0.153 | 52 | 64 | -0.085 |
|
Present | 12 | 3 | 0.369 | 10 | 4 | 0.450 | 14 | 1 | 0.046 | 5 | 6 | 0.334 |
| Tumor budding |
|
Absent | 54 | 27 | -0.174 | 46 | 30 | 0.069 | 62 | 21 | -0.007 | 35 | 40 | -0.006 |
|
Present | 49 | 11 | 0.036 | 29 | 25 | 0.433 | 44 | 16 | 0.927 | 25 | 30 | 0.938 |
| Necrosis |
|
Absent | 32 | 7 | 0.189 | 19 | 13 | -0.105 | 27 | 12 | -0.000 | 15 | 16 | 0.089 |
|
Focal | 39 | 14 | 0.023 | 25 | 28 | 0.229 | 42 | 13 | 0.994 | 27 | 26 | 0.309 |
|
Moderate | 25 | 9 | | 21 | 10 | | 27 | 7 | | 15 | 11 | |
|
Extensive | 7 | 8 | | 10 | 4 | | 10 | 5 | | 3 | 17 | |
 | Table IIICorrelation between NLR and
clinicopathological characteristics. |
Table III
Correlation between NLR and
clinicopathological characteristics.
| | NLR |
|---|
| | Preoperative | | Postoperative | |
|---|
| Parameters | L | H | R P-value | L | H | R P-value |
|---|
| Age, years |
|
<60 | 23 | 15 | -0.225 | 12 | 23 | -0.114 |
|
>60 | 75 | 31 | 0.007 | 43 | 51 | 0.196 |
| Sex |
|
Female | 45 | 11 | 0.104 | 19 | 31 | -0.046 |
|
Male | 53 | 46 | 0.214 | 36 | 43 | 0.602 |
| Localization |
|
Right
colon | 13 | 4 | -0.132 | 9 | 8 | -0.009 |
|
Transverse
colon | 4 | 2 | 0.128 | 2 | 2 | 0.922 |
|
Left
colon | 13 | 0 | | 6 | 8 | |
|
Sigmoid
colon | 16 | 6 | | 5 | 14 | |
|
Rectum | 49 | 27 | | 28 | 39 | |
| Tumor growth |
|
Expanding | 80 | 38 | -0.030 | 49 | 59 | 0.212 |
|
Infiltrative | 18 | 8 | 0.720 | 6 | 15 | 0.016 |
| Tumor size, cm |
|
<2.5 | 16 | 8 | 0.004 | 9 | 11 | 0.111 |
|
2.5-5.0 | 65 | 31 | 0.961 | 36 | 50 | 0.207 |
|
>5.0 | 17 | 7 | | 10 | 13 | |
| TNM stage |
|
1 | 28 | 11 | -0.065 | 18 | 18 | 0.124 |
|
2 | 16 | 12 | 0.438 | 11 | 15 | 0.161 |
|
3 | 39 | 21 | | 19 | 31 | |
|
4 | 15 | 2 | | 7 | 10 | |
| Adenocarcinoma
type |
|
Mucinous
component | 18 | 11 | -0.018 | 10 | 58 | -0.006 |
|
Non-mucinous | 80 | 35 | 0.831 | 45 | 16 | 0.945 |
| Grade of
malignancy |
|
2 | 93 | 43 | 0.134 | 51 | 72 | -0.015 |
|
3 | 5 | 3 | 0.108 | 4 | 2 | 0.866 |
| pT stage |
|
1 | 1 | 2 | -0.052 | 2 | 1 | 0.037 |
|
2 | 40 | 15 | 0.531 | 22 | 28 | 0.676 |
|
3 | 55 | 28 | | 29 | 44 | |
|
4 | 2 | 1 | | 2 | 1 | |
| Lymphatic
invasion |
|
Absent | 76 | 30 | 0.181 | 41 | 57 | 0.023 |
|
Present | 22 | 16 | 0.030 | 14 | 17 | 0.793 |
| Lymph node
metastasis |
|
Absent | 56 | 28 | -0.030 | 36 | 42 | 0.200 |
|
Present | 42 | 18 | 0.715 | 19 | 32 | 0.023 |
| Number of
metastatic lymph nodes |
|
<5 | 30 | 11 | -0.014 | 12 | 23 | 0.175 |
|
>5 | 12 | 7 | 0.861 | 7 | 9 | 0.047 |
| Lymph node pouch
invasion |
|
Absent | 64 | 31 | 0.002 | 41 | 47 | 0.232 |
|
Present | 34 | 15 | 0.981 | 14 | 27 | 0.008 |
| Distant
metastasis |
|
Absent | 85 | 44 | -0.104 | 49 | 66 | -0.018 |
|
Present | 13 | 2 | 0.212 | 6 | 9 | 0.832 |
| Tumor budding |
|
Absent | 57 | 27 | -0.060 | 39 | 41 | 0.152 |
|
Present | 41 | 19 | 0.474 | 16 | 33 | 0.084 |
| Necrosis |
|
Absent | 24 | 15 | -0.004 | 19 | 15 | -0.033 |
|
Focal | 39 | 16 | 0.962 | 15 | 34 | 0.703 |
|
Moderate | 27 | 8 | | 14 | 19 | |
|
Extensive | 8 | 7 | | 7 | 6 | |
Prognostic values of neutrophil count,
lymphocyte count and NLR
The mean DFS of preNEU was 11.67 months in the low
group and 9.97 months in the high group. Moreover, the mean DFS of
postNEU was 9.57 in the low group and 11.79 in the high group. The
postNEU in the low group was associated with a shorter DFS
(P=0.055). The mean DFS of the preLYMPH group was similar in both
groups and was estimated to be 11 months. The mean DFS of postLYMPH
was 11.06 in the low group and 10.42 in the high group. The mean
