Introduction
Inflammatory myopathies, i.e., dermatomyositis (DM)
and polymyositis (PM), are well recognized in association with
malignancies. The first case of malignancy-associated inflammatory
myopathy was described in 1916, which occurred with gastric cancer
(1). Subsequently, ovarian cancer
cases were reported in 1935(2).
Previous studies summarized the type of cancer with inflammatory
myopathy. Ovarian, gastrointestinal, and lung cancers and lymphoma
are relatively common, but germ cell tumors (GCTs) are rare
(3-5).
The typical clinical course of paraneoplastic inflammatory myopathy
is that dermal and muscular symptoms precede the diagnosis of
malignancy. In more than half of cases, the diagnosis of
inflammatory myopathy precedes the diagnosis of malignancy
(3,5). However, a case of extragonadal GCT
with DM that occurred after completing chemotherapy for GCT is
presented. On reviewing previous literature describing GCT with
inflammatory myopathy, this is the first case of GCT whose primary
site was extragonadal and the diagnosis of DM was made after the
treatment for cancer. Moreover, its unique clinical course would be
useful to inform oncologists that, even after treatment for the
cancer itself, dermal and muscular symptoms such as facial erythema
can be useful as signs for the diagnosis of paraneoplastic
inflammatory myopathy.
Materials and methods
Information about this case was extracted from the
medical records of The Cancer Institute Hospital of the Japanese
Foundation for Cancer Research. For the literature review, the
search terms to identify previous cases on PubMed were:
‘Dermatomyositis, germ cell tumor’; ‘dermatomyositis, testicular’;
