Introduction
Prostate cancer is a significant health concern
worldwide. For instance, prostate cancer remains the most commonly
diagnosed solid organ tumor in the United States, with an estimated
161,360 new cases and 26,730 deaths in 2016(1). The incidence of prostate cancer is
also increasing in most Asian countries possibly due to growing
implementation of prostate-specific antigen (PSA) testing (2), with the morbidity ranking 4th among
Japanese men in 2014(3). Prostate
cancer comprises a wide spectrum of diseases with some tumors being
indolent and manageable with a monitoring strategy and others being
so aggressive that radical treatments are required to reduce the
risk of death from the disease (4).
Therefore, the ideal management of prostate cancer requires an
individualized treatment strategy. As a definitive diagnosis of
prostate cancer is usually made through needle biopsy of the
prostate gland, precise differential diagnosis on biopsy specimens
to distinguish indolent tumors from aggressive disease is a key to
offering proper management for individual patients to avoid over-
or undertreatment of prostate cancer (5).
Atypical small acinar proliferation (ASAP) is
defined as a lesion without an adequate amount of histologic atypia
to establish a definitive diagnosis of prostate adenocarcinoma
(6). ASAP was reported to be found
in 5-10% of needle biopsies of prostate glands (7). Previous studies showed that on
average, 40% of patients with ASAP on initial biopsy were found to
harbor adenocarcinoma on repeat biopsy (8,9).
Accordingly, the current US and European guidelines recommend
immediate repeat biopsy within 3-6 months after an initial
diagnosis of ASAP (10,11). However, the evidence on ASAP is
still limited, and no prospective study on ASAP has been reported
so far. Remarkably, the probability of cancer found on repeat
biopsy ranges from 20.0 to 71.6% (12-22).
Clinical and pathological predictors reported for cancer detection
on repeat biopsy after an ASAP diagnosis vary among studies
(7,12,16,18,23,24),
but these variations could result from the retrospective nature of
the studies. Of note, most of the studies lacked information on
factors that could influence patient selection for repeat biopsy
after a diagnosis of ASAP on initial biopsy, which would be crucial
to interpreting the results.
In general, recommendations from the guidelines are
not fully implemented in daily practice, where a variety of
factors, including physician's discretion and patient's preference,
influence clinical decisions. In this study, we first sought to
investigate practice patterns after a diagnosis of ASAP in a
real-world setting, focusing on factors that could affect patient
selection for repeat biopsy after the ASAP diagnosis. We also
analyzed clinical factors associated with the detection of cancer
on repeat biopsy after a diagnosis of ASAP and pathologically
characterized the cancer detected on repeat biopsy.
Materials and methods
Patient population
We retrospectively searched the departmental
database on pathological reports of prostate biopsy performed at
the Hyogo Prefectural Nishinomiya Hospital from January 2011 to
December 2016. From 1,218 initial prostate biopsies, we identified
97 patients with a diagnosis of ASAP without concomitant cancer.
All 97 patients were enrolled in this study. Clinical and
pathological information of the patients was collected from the
electronic medical record. This study was approved by the local
institutional research ethics board (H30-37).
Prostate biopsy
All patients underwent transperineal prostate biopsy
under the guidance of transrectal ultrasonography. Until March
2013, we performed biopsy with a standard 10-core template, but the
biopsy protocol was changed to a 12-core template in April 2014.
Additional targeted biopsy was carried out when a suspicious lesion
was pointed out by an institutional radiologist on pre-biopsy
magnetic resonance imaging. The diagnosis of ASAP and cancer was
confirmed by a single certified pathologist at our institution
throughout the study period. The presence of basal cells was
routinely assessed using 34βE12 immunostaining. A repeat biopsy was
performed based on the urologists' discretion and patients'
preference.
PSA kinetics
PSA kinetics including velocity and doubling time
were calculated using the web-based calculation tool of the
Memorial Sloan Kettering Cancer Center (25). All PSA values measured from the time
of initial biopsy to the time of repeat biopsy were used to
calculate PSA kinetics for patients with repeat biopsy. Patients
with a negative PSA doubling time were assigned a PSA doubling time
of 100 months so as to be included for the linear analyses
following preceding studies (26).
