Introduction
Adenocarcinoma of the esophagogastric junction
(AEG), an entity distinct from non-cardiac gastric cancer, is
defined as a type of cancer located within 2 cm proximal and distal
of the anatomical cardia (1). In
recent decades, there has been a notable increase in the incidence
of AEG, not only in Western (2,3), but
also in Asian countries (4-6).
In China, a significantly increasing trend in the incidence of AEG
was reported from 1988 to 2013 in a population-based study, while
the incidence of non-cardiac gastric cancer decreased (7).
Although Siewert and Stein proposed an AEG
classification system, including types I-III, to aid clinicians in
deciding on the surgical approach (8), the surgical strategies for AEG cases
remain controversial. Total gastrectomy (TG) is considered as a
standard procedure, with the benefits of sufficient resection
margins and more radical lymphadenectomy (9). Some recent studies have reported that
proximal gastrectomy (PG) achieves survival rates equivalent to
those of TG, while preserving the physiological functions of the
gastric remnant (10-14).
However, others questioned whether the advantages of PG outweigh
the functional drawbacks of esophageal reflux, which markedly
affects the quality of life of the patients (15,16),
as several reconstruction methods after PG, including
esophagogastrostomy and jejunal interposition, may carry a high
risk of reflux esophagitis and gastroesophageal anastomotic
stenosis (16-20).
Hayami et al applied a novel double-flap technique, invented
by Kamikawa et al (21), to
laparoscopic proximal gastrectomy (LPG-DFT) in order to prevent
reflux. Their results indicated that LPG-DFT is a better surgical
procedure for upper-third early gastric cancer compared with
laparoscopic TG in terms of morbidity, postoperative hospital stay
and postoperative nutritional status (22). In addition, Tanioka et al
indicated that LPG may be more beneficial compared with
laparoscopic TG (LTG) in terms of perioperative and nutritional
outcomes for early-stage gastric cancer (23). However, the operative time was
significantly longer in the LPG-DFT group due to the complexity of
valvuloplasty, which requires masterful intracorporeal
suturing.
The aim of the present study was to investigate a
simple and safe anti-reflux anastomosis technique, the
triangle-valve technique (TVT), in PG. This valve technique was
designed to prevent reflux. It was hypothesized that the TVT may be
time-saving due to its easiness and simplicity and, if the clinical
outcomes of PG-TVT and TG were found to be comparable in terms of
postoperative complications, PG-TVT may improve their nutritional
status by conserving half of the stomach.
Materials and methods
Patients
A total of 74 patients with AEG (Siewert II or III)
were recruited consecutively at the First Affiliated Hospital of
Zhengzhou University between July 2013 and December 2017. From May
2015 to December 2017, PG-TVT was performed on 44 patients with a
clinical diagnosis of T1-4N0-3M0
AEG at the preoperative evaluation according to the 8th edition of
American Joint Committee on Cancer-TNM Staging System of Gastric
Carcinoma (24). A total of 30
patients with T1-4N0-3M0 AEG
located in or involving the upper third of the stomach who received
TG between July 2013 and December 2015 were considered as the
control group. Between January 2016 and January 2017, TG was also
performed in a further 17 patients with the same indications as in
the previous period. However, the latter period was not included,
as the number of TG-TVT cases had gradually increased during that
period. Certain settings, including the surgeon's preference in
relation to adopting the procedure, had been taken into
consideration to avoid selection bias. All the procedures were
performed by the same surgical team.
All the patients were in stable condition and were
considered as operable. Written informed consent was obtained from
each patient. This was a retrospective study using
clinicopathological, surgical and follow-up data, and the study
protocol was approved by the Institutional Review Board at the
First Affiliated Hospital of Zhengzhou University (Zhengzhou,
China).
Surgical procedure of PG and PG-TVT
reconstruction
Lymph node dissection was performed laparoscopically
and the TVT reconstruction was performed with an open technique.