‘polymyositis, germ cell tumor’; and ‘polymyositis, testicular’.
Articles without abstracts or information about the clinical course
were excluded. Articles written in languages other than English
were also excluded.
Results
A 53-year-old man was referred to our hospital with
suspected cancer of unknown origin. He complained of cervical lymph
node enlargement and underwent contrast computed tomography (CT),
positron emission tomography (PET), and diagnostic cytology.
Although poorly differentiated cancer was suspected on cytology,
the primary site was not detected by imaging modalities. His past
medical history included inguinal hernia and appendicitis in
childhood, a ureterocele at age 15 years, fracture of the fibula at
age 20 years, and ongoing hypertension and hyperuricemia. The
patient's medications included amlodipine, valsartan, allopurinol,
and diclofenac as needed. His father had prostate cancer at the age
of 60 years. He drank alcohol every day, had no smoking history,
and had not been exposed to toxins or illegal drugs. He worked as a
bank clerk without any contacts with sick people. His vital signs
were stable. On physical examination, cervical lymph nodes, 2x2 cm
in size, were palpable bilaterally. Other examinations including
chest, abdomen, genitals, extremities, and a neurological
examination showed no abnormal findings. Laboratory data showed
elevated lactate dehydrogenase (727 U/L, normal range: 124-222
U/L). The blood cell count was normal, and the chemistry panel
showed no other abnormalities. α-fetoprotein (AFP) and human
chorionic gonadotropin (HCG) were both under the lower limits
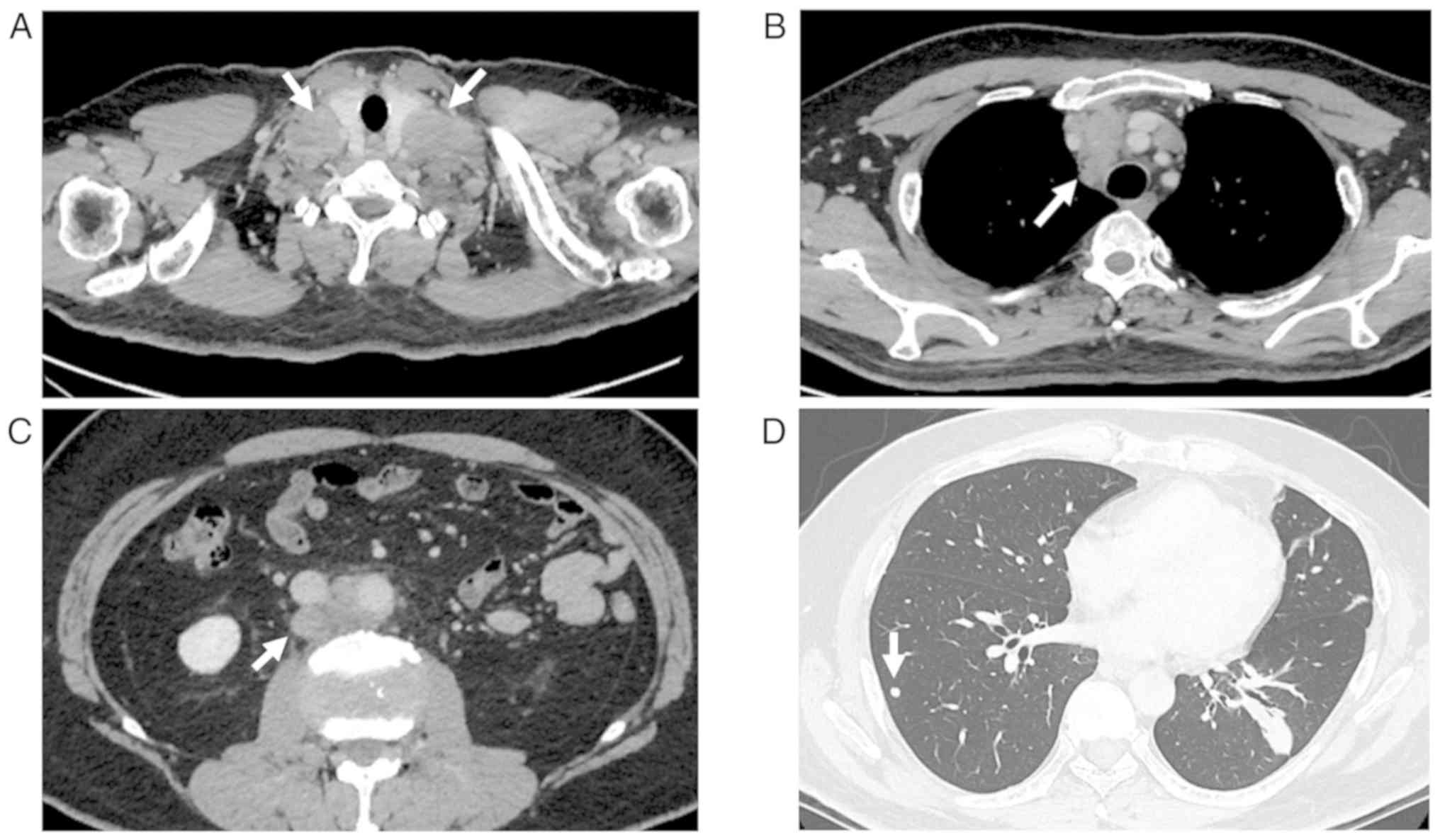
(Table I). Contrast CT showed