DFS of NLR was similar in both groups and was estimated to be ~11
months for preNLR and ~10 months for postNLR. DFS did not differ
significantly among the preNEU, preLYMPH, postLYMPH, preNLR and
postNLR groups (P=0.224, P=0.273, P=0.470, P=0.297 and P=0.554,
respectively; Fig. 2A-F).
Discussion
The identification of chemotherapeutic targets for
the treatment of advanced-stage CRC patients may increase the
survival time of these subjects. However, the examination of the
molecular predictive factors is costly and requires sophisticated
laboratory equipment. There is a continuous search for low-cost,
easy-to-obtain specific parameters that can detect early recurrence
of cancer and monitor treatment efficacy (1). In the present study, the diagnostic
and predictive value of specific parameters, such as neutrophil
count, lymphocyte count and the combination of those parameters
(NLR), pre- and postoperatively were determined from whole blood
samples of patients with CRC. Neutrophils have been reported to
have multiple functions in different types of tumors (16). Neutrophils are functionally
classified into two subtypes: Tumor-suppressing (N1) and
tumor-promoting (N2) neutrophils. They are located at the margin of
the tumor site and are present at the early stages of cancer
progression. They can also infiltrate into the center of the tumor
in advanced lesions. A specific classification of circulating
neutrophils has been characterized in the whole blood of patients
with cancer; these can be divided based on their density into
high-density neutrophils (HDNs) and low-density neutrophils (LDNs)
(17). HDNs are functionally
similar to N1 neutrophils, while LDNs resemble N2 neutrophils
(18). Type N1 neutrophils have
potent antitumor activity and release immunostimulatory cytokines,
such as interleukin-12 and tumor necrosis factor-α (19). In contrast to these observations, N2
phenotype cells induce strong immunosuppressive and tumor-promoting
activity, which produces pro-angiogenic chemokines and cytokines
that are involved in tumor cell proliferation, invasion and
vascularization (20). In the
present study, the cut-off values of preNEU and postNEU were
examined in whole blood samples of patients with CRC. The cut-off
values exhibited moderate sensitivity and specificity, with ~77-79%
of disease prevalence. Moreover, preNEU was correlated with tumor
size and the presence of necrosis. In the first step, neutrophils
are able to produce reactive oxygen species and reactive nitrogen
species that cause DNA damage and genetic instability in cancer
cells (21). Furthermore,
neutrophils can secrete various mediators, such as hepatocyte
growth factor, that lead to tumor growth and progression (22). Neutrophils are also involved in
remodeling of the tumor extracellular matrix by the release of
matrix metalloproteinase-9(23).
The aforementioned properties of neutrophils were compared with
tumor size and necrosis in previous studies and the data reported
were consistent with our observations. However, the high neutrophil
count noted in preoperative blood samples of patients with CRC
exhibited a negative correlation with tumor budding. Tumor budding
is defined as small clusters of cancer cells that are localized in
the invasive margin of the primary tumor mass and they have
important prognostic value. Ueno et al (24) demonstrated that tumor budding was
observed in the margin of tumors with unfavorable fibrotic stroma.