The detailed surgical procedure of PG and PG-TVT reconstruction is
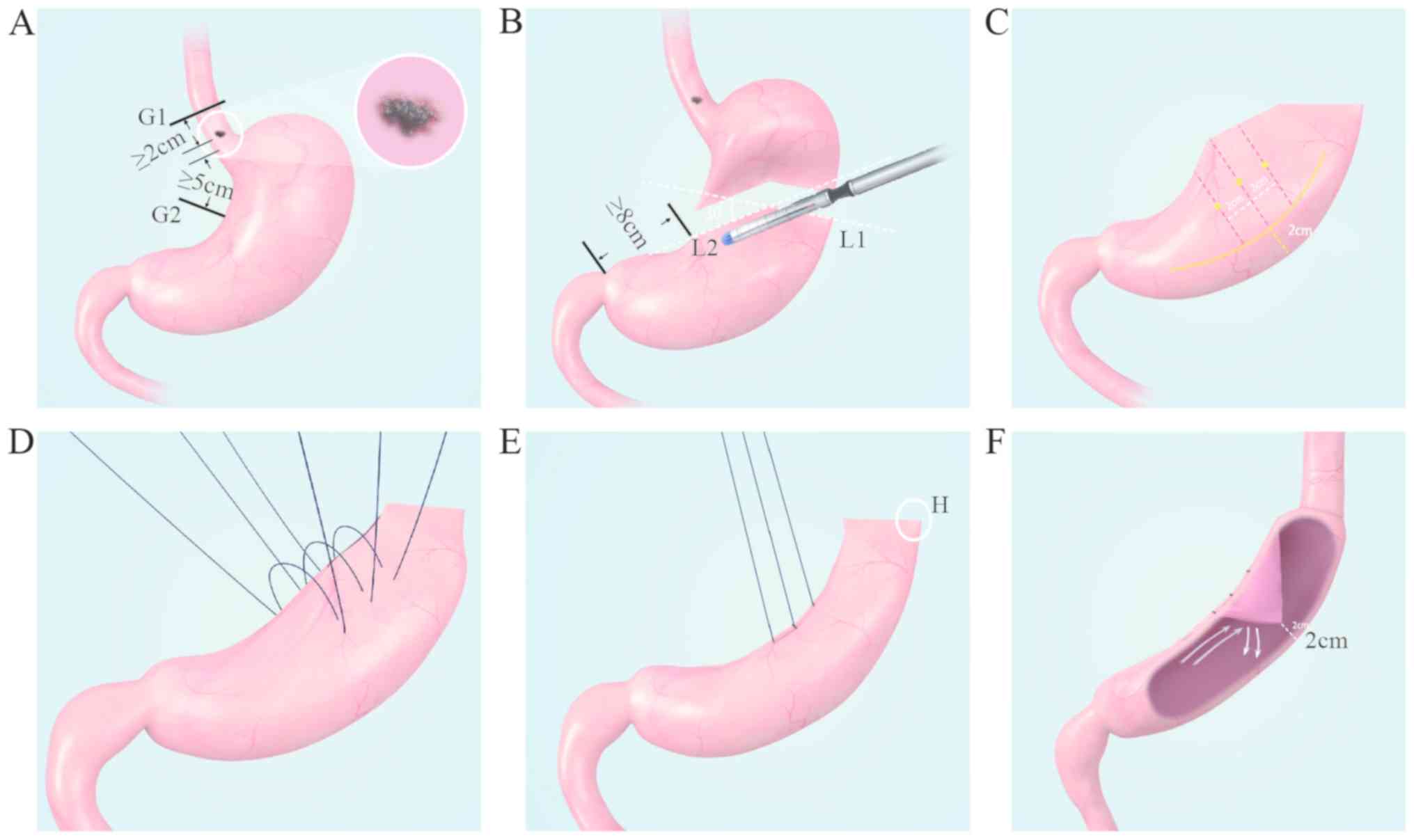
described below (Fig. 1). After
lymph node dissection is completed, the first step of the procedure
is to locate the tumor lesion and determine the area of resection,
including upper (G1) and lower (G2) resection margins. As shown in
Fig. 1A, an example of a primary
tumor lesion (indicated by the white circle) located along the
lesser curvature is used to present the resection region with the
G1 and G2 margins at a distance of no less than 2 and 5 cm,
respectively, from the tumor. The aforementioned distances are
measured on the tension-free gastric body. Based on the
after-mentioned estimation of the resection area, the upper
resection line is defined with cutting line G1 (black), and then a
lower resection line is defined with the first cutting line L1,
which was perpendicular to the greater curvature, and the second
cutting line L2, which is at an angle of 30˚ relative to L1 (both
indicated with white dotted lines; Fig.
1B). The crossing point of line L2 on the lesser curvature must
be no less than 8 cm from the pyloric sphincter. Consequently, a
solid linear path of L1 and L2, as shown in Fig. 1B, indicates the lower resection
line. A proximal gastrectomy is then performed along the
aforementioned solid linear path with a linear cutting closure
(Fig. 1B). Third, as shown in
Fig. 1C, on the exposed flattened
side of the remnant gastric body, a curved line (yellow continuous
line) is drawn 2 cm from the greater curvature, with 3 parallel
lines (red dotted lines, 2 cm from each other) perpendicular to it.