enlargement of bilateral cervical lymph nodes (35 mm x 29 mm),
anterior mediastinal lymph nodes (49 mm x 30 mm), and paraaortic
lymph nodes at the L2 vertebral level (25 mm x 18 mm) and multiple
lung nodules, but no tumor in the testis (Fig. 1). Gastrointestinal and colorectal
endoscopy found no evidence of malignancy. A needle biopsy was
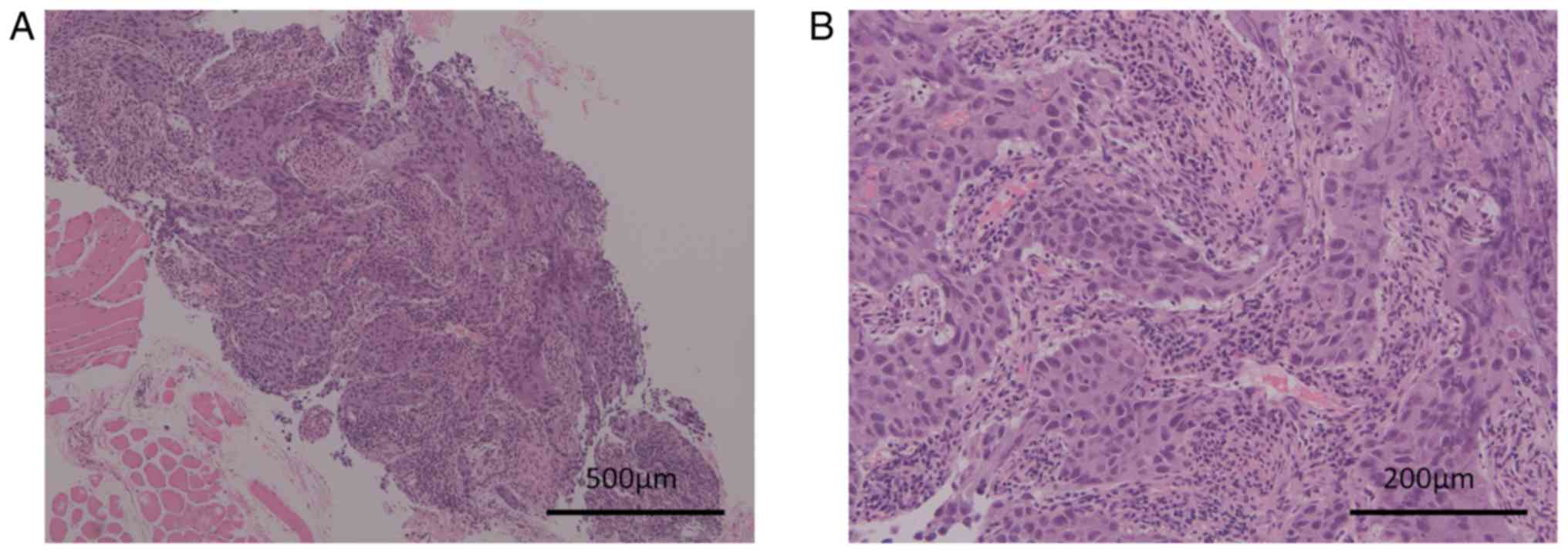
performed from a cervical lymph node twice. Histologically, the
biopsy specimen showed proliferation of cancer cells forming a
sheet-like structure with coagulative necrosis (Fig. 2A). The cancer cells showed enlarged
oval nuclei with conspicuous nucleoli and pale cytoplasm (Fig. 2B). The differential diagnoses were
squamous cell carcinoma, undifferentiated carcinoma and GCT, and
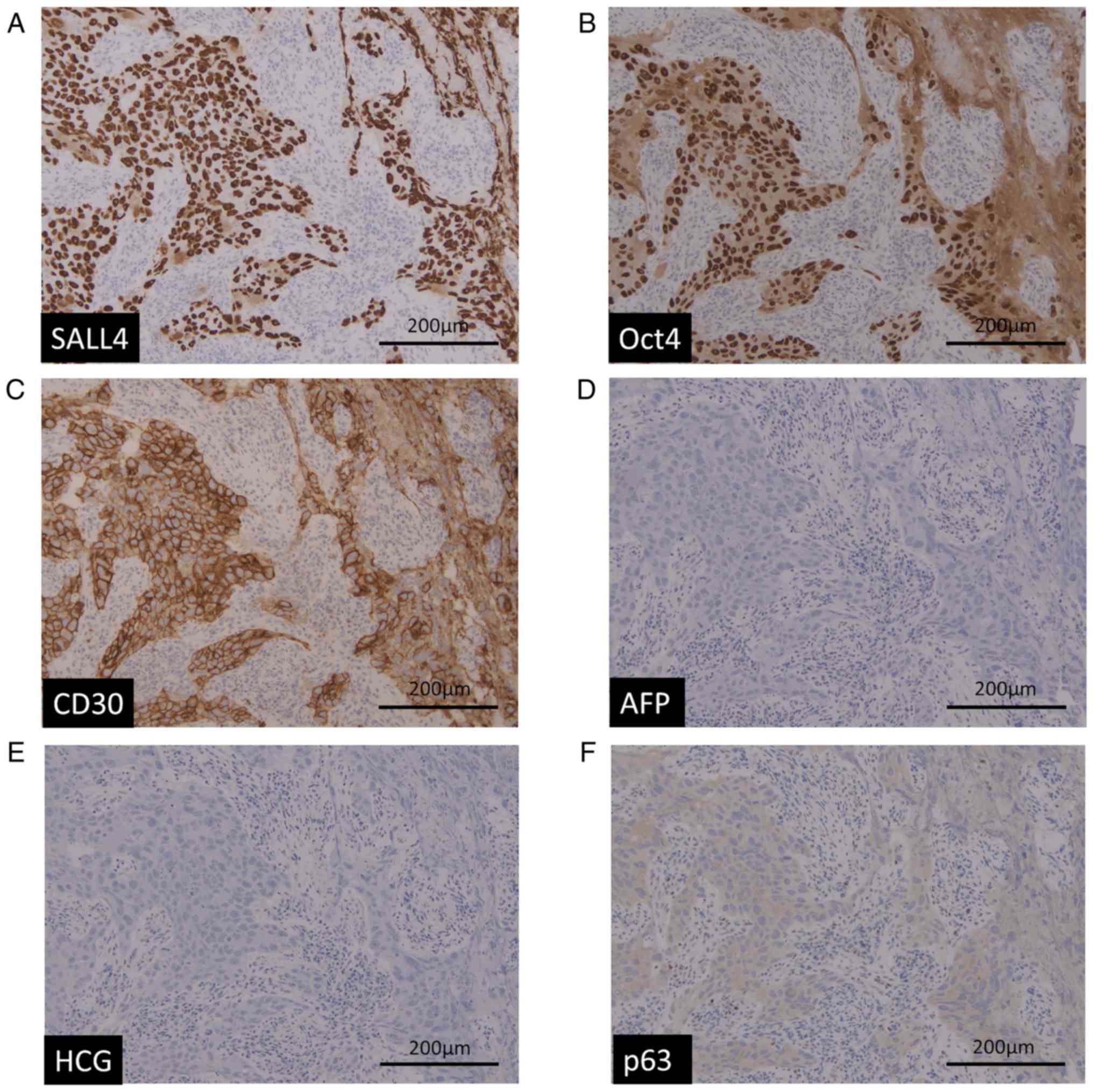
immunohistochemistry showed that the cancer cells were positive for
SALL4, OCT4, and CD30 (Fig. 3A-C).
AFP, HCG and p63 were totally negative (Fig. 3D-F). Morphologically and
immunohistochemically, GCT (embryonal carcinoma) was highly
suspected (Figs. 2 and 3). Based on these results, this patient
was diagnosed with extragonadal embryonal carcinoma with multiple
lymph node and lung metastases. Although isochromosome 12p was not
checked and the primary site could not be confirmed, the primary
site was considered to be the mediastinum because the size of the
mediastinal lymph node metastases was the largest and cervical
lymph node metastases were symmetrical in this patient.
 | Table IInitial laboratory data. |
Table I
Initial laboratory data.
| A, Complete blood
cell |
|---|
| Variable | Value | Unit |
|---|
| WBC | 5,100 | /µl |
|
Stab+Seg | 54.3 | % |
|
Lymphocyte | 31.2 | % |