For comparison, PSA values measured within one year after initial
biopsy were used to calculate PSA kinetics for patients without
repeat biopsy because the median time from initial biopsy to repeat
biopsy in the patients with repeat biopsy was 354.5 days.
Statistical analysis
Continuous variables are presented as the median
value and range. Continuous and categorical variables were compared
using the Mann-Whitney U test and the Chi-square test,
respectively. Multivariate analysis was conducted by logistic
regression analysis. Variables were entered into multivariate
analysis if a P-value was <0.1 according to the univariate
analysis. A P-value <0.05 was considered statistically
significant. All statistical analyses were performed using JMP
version 11.0.0 (SAS Institute Inc.).
Results
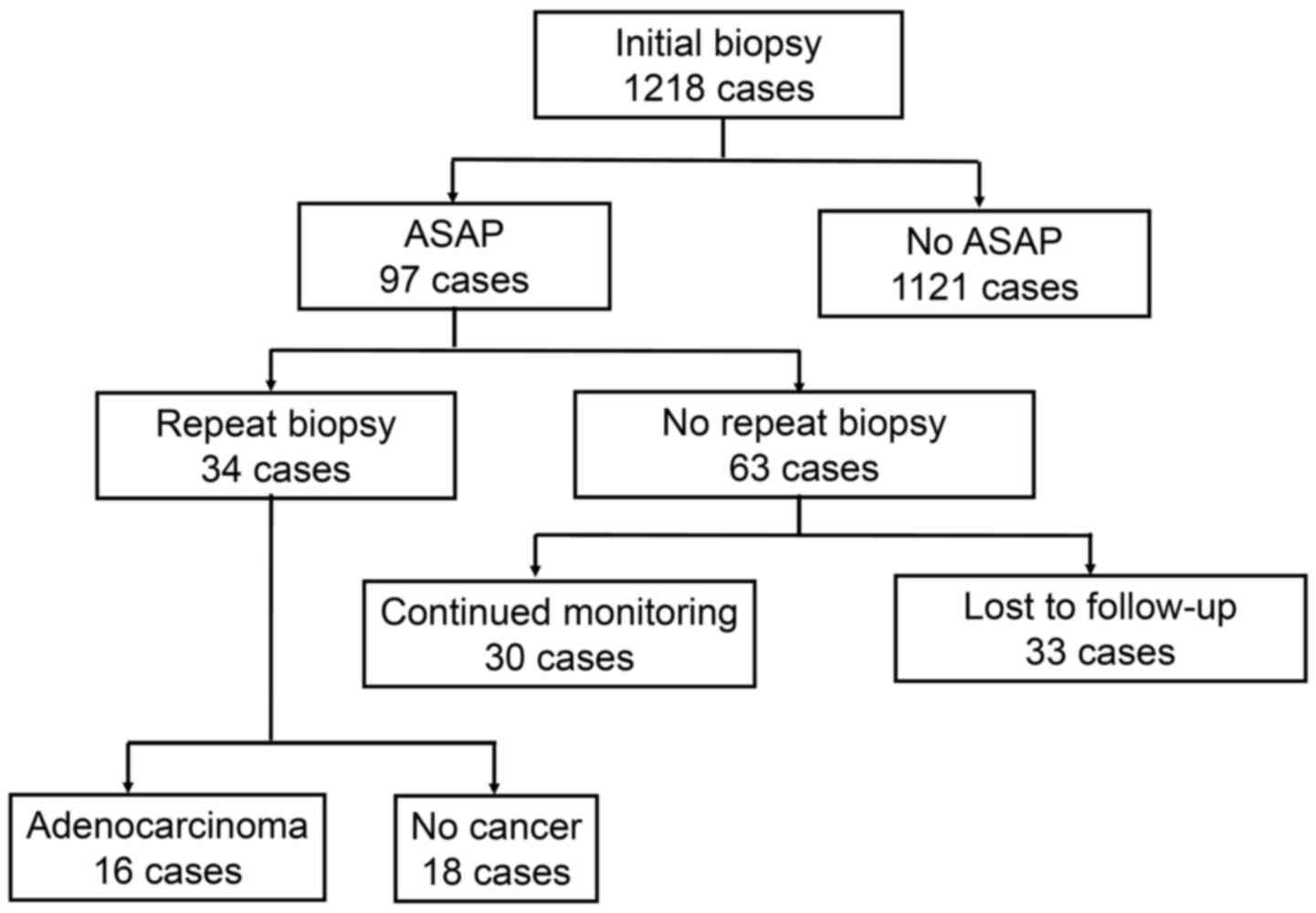
Fig. 1 shows a
diagrammatic representation of the clinical courses of the patients
who were diagnosed as having ASAP on initial biopsy of the
prostate. In total, 1,218 patients who received an initial biopsy
were identified, and 97 (8.0%) were diagnosed as having ASAP.