The midpoints of those three parallel lines are marked with yellow
dots (Fig. 1C). A similar curved
line, three parallel lines and midpoints are also marked on the
posterior side of the remnant gastric body (not shown in the
figure). Then, three stitches are made along these midpoints on
both sides (Fig. 1D), so that the
gastric wall between those midpoints of both sides will be folded
towards the gastric cavity to form a triangle-valve shaped bulge
when those sutures are knotted (Fig.
1E and F). Finally, the distal
gastric remnant is anastomosed to the esophageal end through point
H (Fig. 1E). The triangle
valve-shaped bulge (Fig. 1F)
functions similarly to the cardia as an anti-reflux mechanism.
Surgical procedure of TG and Roux-en-Y
(R-Y) reconstruction
Radical TG was performed following the Japanese
gastric cancer treatment guidelines (25). The resection distance from the upper
and lower margins of the tumor was ≥2 cm, and D2 lymph node
dissection was ensured (26,27).
After that, Roux-en-Y reconstruction was completed (28). The jejunum was separated 20 cm below
the ligament of Treitz and esophageal-distal jejunal anastomosis
was performed. subsequently, the proximal jejunum was anastomosed
with the distal jejunum 40 cm below the esophagojejunal
anastomosis.
Clinical parameters and surgical
outcomes
The patients' clinical characteristics, including
age, sex, body mass index (BMI), Siewert type, tumor size,
histological type, pathological TNM stage, history of abdominal
surgery, preoperative chemotherapy and postoperative adjuvant
chemotherapy, were obtained from their medical records. Surgical
parameters, such as operative time, estimated blood loss,
laparoscopy assistance, extent of lymph node dissection, number of
retrieved lymph nodes, residual tumor (R), postoperative
complications and postoperative hospital stay, were also retrieved
from the medical records.
Follow-up and postoperative
nutritional status
All the patients were followed up for 6 months. The
Reflux Disease Questionnaire (RDQ) was used to evaluate reflux
esophagitis. Information on the frequency and severity of upper
gastrointestinal symptoms (heartburn, regurgitation and
non-cardiogenic chest pain) were obtained in the 6 months after
surgery. Patients with RDQ scores of ≥12 points were diagnosed with
gastroesophageal reflux disease (GERD) (29). To evaluate postoperative nutritional
status, changes in body weight and biochemical data, such as serum
concentrations of total protein (TP), albumin (Alb), hemoglobin
(Hb) and prealbumin (PA), were examined at 7 days and at 6 months
after surgery.
Statistical analysis
Categorical variables were compared by using the
χ2 test or Fisher's exact test, and continuous data were
compared by using the Student's t-test or Mann-Whitney U test.
Postoperative changes in weight, TP, Alb, Hb and PA were compared
using repeated measures ANOVA and the Least-Significant Difference
method was used for pairwise comparisons. All analyses were
conducted using RStudio software (version 1.1.456, 2009-2018
RStudio Inc.). All statistical tests were two-sided, and P<0.05
was considered to indicate statistically significant
differences.
Results
Patient characteristics
The characteristics of the patients are listed in
Table I. There were no significant
differences in age, sex, BMI, previous abdominal operations,
Siewert type, histological type, pathological TNM stage,
preoperative and postoperative chemotherapy between the two groups.
The median tumor size was significantly larger for patients with TG
(4.6 cm) compared with those undergoing PG-TVT (3.5 cm).
 | Table ICharacteristics of the study
population. |
Table I
Characteristics of the study
population.