|
Monocyte | 11.9 | % |
|
Eosinophil | 1.5 | % |
|
Basophil | 1.1 | |
| RBC | 477 |
104/µl |
| Hgb | 15.9 | g/dl |
| PLT | 217,000 | /µl |
| B, Biochemistry |
| TP | 7.7 | g/dl |
| Alb | 4.5 | g/dl |
| LDH | 727 | IU/l |
| T-Bil | 0.8 | mg/dl |
| AST | 32 | U/l |
| ALT | 32 | U/l |
| ALP | 294 | U/l |
| γGTP | 50 | U/ |
| CK | 65 | U/Ll |
| BUN | 12.0 | mg/dl |
| Cr | 0.84 | mg/dl |
| eGFR | 75.1 | ml/min/l |
| Na | 141 | mEq/l |
| K | 4.4 | mEq/l |
| Cl | 105 | mEq/l |
| Ca | 9.1 | mg/dl |
| CRP | 0.06 | mg/dl |
| C, Infectious
diseases |
| Variable | Value | Unit |
| HBs Ag | 0.0 | IU/ml |
| HBs Ab | 0.45 | mIU/ml |
| HCV Ab | Negative | |
| HIV Ab | Negative | |
| D, Tumor markers |
| Variable | Value | Unit |
| CEA | 1.8 | ng/ml |
| SCC | 1.1 | ng/ml |
| AFP | 1.1 | ng/ml |
| HCG | <1.0 | mIU/ml |
| β-HCG | <0.1 | ng/ml |
| E, Coagulation
test |
| Variable | Value | Unit |
| PT | 13.6 | Second |
| PT-INR | 0.98 | |
| APTT | 33.2 | Second |
| Fibrinogen | 386 | mg/dl |
| D-Dimer | 0.38 | µg/ml |
About one month after referral to our hospital, this
patient developed bilateral recurrent laryngeal nerve paralyses due
to enlarged cervical lymph nodes and was hospitalized. Aspiration
pneumonia and respiratory failure occurred. Thus, a tracheotomy was
performed, and respiratory support by continuous positive airway
pressure was started. On the following day, chemotherapy (BEP:
Bleomycin 30 mg on days 1, 8, and 15; etoposide 100
mg/m2 on days 1-5; and cisplatin 20 mg/m2 on
days 1-5) for extragonadal GCT was started. Administration of
granulocyte colony-stimulating factor (G-CSF; filgrastim, 75
µg/day) was begun on day 6 of the first course of BEP therapy.