This cellular morphology was characterized by the presence of
keloid-like collagen with random orientation of the fibrils and
pervasive distribution of myofibroblasts. These histological
characteristics of the tumor microenvironment likely determine the
inhibition of the local and systemic immune response observed in
patients with CRC. Moreover, high levels of neutrophil counts in
whole blood samples were observed postoperatively in patients with
non-mucinous CRC. The histological type of CRC was characterized by
glandular cancer clusters in well-formed rich stroma, as opposed to
the mucinous cancer type that exhibits spontaneous formation of
cancer cells in mucin. The presence of the rich stroma may affect
the immune response development and its effectiveness.
Lymphocytes are also involved in the organization of
the immune response in malignant neoplasms (25). Our previous study indicated that
patients with CRC who did not have intraepihethial
tumor-infiltrating lymphocytes (TILs) in the center of the primary
tumor mass exhibited shorter DFS (26). Moreover, the infiltration and
distribution of TILs in tumor tissues of patients with CRC were
associated with the invasion and progression of the disease. In the
present study, the correlation between preLYMPH and distant
metastasis was confirmed. Therefore, the NLR may be a factor
reflecting the balance between the tumor-promoting property of
neutrophils and the host antitumor immune response of lymphocytes.
A total of 144 samples of whole blood were obtained prior to and
following surgery. A ROC curve was used to estimate the cut-off
values of NLR. The present analysis indicated that the cut-off of
preNLR was 2.5 and that of postNLR was 3.3. These results are
consistent with the observations of other studies that reported
variations in these parameters between 2.5 and 5.0 (27-29).
The present study demonstrated high sensitivity and specificity
with positive disease prevalence for ~78% of the participants. Zhou
et al (30) reported that
the sensitivity and specificity of NLR for CRC were 66.9 and 77.6%,
respectively; moreover, the NLR values were significantly higher
compared with those in healthy volunteers. Pedrazzani et al
(31) observed that the
distribution of NLR was different between CRC patients and control
subjects. Moreover, the NLR value was correlated with age, TNM
stage, systemic metastasis and serum CEA levels. In the present
study, preNLR and postNLR were correlated with age and various
morphological characteristics of disease progression, such as
lymphatic invasion, tumor growth, lymph node metastasis, number of
invaded lymph nodes and invasion of the lymph node pouch.
Furthermore, Chen et al (32) highlighted that high NLR was more
prevalent in the elderly (>60 years) population and that it was
associated with larger tumor size, advanced pT stage and positive N
and M. Özgehan et al (33)
demonstrated that NLR was higher in CRC patients with T3/4-N1/2-M1
stage compared with the corresponding value noted in patients with
T1/1-N0-M0.
The present study analyzed the DFS of CRC patients
in association with neutrophil count, lymphocyte count and NLR. The
patients with low postNEU exhibited a higher tendency for shorter
DFS. These results did not confirm the association of DFS with
preLYMPH and postLYMPH in CRC patients. However, Iseki et al
(34) confirmed that patients with
higher lymphocyte count demonstrated a tendency for higher 5-year
relapse-free survival rate. Moreover, patients with a high preLYMPH
exhibited significantly longer overall survival. An association
between DFS and NLR was not observed in blood samples between pre-
and postoperative CRC patients. Jankova et al (35) demonstrated that preNLR could predict
overall survival, although it was not specific to recurrence and
cancer-specific survival. In contrast to these observations, Ying
et al (36) indicated that
elevated NLR levels were of prognostic value for recurrence-free,
overall and cancer-specific survival in CRC patients who had
undergone surgery. Balde et al (37) reported that the high preNLR group
exhibited a higher recurrence rate compared with that of the low
preNLR group. Li et al (38)
demonstrated that a high preoperative NLR may be considered as a
negative independent prognostic factor in non-metastatic rectal
cancer. In addition, a previous study confirmed that a high preNLR
was independently associated with poor prognosis of patients with
CRC (39).
In conclusion, the findings of the present study
indicated a significant correlation between specific blood indices
in patients with CRC and disease progression markers, with only
postNEU exhibiting a tendency of association disease prognosis.
However, this preliminary evidence requires confirmation in studies
with larger sample sizes.
Acknowledgements
Not applicable.
Funding
The author(s) received funding support from Medical
University of Bialystok for the present study (grant no.
N/ST/ZB/18/002/1194).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KJ collected data, performed analysis, wrote the
manuscript, reviewed the literature, acquired the data and
contributed to manuscript drafting; MK analysed and interpreted the
pathological examination; WF, WK and LKK collected data; MG wrote
the manuscript, reviewed the literature, acquired data and
contributed to manuscript drafting. All authors reviewed and
approved the final manuscript.
Ethics approval and consent to
participate
The present study conformed to the principles
outlined in the Declaration of Helsinki for human experimentation
and the protocol was approved by the Bioethics Committee of the
Medical University of Bialystok (approval no. R-I-002/353/2016).