| Characteristics | PG-TVT (n=44) | TG (n=30) | P-valuea |
|---|
| Age (years) | 64 (45-79) | 62 (37-77) | 0.574 |
| Sex
(male/female) | 35/9 | 28/2 | 0.182 |
| BMI
(kg/m2) | 23.8 (17.3-28.4) | 24.5 (19.8-29.0) | 0.216 |
| Siewert (II/III) | 27/17 | 16/14 | 0.655 |
| Preoperative
chemotherapy | | | 0.336 |
|
Yes | 5 (11.4) | 6 (20.0) | |
|
No | 39 (88.6) | 24 (80.0) | |
| Previous abdominal
surgery | | | 0.336 |
|
Yes | 5 (11.4) | 6 (20.0) | |
|
No | 39 (88.6) | 24 (80.0) | |
| Tumor size
(cm) | 3.5 (0.6-10.0) | 4.6 (1.0-10.0) | <0.001 |
| T
stageb | | | 0.095 |
|
T1 | 13 (29.5) | 3 (10.0) | |
|
T2 | 8 (18.2) | 5 (16.7) | |
|
T3 | 7 (15.9) | 3 (10.0) | |
|
T4 | 16 (36.4) | 19 (63.3) | |
| N
stageb | | | 0.507 |
|
N0 | 27 (61.4) | 20 (66.6) | |
|
N1 | 5 (11.4) | 2 (6.7) | |
|
N2 | 5 (11.4) | 6 (20.0) | |
|
N3a/N3b | 7 (15.8) | 2 (6.7) | |
| M
stageb | | | 1.000 |
|
M0 | 44 (100.0) | 30 (100.0) | |
| Proximal resection
margin, cm | 4.6 (2.5-10.0) | 4.9 (2.0-10.0) | 0.796 |
| Distal resection
margin, cm | 7.4 (2.5-10.5) | 7.0 (2.0-15.0) | 0.093 |
| Histological
grading | | | 1.000 |
|
Well-differentiated | 6 (13.6) | 4 (13.3) | |
|
Moderately
differentiated | 21 (47.7) | 14 (46.7) | |
|
Poorly
differentiated | 17 (38.6) | 12 (40.0) | |
| Postoperative
adjuvant chemotherapy | | | 0.210 |
|
Yes | 28 (63.6) | 24 (80.0) | |
|
No | 16 (36.4) | 6 (20.0) | |
Surgical outcome
The operative and early postoperative outcomes of
patients undergoing PG-TVT and TG are shown in Table II. The mean operative time was
significantly shorter in the PG-TVT group (242.6 min) compared with
that in the TG group (288.1 min). There was no significant
difference in the estimated blood loss, transfusion, laparoscopy
assistance, extent of lymph node dissection or the number of
retrieved lymph nodes between the two groups. R0 resection was
performed in all patients and no fatalities were recorded. The
overall postoperative complication rate did not differ
significantly between the PG-TVT and TG groups (22.7 vs. 20.0%,
respectively; P=1.000), including the frequency of anastomotic
complications, infection and lymphatic fistula. The mean
postoperative hospital stay of the patients was shorter in the
PG-TVT group (16 days) compared with that in the TG group (17
days), but the difference was not statistically significant.
 | Table IISurgical outcomes of patients
undergoing PG-TVT and TG. |
Table II
Surgical outcomes of patients
undergoing PG-TVT and TG.
|
Characteristics | PG-TVT (n=44) | TG (n=30) |
P-valuea |
|---|
| Operative time
(min) | 242.6±55.3
(85-355) | 288.1±58.3
(210-470) | 0.002 |
| Estimated blood
loss (ml) | 239±208
(50-1200) | 297±214
(100-1000) | 0.100 |
| Transfusion | | | |
|
Yes | 2 (4.5) | 4 (13.3) | 0.215 |
|
No | 42 (95.5) | 26 (86.7) | |
| Laparoscopy
assistance | | | |
|
Yes | 41 (93.2) | 24 (80.0) | 0.146 |
|
No | 3 (6.8) | 6 (20.0) | |
| Lymph node
metastasis | | | |
|
Positive | 27 (61.4) | 20 (66.7) | 0.826 |
|
Negative | 17 (38.6) | 10 (33.3) | |
| No. of retrieved
lymph nodes | 32±13 (9-70) | 36±17 (18-87) | 0.585 |
| R0 resection | 44(100) | 30(100) | |
| Morbidity | 10 (22.7) | 6 (20.0) | 1.000 |
| Anastomotic
complications | 9 (20.5) | 5 (16.7) | |
|
Bleeding | 2 (4.5) | 2 (6.7) | |
|
Leakage | 3 (6.8) | 1 (3.3) | |
|
Stricture | 4 (9.1) | 2 (6.7) | |
| Infection | 1 (2.3) | 0 (0) | |
| Lymphatic
fistula | 0 | 1 (3.3) | |
| Mortality | 0 | 0 | |
| Postoperative
hospital stay (days) | 16±7 (8-41) | 17±7 (8-36) | 0.663 |
Follow-up and postoperative
nutritional status
All the patients were followed up for 6 months. None
of the patients developed cancer recurrence in distant organs,
gastric remnant, or lymph nodes. As regards the incidence of GERD
within 6 months after PG-TVT and TG, GERD was observed in 7 of the
44 PG-TVT patients (15.9%), compared with 4 of the 30 TG patients
(13.3%), but the difference was not statistically significant
(P=1.00).