During this course, the patient's neutrophil count decreased (Grade
4) on day 12, and he developed pneumonia requiring management in
the intensive care unit. On the same day, the dose of filgrastim
was increased to 150 µg/day, and antibiotics including vancomycin
and piperacillin/tazobactam were started with mechanical
ventilation support. He recovered quickly on day 15, and the
subsequent courses of BEP treatment were continued with
prophylactic G-CSF (filgrastim 150 µg/day) support. During the
first and second courses of BEP treatment, bleomycin administration
was skipped on day 15 due to pneumonia and grade 4 neutropenia.
However, four courses of the BEP regimen were completed as
scheduled two months later. Contrast CT after three weeks of these
interventions showed partial response according to the Response
Evaluation Criteria in Solid Tumors (RECIST). PET-CT showed no
uptake around the tumor lesions, and this patient was followed
closely without further treatment. Due to dysphagia caused by
bilateral recurrent laryngeal nerve paralyses, percutaneous
endoscopic gastrostomy was performed one month after BEP therapy
was ended. Swallowing training was started, and the patient was
discharged four months after chemotherapy was started.
One month after discharge, this patient developed
facial erythema aggravated by sunlight exposure. The use of
allopurinol was stopped due to a suspected drug-induced
dermatologic adverse event, but the erythema was not improved.
Bilateral periorbital edema and an erythematous eruption over the
extensor surfaces of bilateral fingers, or Gottron's sign, appeared
subsequently with an elevated CK level (562 U/L). Skin biopsy of
the facial erythema showed superficial perivascular dermatitis with
mucin deposition, consistent with DM. This patient was referred to
another hospital for investigation and treatment. Although the
embryonal carcinoma maintained partial response at this time, the
distinct skin lesions with the results of the biopsy and the
positive anti-TIF-1γ-antibody result confirmed the diagnosis of DM.
Laboratory data at that time are shown in Table II. After starting prednisolone (1
mg/kg=70 mg/body daily), the erythema improved, and his
prednisolone is being carefully tapered to prevent the recurrence
of DM. The post-chemotherapy surgery was omitted because there were
multiple lymph node metastases that had been shrinking without
uptake on PET-CT. Over the course of the DM, the extragonadal GCT
has maintained a partial response. So far, the relapse-free
survival and overall survival of this patient after BEP treatment
are both sixteen months without recurrence or death.
 | Table IILaboratory data when dermatomyositis
occurred. |
Table II
Laboratory data when dermatomyositis
occurred.
| A, Complete blood
cell |
|---|
| Variable | Value | Unit |
|---|
| WBC | 6,100 | /µl |
|
Stab+Seg | 51.6 | % |
|
Lymphocyte | 28.9 | % |
|
Monocyte | 16.9 | % |
|
Eosinophil | 2.1 | % |
|
Basophil | 0.5 | |
| RBC | 373 |
104/µl |
| Hgb | 12.2 | g/dl |
| PLT | 192,000 | /µl |
| B,
Biochemistry |
| Variable | Value | Unit |
| TP | 6.7 | g/dl |
| Alb | 3.6 | g/dl |
| LDH | 273 | IU/l |
| T-Bil | 0.5 | mg/dl |
| AST | 55 | U/l |
| ALT | 27 | U/l |
| ALP | 229 | U/l |
| γGTP | 30 | U/l |
| CK | 562 | U/l |
| BUN | 22.0 | mg/dl |
| Cr | 0.88 | mg/dl |
| eGFR | 71.4 | ml/min/l |
| Na | 138 | mEq/l |
| K | 4.7 | mEq/l |
| Cl | 102 | mEq/l |
| Ca | 9.7 | mg/dl |
| CRP | 0.88 | mg/dl |
Discussion
It is well known that the risk of malignancy in
inflammatory myopathy patients is high (6). Although the pathophysiology of
malignancy-associated inflammatory myopathy has not yet been
determined, many lines of evidence support the relationship of
these two different diseases and have shown the incidence, outcome,
and prognosis. In inflammatory myopathy, the risk of malignancy
differs between DM and PM. The relative ratio of malignancy is
approximately 2.4-7.7 times in DM patients and 1.4-2.1 times in PM,
respectively. Thus, the relative risk of malignancy is generally
higher in DM patients than in PM patients (3-5).