Written informed consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maeda K, Shibutani M, Otani H, Nagahara H,
Ikeya T, Iseki Y, Tanaka H, Muguruma K and Hirakawa K:
Inflammation-based factors and prognosis in patients with
colorectal cancer. World J Gastrointest Oncol. 7:111–117.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hamilton S and Aaltonen L: Tumors of the
colon and rectum. In: World Health Organization Classification of
Tumors. Pathol Genet Tumors Dig Syst IARC Press Lyon pp103-104,
2000.
|
|
3
|
Laird BJ, Kaasa S, McMillan DC, Fallon MT,
Hjermstad MJ, Fayers P and Klepstad P: Prognostic factors in
patients with advanced cancer: A comparison of clinicopathological
factors and the development of an inflammation-based prognostic
system. Clin Cancer Res. 19:5456–5464. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lu H, Ouyang W and Huang C: Inflammation,
a key event in cancer development. Mol Cancer Res. 4:221–233.
2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
McMillan DC: The systemic
inflammation-based Glasgow prognostic score: A decade of experience
in patients with cancer. Cancer Treat Rev. 39:534–540.
2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shibutani M, Maeda K, Nagahara H, Ohtani
H, Sakurai K, Yamazoe S, Kimura K, Toyokawa T, Amano R, Tanaka H,
et al: Prognostic significance of the lymphocyte-to-monocyte ratio
in patients with metastatic colorectal cancer. World J
Gastroenterol. 21:9966–9973. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rasic I, Rebic V, Rasic A, Aksamija G and
Radovic S: The association of simultaneous increase in
interleukin-6, C reactive protein, and matrix metalloproteinase-9
serum levels with increasing stages of colorectal cancer. J Oncol.
2018(2830503)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yamamoto M, Saito H, Uejima C, Tanio A,
Takaya S, Sakamoto T, Honjo S, Maeta Y, Ashida K and Fujiwara Y:
Prognostic value of the combination of pre- and postoperative
C-reactive protein in colorectal cancer patients. Surg Today.
48:986–993. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chua W, Charles KA, Baracos VE and Clarke
SJ: Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in
patients with advanced colorectal cancer. Br J Cancer.
104:1288–1295. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Maeda K, Shibutani M, Otani H, Nagahara H,
Sugano K, Ikeya T, Kubo N, Amano R, Kimura K, Muguruma K, et al:
Low nutritional prognostic index correlates with poor survival in
patients with stage IV colorectal cancer following palliative
resection of the primary tumor. World J Surg. 38:1217–1222.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhou ZQ, Pang S, Yu XC, Xue Q, Jiang HY,
Liang XJ and Liu L: Predictive values of postoperative and dynamic
changes of inflammation indexes in survival of patients with
resected colorectal cancer. Curr Med Sci. 38:798–808.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
He WZ, Hu WM, Kong PF, Yang L, Yang YZ,
Xie QK, Jiang C, Yin CX, Qiu HJ, Zhang B, et al: Systemic
neutrophil lymphocyte ratio and mismatch repair status in
colorectal cancer patients: Correlation and prognostic value. J
Cancer. 9:3093–3100. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982.PubMed/NCBI
|
|
14
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. J Natl Cancer
Inst. 92:205–216. 2000.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jakubowska K, Kisielewski W, Kańczuga-Koda
L, Koda M and Famulski W: Diagnostic value of inflammatory cell
infiltrates, tumor stroma percentage and disease-free survival in
patients with colorectal cancer. Oncol Lett. 14:3869–3877.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mayadas TN, Cullere X and Lowell CA: The
multifaceted functions of neutrophils. Annu Rev Pathol Mech Dis.
9:181–218. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sagiv JY, Voels S and Granot Z: Isolation
and characterization of low- vs. High-density neutrophils in
cancer. Methods Mol Biol. 1458:179–193. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fridlender ZG and Albelda SM:
Tumor-associated neutrophils: Friend or foe? Carcinogenesis.
33:949–955. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Coffelt SB, Wellenstein MD and De Visser
KE: Neutrophils in cancer: Neutral no more. Nat Rev Cancer.
16:431–446. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gregory AD and Houghton AM:
Tumor-associated neutrophils: New targets for cancer therapy.
Cancer Res. 71:2411–2416. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Powell DR and Huttenlocher A: Neutrophils
in the tumor microenvironment. Trends Immunol. 37:41–52.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Brandau S, Moses K and Lang S: The kinship
of neutrophils and granulocytic myeloid-derived suppressor cells in
cancer: Cousins, siblings or twins? Semin Cancer Biol. 23:171–182.