The mean weight loss of the patients at 6 months was
1.9 kg in the TG group and 2.0 kg in the PG-TVT group, but the
difference was not statistically significant (P=0.743, data not
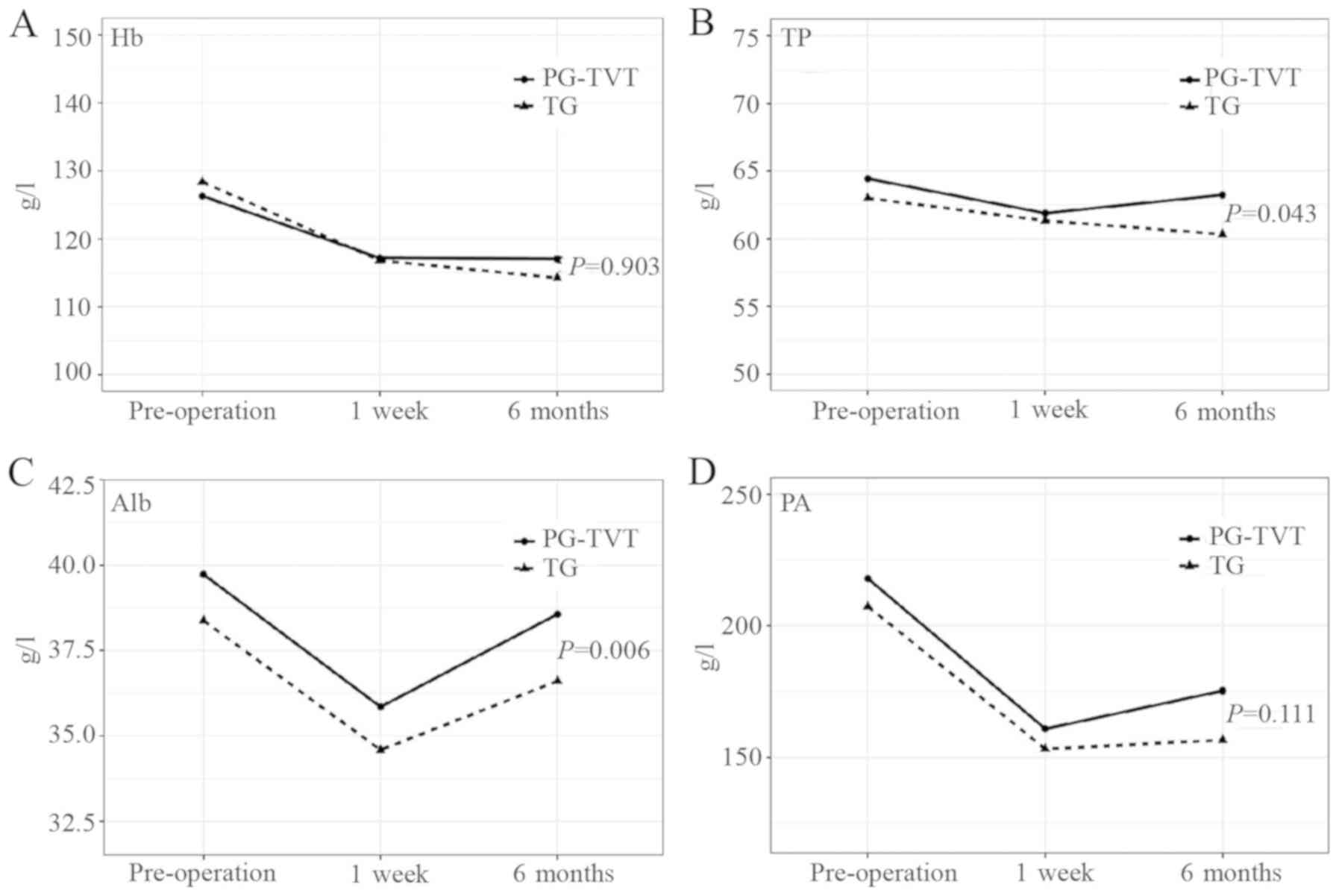
shown). The means of Hb, Alb, TP and PA at 3 timepoints
(pre-operatively, and at 1 week and 6 months postoperatively) in
the PG-TVT and TG groups are shown in Fig. 2. Adjusting the time effect and
interaction of time and surgical methods, the mean levels of TP and
Alb in 6 months were significantly higher in the PG-TVT compared
with those in the TG group. Furthermore, the level of TP was
significantly increased at 6 months in the PG-TVT group and
decreased in the TG group. The mean levels of Hb and PA at 6 months
were also higher in the PG-TVT group compared with those in the TG
group, but the difference was not statistically significant. As
shown in Fig. 2, the levels of all
the biomarkers decreased after surgery, but gradually increased
over 6 months in the PG-TVT group. However, the levels of all the
biomarkers, with the exception of Alb, decreased over 6 months in
the TG group. Detailed information is provided in supplementary
Table SI.