The type of malignancy associated with DM and PM is wide-ranging.
Ovarian, lung, gastrointestinal, and pancreatic cancers and
non-Hodgkin lymphoma are frequently seen in DM patients, and
non-Hodgkin lymphoma and lung and bladder cancers are relatively
common in PM patients (5). In
Japanese patients, gastric, colon, and ovarian cancers are
frequently seen in inflammatory myopathy patients (7). However, these population-based studies
did not identify GCT patients, and GCT with these myopathies is
extremely rare. Only a few cases of GCT including testicular tumors
have been reported in association with DM and PM (Table III).
 | Table IIIReported cases of germ cell tumor
with inflammatory myopathy. |
Table III
Reported cases of germ cell tumor
with inflammatory myopathy.
| Case | Age | Stage | Pathological
diagnosis | Primary site | DM/PM | Treatment for
myopathy | Control of
myopathy | Best response of
cancer | Myopathy before/
after malignancy | (Refs.) |
|---|
| 1 | 30 | III | Non-seminomatous
GCT | Testis | DM | PSL | Bad | CR | After | (12) |
| 2 | 30 | III | Non-seminomatous
GCT | Testis | DM | PSL+AZA+CPA | Good but
relapsed | CR but
relapsed | Before | (13) |
| 3 | 28 | I | Teratoma,
undifferentiated | Testis | DM | PSL | Good | PD | After | (14) |
| 4 | 30 | IIIB | Teratoma,
undifferentiated | Testis | DM | PSL | Good | CR | Before | (14) |
| 5 | 24 | III | Mature teratoma,
intestinal epithelium and intratubular GCT | Testis | DM | PSL,
Chemotherapy | Good | CR | Before | (15) |
| 6 | 24 | III | Intratubular
GCT | Testis | DM | PSL | Good | CR | Before | (16) |
| 7 | 31 | IIIa | Embryonal
cancer | Testis | DM | Chemotherapy | Gooda | CR | After | (17) |
| 8 | 46 | IIA | Seminoma | Testis | DM | PSL | Good | CR | Before | (18) |
| 9 | 29 | III | Seminoma or
Embryonal carcinoma |
Retroperitoneum | DM | PSL+MTX,
Chemotherapy | Relatively
Good | CR but
relapsed | Before | (19) |
| 10 | 31 | IIIB | Embryonal
carcinoma | Testis | DM | Chemotherapy | Good | CR | Before | (20) |
| 11 | 34 | Ia | Testicular
cancerb | Testis | DM |
Immunosuppressionb | Good | CR | After | (21) |
| 12 | 50 | I | Seminoma | Testis | PM | PSL+MTX | Good | CR | Before | (22) |
| 13 | 37 | I | Seminoma | Testis | DM | Orchiectomy,
PSL+AZA | Good | CR | Before | (23) |
| 14 | 36 | IB | Mixed (Seminoma and
embryonal carcinoma) | Testis | DM | PSL+MTX | Not good | CR | After | (24) |
| 15 | 32 | II | Embryonal carcinoma
with intratubular germ cell neoplasia | Testis | PM | Chemotherapy, PSL,
IVIg | Not good | CR | Before | (25) |
| 16 | 30 | I | Seminoma | Testis | DM | mPSL+ AZA | Bad | CR | Before | (26) |
| 17 | 22 | III | Non-seminomatous
GCTb |
Retroperitoneum | DM | PSL, Surgery,
Chemotherapyb | Good | CR | Before | (11) |
| 18 | 53 | III | Embryonal
carcinoma |
Extragonadc | DM | PSL | Good | PR | After | This case |
In inflammatory myopathies, several auto-antibodies
have been detected. In particular, anti-TIF1γ antibody and
anti-NXP2 antibody are known to be associated with malignancy
(8). A meta-analysis of six studies
involving 312 adult DM patients with malignancy showed that
anti-TIF1γ antibody has sensitivity of 78% and specificity of 89%
for making a diagnosis of cancer-associated DM (9). The positive anti-TIF1γ antibody result
in the present case supports the diagnosis of paraneoplastic
inflammatory myopathy though the causal relationship cannot be
confirmed only through this test (9). There has been only one case of GCT
with autoinflammatory myopathy that was anti-TIF1γ
antibody-positive, and more cases are needed to discuss the
relationship between this antibody and DM in GCT patients (10).