2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nozawa H, Chiu C and Hanahan D:
Infiltrating neutrophils mediate the initial angiogenic switch in a
mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA.
103:12493–12498. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ueno H, Jones AM, Wilkinson KH, Jass JR
and Talbot IC: Histological categorisation of fibrotic cancer
stroma in advanced rectal cancer. Gut. 53:581–586. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Najafi M, Farhood B and Mortezaee K:
Contribution of regulatory T cells to cancer: A review. J Cell
Physiol. 234:7983–7993. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jakubowska K, Kisielewski W, Kańczuga-Koda
L, Koda M and Famulski W: Stromal and intraepithelial
tumor-infiltrating lymphocytes in colorectal carcinoma. Oncol Lett.
14:6421–6432. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nagasaki T, Akiyoshi T, Fujimoto Y,
Konishi T, Nagayama S, Fukunaga Y and Ueno M: Prognostic impact of
neutrophil-to-lymphocyte ratio in patients with advanced low rectal
cancer treated with preoperative chemoradiotherapy. Dig Surg.
32:496–503. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Palin RP, Devine AT, Hicks G and Burke D:
Association of pretreatment neutrophil-lymphocyte ratio and outcome
in emergency colorectal cancer care. Ann R Coll Surg Engl.
100:308–315. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Artaç M, Uysal M, Karaağaç M, Korkmaz L,
Er Z, Güler T, Börüban MC and Bozcuk H: Prognostic impact of
neutrophil/lymphocyte ratio, platelet count, CRP, and albumin
levels in metastatic colorectal cancer patients treated with
FOLFIRI-bevacizumab. J Gastrointest Cancer. 48:176–180.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhou WW, Chu YP and An GY: Significant
difference of neutrophil-lymphocyte ratio between colorectal
cancer, adenomatous polyp and healthy people. Eur Rev Med Pharmacol
Sci. 21:5386–5391. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pedrazzani C, Mantovani G, Fernandes E,
Bagante F, Luca Salvagno G, Surci N, Campagnaro T, Ruzzenente A,
Danese E, Lippi G and Guglielmi A: Assessment of
neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and
platelet count as predictors of long-term outcome after R0
resection for colorectal cancer. Sci Rep. 7(1494)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen L, Yan Y, Zhu L, Cong X, Li S, Song
S, Song H and Xue Y: Systemic immune-inflammation index as a useful
prognostic indicator predicts survival in patients with advanced
gastric cancer treated with neoadjuvant chemotherapy. Cancer Manag
Res. 9:849–867. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Özgehan G, Kahramanca Ş, Kaya IO, Bilgen
K, Bostanci H, Güzel H, Küçükpinar T and Kargici H:
Neutrophil-lymphocyte ratio as a predictive factor for tumor
staging in colorectal cancer. Turkish J Med Sci. 44:365–368.
2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Iseki Y, Shibutani M, Maeda K, Nagahara H,
Tamura T, Ohira G, Yamazoe S, Kimura K, Toyokawa T, Amano R, et al:
The impact of the preoperative peripheral lymphocyte count and
lymphocyte percentage in patients with colorectal cancer. Surg
Today. 47:743–754. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jankova L, Dent OF, Chan C, Chapuis P and
Clarke SJ: Preoperative neutrophil/lymphocyte ratio predicts
overall survival but does not predict recurrence or cancer-specific
survival after curative resection of node-positive colorectal
cancer. BMC Cancer. 13(442)2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ying HQ, Deng QW, He BS, Pan YQ, Wang F,
Sun HL, Chen J, Liu X and Wang SK: The prognostic value of
preoperative NLR, d-NLR, PLR and LMR for predicting clinical
outcome in surgical colorectal cancer patients. Med Oncol.
31(305)2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Balde AI, Fang S, He L, Cai Z, Han S, Wang
W, Li Z and Kang L: Propensity score analysis of recurrence for
neutrophil-to-lymphocyte ratio in colorectal cancer. J Surg Res.
219:244–252. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li H, Song J, Cao M, Wang G, Li L, Zhang
B, Li Y, Xu W and Zheng J: Preoperative neutrophil-to-lymphocyte
ratio is a more valuable prognostic factor than
platelet-to-lymphocyte ratio for nonmetastatic rectal cancer. Int
Immunopharmacol. 40:327–331. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ozdemir Y, Akin ML, Sucullu I, Balta AZ
and Yuce E: Pretreatment neutrophil/lymphocyte ratio as a
prognostic aid in colorectal cancer. Asian Pacific J Cancer Prev.
15:2647–2650. 2014.PubMed/NCBI View Article : Google Scholar
|