Discussion
In the present study, the operative time for PG-TVT
was markedly shorter compared with that for TG. The nutritional
status of patients in the PG-TVT group was superior to that of the
TG group. There were no significant differences between the two
groups in the frequency of complications, including reflux
esophagitis, and the postoperative weight loss at 6 months. Thus,
PG-TVT achieved a good clinical result in patients with AEG.
PG for patients with AEG is controversial. Important
considerations include curability and prognosis related to the
surgical treatment, as well as the development of complications and
postoperative quality of life. It was widely believed that PG
reduces postoperative weight loss (11) due to conserving half of the stomach
and achieves survival rates equivalent to those of TG (30). The reported incidence of anastomotic
leakage was 1.5-7.4% and that of stricture 3.4-21.2% after
gastrectomy (31,32). In the present study, the incidence
of leakage and stricture were also in this range. However, several
studies reported that PG was associated with a markedly higher
complication rate and the functional drawbacks of gastroesophageal
reflux, which substantially affects the quality of life, compared
with the TG (16-20).
However, the postoperative morbidity of the PG-TVT group did not
differ from that of the TG group in the present study.
The most common reported problem after PG is reflux.
Kim et al reported the rate of reflux esophagitis at 48%
after PG (33), and Katsoulis et
al reported that 100% of patients experienced reflux symptoms
after PG (15). In a recent study,
Hayami et al reported no severe reflux esophagitis observed
after the novel double-flap technique, LPG-DFT (22). In the LPG-DFT procedure,
valvuloplasty preserves the backflow prevention valve embedded
between the submucosal layer and the seromuscular flap of the
stomach. However, due to the complex valvuloplasty, it demands
masterful intracorporeal suturing and the operative time was
notably longer compared with that of the TG.
In the present study, TVT was applied in PG, and no
patients developed reflux esophagitis in the PG-TVT group in 6
months of follow-up, compared to 3 patients in the TG group. TVT
was designed based on the anti-reflux principle. The stomach wall
on the side of the lesser curvature was sewed into the stomach to
form a triangular valve, resembling the cardia, which prevents
gastric juice reflux through the narrow threshold. When the stomach
is dilated, the valve resembles a peaked hillock, which has
anti-reflux function. When the gastric fluid flows upwards,
according to physics principles, the collision with the peak
generates a vortex phenomenon, which greatly reduces the reflux and
also prevents the gastric juice from irritating the relatively
fragile anastomotic region. The procedure of TVT is simple and easy
to perform, and does not demand complex suturing skills; therefore,
the operative time for PG-TVT was markedly shorter compared with
that for TG.
In terms of the nutritional status, the
postoperative levels of TP and Alb were significantly higher in the
PG-TVT compared with those in the TG group. No significant
difference in Hb and PA was observed between the two groups.
However, the Hb level increased slightly within 6 months in the
PG-TVT group and decreased in the TG group, which is one of the
benefits of the presence of the gastric remnant. The levels of
several hormones, such as ghrelin and gastrin, decrease after
gastrectomy (34,35). However, the reduction in the serum
levels of vitamin B12 and these hormones is less notable in the
PG-TVT group. In addition, the patients' appetite improves due to
the lower incidence of GERD. These results indicate that PG-TVT had
important advantages compared with TG. Tanioka et al also
suggested that LPG may be more beneficial compared with LTG in
terms of perioperative and nutritional outcomes for early-stage
gastric cancer (23).
Although weight loss and other biomarkers did not
differ significantly between the two groups, there was a positive
trend observed in the PG-TVT group. These results are consistent
with those of other studies (22).
However, as the patients were only followed up for 6 months in the
present study, long-term follow-up evaluation is also required.
There were certain limitations to the present study.
First, this was a retrospective study with a small sample size that
was conducted in a single institution. However, the two operative
procedures were performed by the same surgical team in the same
institution. Clinicopathological and treatment factors, Siewert
type, degree of lymph node dissection and the degree of lymph node
involvement were similar between the two groups studied. Thus, the
bias from patients and surgeons were minimized. Second, a
randomized clinical trial with equivalent background
characteristics among the reconstructions after PG is required to
further analyze the advantages of PG-TVT. Third, as shown in
Table I, the maximum proximal
resection margins for both operations were 10 cm. The reason for
this is that the actual measurements demonstrated that the length
of the lesser curvature was ~22-28 cm, and the length of the
greater curvature was ~25-32 cm. The center of Siewert Ⅲ AEG is
located 2-5 cm below the dentate line. If the upward infiltration
distance of the tumor is not long, the resection distance of the
upper margin may be 10 cm, provided that sufficient residual
stomach (lesser curvature ≥10 cm) and a safe resection distance of
the upper margin are ensured. Another limitation was the relatively
short follow-up time. A longer follow-up period and more
nutritional indices should be included in future analyses.