The prognosis of DM and PM with malignancy has been
reported to be worse than with typical DM/PM without malignancy
(11). However, rather than other
solid tumors in the advanced stage, the prognosis of GCT is
relatively better. Thus, the prognosis and complications of this
patient group should be more precisely determined.
In our review of previous reports, 17 cases
mentioning the relationship between GCT and inflammatory myopathy
were identified (Table III). The
primary site of 15 cases was testis, and that of two cases was
extragonadal (retroperitoneum). Pathological results included
seminoma, teratoma, intratubular GCT, mixed GCT, and embryonal
carcinoma. Only two cases (12%) had PM, and most cases were
associated with DM. As for the timing of onset, five cases (29%)
developed myopathy after treatment for the malignancy. Given these
results, the present case appears to be the first case of
extragonadal GCT with DM that occurred after treatment for the
malignancy. The treatment strategy for paraneoplastic inflammatory
myopathy varies from corticosteroid administration to treatment of
the malignancy, depending on each case. Although the prognosis of
inflammatory myopathy with malignancy is generally poor, cases with
GCT seem to have a good prognosis, presumably due to the good
prognosis of the GCT itself.
To the best of our knowledge, this is the first case
of extragonadal GCT with DM that occurred after chemotherapy was
completed and during the period of tumor responsiveness. A delayed
diagnosis of DM and PM would decrease the quality of life and
negatively affect the survival of a patient. The unique clinical
course of the present case reminds us that we should consider the
possibility of malignancy-associated inflammatory myopathies when
we see dermal and muscular symptoms, even after treatment for the
malignancy. Because of its rarity and the lack of clarity regarding
its pathophysiology, further cases are needed to understand this
paraneoplastic syndrome in GCT.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YF mainly wrote the manuscript and created the
figures and tables; NF cared for the patient, provided professional
opinions about writing this report, and revised it; AO, KN, MO,
STai and JT cared for the patient and reviewed the manuscript; MT
contributed to pathological diagnosis, provided professional
opinions about pathological discussion and reviewed the manuscript;
STak managed the whole project, and reviewed and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Consent for publication was obtained from the
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sterz G: Polymyositis. Berl Klin
Wochenschr. 53(489)1916.
|
|
2
|
Bezecny R: Dermatomyositis. Arch Dermat
Syph. 171:242–251. 1935.
|
|
3
|
Buchbinder R, Forbes A, Hall S, Dennett X
and Giles G: Incidence of malignant disease in biopsy-proven
inflammatory myopathy. A population-based cohort study. Ann Intern
Med. 134:1087–1095. 2001.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Stockton D, Doherty VR and Brewster DH:
Risk of cancer in patients with dermatomyositis or polymyositis,
and follow-up implications: A Scottish population-based cohort
study. Br J Cancer. 85:41–45. 2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hill CL, Zhang Y, Sigurgeirsson B, Pukkala
E, Mellemkjaer L, Airio A, Evans SR and Felson DT: Frequency of
specific cancer types in dermatomyositis and polymyositis: A
population-based study. Lancet. 357:96–100. 2001.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Qiang JK, Kim WB, Baibergenova A and
Alhusayen R: Risk of malignancy in dermatomyositis and
polymyositis. J Cutan Med Surg. 21:131–136. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Azuma K, Yamada H, Ohkubo M, Yamasaki Y,
Yamasaki M, Mizushima M and Ozaki S: Incidence and predictive
factors for malignancies in 136 Japanese patients with
dermatomyositis, polymyositis and clinically amyopathic
dermatomyositis. Mod Rheumatol. 21:178–183. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fiorentino DF, Chung LS, Christopher-Stine
L, Zaba L, Li S, Mammen AL, Rosen A and Casciola-Rosen L: Most
patients with cancer-associated dermatomyositis have antibodies to
nuclear matrix protein NXP-2 or transcription intermediary factor
1γ. Arthritis Rheum. 65:2954–2962. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Trallero-Araguás E, Rodrigo-Pendás JÁ,
Selva-O'Callaghan A, Martínez-Gómez X, Bosch X, Labrador-Horrillo
M, Grau-Junyent JM and Vilardell-Tarrés M: Usefulness of anti-p155
autoantibody for diagnosing cancer-associated dermatomyositis: A
systematic review and meta-analysis. Arthritis Rheum. 64:523–532.