In conclusion, PG-TVT has several advantages over TG
for patients with AEG, including a shorter operative time, better
postoperative nutritional status, with a similar incidence of GERD.
Further randomized clinical trials with a larger sample size are
required to fully investigate the comparative benefits of PG-TVT.
In addition, further evaluation of the patients' quality of life
and survival analysis compared with that after traditional TG
should be performed in future studies.
Supplementary Material
Comparison of nutritional biomarkers
between patients of the PG-TVT and TG groups.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
JS and YG performed the surgical procedures and
wrote the manuscript. YZ helped to analyze the data. YG, JS, YC,
YZ, PC, LZ, JHu, JHa and XC designed this study. YC performed a
linguistic review and editing of the manuscript. All the authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of the First Affiliated Hospital of Zhengzhou University.
All clinical samples were obtained from patients who had provided
written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brierley JD, Gospodarowicz MK and
Wittekind C (eds): TNM classification of malignant tumours. 8th
edition. Oxford, Wiley Blackwell, 2017.
|
|
2
|
Buas MF and Vaughan TL: Epidemiology and
risk factors for gastroesophageal junction tumors: Understanding
the rising incidence of this disease. Semin Radiat Oncol. 23:3–9.
2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Devesa SS, Blot WJ and Fraumeni JF Jr:
Changing patterns in the incidence of esophageal and gastric
carcinoma in the United States. Cancer. 83:2049–2053.
1998.PubMed/NCBI
|
|
4
|
Kim JJ: Epidemiology of gastroesophageal
junction adenocarcinoma in Korea. J Gastric Cancer. 18:328–338.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Blaser MJ and Saito D: Trends in reported
adenocarcinomas of the oesophagus and gastric cardia in Japan. Eur
J Gastroenterol Hepatol. 14:107–113. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhou Y, Zhang Z, Zhang Z, Wu J, Ren D, Yan
X, Wang Q, Wang Y, Wang H, Zhang J, et al: A rising trend of
gastric cardia cancer in Gansu Province of China. Cancer Lett.
269:18–25. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liang D, Liang S, Jin J, Li D, Shi J and
He Y: Gastric cancer burden of last 40 years in North China (Hebei
Province): A population-based study. Medicine (Baltimore).
96(e5887)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Siewert JR and Stein HJ: Classification of
adenocarcinoma of the oesophagogastric junction. Br J Surg.
85:1457–1459. 1998.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Isgüder AS, Nazli O, Tansug T, Bozdag AD
and Onal MA: Total gastrectomy for gastric carcinoma.
Hepatogastroenterology. 52:302–304. 2005.PubMed/NCBI
|
|
10
|
Harrison LE, Karpeh MS and Brennan MF:
Total gastrectomy is not necessary for proximal gastric cancer.
Surgery. 123:127–130. 1998.PubMed/NCBI
|
|
11
|
Katai H, Sano T, Fukagawa T, Shinohara H
and Sasako M: Prospective study of proximal gastrectomy for early
gastric cancer in the upper third of the stomach. Br J Surg.
90:850–853. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Shiraishi N, Adachi Y, Kitano S, Kakisako
K, Inomata M and Yasuda K: Clinical outcome of proximal versus
total gastrectomy for proximal gastric cancer. World J Surg.
26:1150–1154. 2002.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Solerio D, Camandona M, Gasparri G,
Casalegno PA and Dei Poli M: Adenocarcinoma of the cardia: Surgical
strategies compared. Tumori. 89 (4 Suppl):S143–S148.
2003.PubMed/NCBI
|
|
14
|
Aitaliev MS, Zemlianoi VP and
Nepomniashchaia SL: Evaluation of surgical traumaticity of standard
and extended surgical procedures in cancer of a proximal part of
the stomach. Khirurgiia. (Mosk):23–26. 2005.PubMed/NCBI(In Russian).
|
|
15
|
Katsoulis IE, Robotis JF, Kouraklis G and
Yannopoulos PA: What is the difference between proximal and total
gastrectomy regarding postoperative bile reflux into the
oesophagus? Dig Surg. 23:325–330. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
An JY, Youn HG, Choi MG, Noh JH, Sohn TS
and Kim S: The difficult choice between total and proximal
gastrectomy in proximal early gastric cancer. Am J Surg.
196:587–591. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ronellenfitsch U, Najmeh S, Andalib A,
Perera RM, Rousseau MC, Mulder DS and Ferri LE: Functional outcomes
and quality of life after proximal gastrectomy with
esophagogastrostomy using a narrow gastric conduit. Ann Surg Oncol.