2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Taki E, Shimizu M, Soeda Y, Shirai M and
Muro Y: Anti-TIF1-γ-positive young adult dermatomyositis with germ
cell tumour. Eur J Dermatol. 26:623–624. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Airio A, Kautiainen H and Hakala M:
Prognosis and mortality of polymyositis and dermatomyositis
patients. Clin Rheumatol. 25:234–239. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fife RS, Williams S and Eyanson S:
Dermatomyositis associated with treated testicular carcinoma. J
Rheumatol. 11:397–398. 1984.PubMed/NCBI
|
|
13
|
Barker RA, Currie DC, Horwich A and Spiro
SG: Metastatic non-seminomatous germ cell tumour and
dermatomyositis. Postgrad Med J. 66:59–60. 1990.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Clayton AJ and Mead GM: Germ cell cancer
and dermatomyositis. Clin Oncol (R Coll Radiol). 10:56–58.
1998.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hayami S, Kubota Y, Sasagawa I, Suzuki H,
Nakada T and Motoyama T: Dermatomyositis associated with
intratubular germ cell tumor and metastatic germ cell cancer. J
Urol. 159:2096–2097. 1998.PubMed/NCBI
|
|
16
|
Ishizawa T, Mitsuhashi Y and Kondo S:
Dermatomyositis associated with testicular tumour with elevation of
serum lactate dehydrogenase (LDH) isoenzyme-1. Acta Derm Venereol.
79(167)1999.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Di Stasi SM, Poggi A, Giannantoni A and
Zampa G: Dermatomyositis associated with testicular germ cell
cancer. J Urol. 163(240)2000.PubMed/NCBI View Article : Google Scholar
|
|
18
|
von Heyden B, Kliesch S and Nashan D: Re:
Dermatomyositis associated with testicular germ cell cancer. J
Urol. 164(2030)2000.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Vattemi G, Tonin P, Martignoni G, Filosto
M, Marchioretto F, Rizzuto N and Tomelleri G: Dermatomyositis and
retroperitoneal germ cell cancer. Eur Neurol. 45:52–53.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yoshinaga A, Hayashi T, Ishii N, Ohno R,
Watanabe T and Yamada T: Successful cure of dermatomyositis after
treatment of nonseminomatous testicular cancer. Int J Urol.
12:593–595. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Curiel RV, Brindle KA, Kressel BR and Katz
JD: Dysphagia after testicular cancer. Arthritis Rheum.
52(3712)2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Singhal N, Hissaria P, Joshi R and Nayagam
S: Inflammatory myopathy and cancer: Rare association of seminoma
testes and polymyositis. Intern Med J. 38:295–297. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Dourmishev LA, Popov JM and Rusinova D:
Paraneoplastic dermatomyositis associated with testicular cancer: A
case report and literature review. Acta Dermatovenerol Alp
Pannonica Adriat. 19:39–43. 2010.PubMed/NCBI
|
|
24
|
Tan E, Young D, McLaren B and Wright A:
Early-stage testicular cancer: A rare association with
dermatomyositis. Australas J Dermatol. 51:139–141. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kamei J, Kyono Y, Yamada Y, Shinohara M
and Homma Y: Life-threatening polymyositis associated with
non-seminomatous testicular cancer: A case report. Hinyokika Kiyo.
58:361–364. 2012.PubMed/NCBI(In Japanese).
|
|
26
|
Norrenberg S, Gangji V, Del Marmol V and
Soyfoo MS: Diffuse muscular pain, skin tightening, and nodular
regenerative hyperplasia revealing paraneoplastic amyopathic
dermatomyositis due to testicular cancer. Case Rep Rheumatol.
2012(534236)2012.PubMed/NCBI View Article : Google Scholar
|