22:772–779. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tokunaga M, Ohyama S, Hiki N, Hoshino E,
Nunobe S, Fukunaga T, Seto Y and Yamaguchi T: Endoscopic evaluation
of reflux esophagitis after proximal gastrectomy: Comparison
between esophagogastric anastomosis and jejunal interposition.
World J Surg. 32:1473–1477. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wen L, Chen XZ, Wu B, Chen XL, Wang L,
Yang K, Zhang B, Chen ZX, Chen JP, Zhou ZG, et al: Total vs
proximal gastrectomy for proximal gastric cancer: A systematic
review and meta-analysis. Hepatogastroenterology. 59:633–640.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Hsu CP, Chen CY, Hsieh YH, Hsia JY, Shai
SE and Kao CH: Esophageal reflux after total or proximal
gastrectomy in patients with adenocarcinoma of the gastric cardia.
Am J Gastroenterol. 92:1347–1350. 1997.PubMed/NCBI
|
|
21
|
Kamikawa Y, Kobayashi T and Kamiyama S: A
new procedure of esophagogastrostomy to prevent reflux following
proximalgastrectomy (in Japanese). Shoukakigeka. 24:1053–1060.
2001.
|
|
22
|
Hayami M, Hiki N, Nunobe S, Mine S, Ohashi
M, Kumagai K, Ida S, Watanabe M, Sano T and Yamaguchi T: Clinical
outcomes and evaluation of laparoscopic proximal gastrectomy with
double-flap technique for early gastric cancer in the upper third
of the stomach. Ann Surg Oncol. 24:1635–1642. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tanioka T, Waratchanont R, Fukuyo R, Saito
T, Umebayashi Y, Kanemoto E, Kobayashi K, Nakagawa M and Inokuchi
M: Surgical and nutritional outcomes of laparoscopic proximal
gastrectomy versus total gastrectomy: A meta-analysis. Surg Endosc.
34:1061–1069. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
In H, Solsky I, Palis B, Langdon-Embry M,
Ajani J and Sano T: Validation of the 8th edition of the AJCC TNM
staging system for gastric cancer using the national cancer
database. Ann Surg Oncol. 24:3683–3691. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Japanese Gastric Cancer Association.
Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric
Cancer. 14:113–123. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Giacopuzzi S, Bencivenga M, Weindelmayer
J, Verlato G and de Manzoni G: Western strategy for EGJ carcinoma.
Gastric Cancer. 20 (Suppl 1):S60–S68. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Di Leo A and Zanoni A: Siewert III
adenocarcinoma: Treatment update. Updates Surg. 69:319–325.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Aoki M, Saka M, Morita S, Fukagawa T and
Katai H: Afferent loop obstruction after distal gastrectomy with
Roux-en-Y reconstruction. World J Surg. 34:2389–2392.
2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shaw MJ, Talley NJ, Beebe TJ, Rockwood T,
Carlsson R, Adlis S, Fendrick AM, Jones R, Dent J and Bytzer P:
Initial validation of a diagnostic questionnaire for
gastroesophageal reflux disease. Am J Gastroenterol. 96:52–57.
2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Son MW, Kim YJ, Jeong GA, Cho GS and Lee
MS: Long-term outcomes of proximal gastrectomy versus total
gastrectomy for upper-third gastric cancer. J Gastric Cancer.
14:246–251. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kosuga T, Hiki N, Nunobe S, Ohashi M,
Kubota T, Kamiya S, Sano T and Yamaguchi T: Does the
single-stapling technique for circular-stapled esophagojejunostomy
reduce anastomotic complications after laparoscopic total
gastrectomy? Ann Surg Oncol. 22:3606–3612. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shim JH, Oh SI, Yoo HM, Jeon HM, Park CH
and Song KY: Short-term outcomes of laparoscopic versus open total
gastrectomy: A matched-cohort study. Am J Surg. 206:346–351.
2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kim JH, Park SS, Kim J, Boo YJ, Kim SJ,
Mok YJ and Kim CS: Surgical outcomes for gastric cancer in the
upper third of the stomach. World J Surg. 30:1870–1878.
2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ariyasu H, Takaya K, Tagami T, Ogawa Y,
Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, et al:
Stomach is a major source of circulating ghrelin, and feeding state
determines plasma ghrelin-like immunoreactivity levels in humans. J
Clin Endocrinol Metab. 86:4753–4758. 2001.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Castañeda TR, Tong J, Datta R, Culler M
and Tschöp MH: Ghrelin in the regulation of body weight and
metabolism. Front Neuroendocrinol. 31:44–60. 2010.PubMed/NCBI View Article : Google Scholar
|