Thirty-four (35.1%) of these 97 patients underwent a repeat biopsy,
whereas the remaining 63 did not. Thirty of these 63 patients were
followed after an initial biopsy at our institution, whereas the
remaining 33 were either lost to follow up or were introduced to a
local clinic for follow-up. Of the 34 patients who received a
repeat biopsy, 16 (47.1%) were found to have prostate
adenocarcinoma.
Table I provides
clinical characteristics at the time of the repeat biopsy in the 34
patients receiving repeat biopsy. Because the median time to repeat
biopsy in these patients was 354.5 days, clinical characteristics
at one year after initial biopsy of the 30 patients without repeat
biopsy were also summarized in Table
I for comparison. Of the 34 and 30 patients with and without
repeat biopsy, 3 and 14 had declining PSA values over time,
respectively, and consequently had negative values of PSA velocity
and PSA doubling time. These 17 patients were assigned a PSA
doubling time of 100 months. Age, prostate volume, PSA value, and
PSA density were not significantly different between the patients
with and without repeat biopsy. Among the variables examined, PSA
velocity and PSA doubling time were significantly different between
the two groups (1.4 vs. -0.9 ng/ml/year and 29.3 vs. 100.0 months,
P=0.0002 and 0.0002, respectively) (Table I).
 | Table IDemographic and clinicopathological
characteristics of patients with and without repeat biopsy. |
Table I
Demographic and clinicopathological
characteristics of patients with and without repeat biopsy.
| Characteristic | Repeat biopsy
(n=34) | No repeat biopsy
(n=30) | P-valuea |
|---|
| Age, years median
(range) | 71.5 | (56-82) | 76.0 | (45-86) | 0.1149 |
| PSA, ng/ml median
(range) | 9.6 | (1.7-30.2) | 7.7 | (3.1-30.4) | 0.4432 |
| Volume, ml median
(range) | 38.0 | (20.3-72.1) | 43.8 | (22.0-85.0) | 0.3293 |
| PSAD, ng/ml/ml median
(range) | 0.21 | (0.03-1.02) | 0.18 | (0.05-0.50) | 0.2364 |
| Time to repeat
biopsy, days median (range) | 355 | (19-1994) | - | | |
| PSA velocity,
ng/ml/year median (range) | 1.4 | (-2.4-20.6) | -0.9 | (-20.0-3.9) | 0.0002 |
| PSA doubling time,
months median (range) | 29.3 | (6.4-105.5) | 100.0 | (16.2-694.3) | 0.0002 |
Next, we compared clinical characteristics of the
patients with and without cancer on repeat biopsy (Table II). Compared to the patients
without cancer, those with cancer had a significantly smaller
prostate volume (33.2 vs. 47.9 ml, P=0.0473) and higher PSA density
(0.28 vs. 0.17 ng/ml/ml, P=0.0324) on univariate analysis.
Multivariate analysis subsequently identified increasing age (OR
1.20; 95% CI, 1.02-1.50; P=0.0297) and small prostate volume (OR
0.92; 95% CI, 0.86-0.99; P=0.0250) to be significantly associated
with cancer detection on repeat biopsy (Table III).
 | Table IIClinical factors of cohorts with and
without cancer on repeat biopsy following a diagnosis of atypical
small acinar proliferation. |
Table II
Clinical factors of cohorts with and
without cancer on repeat biopsy following a diagnosis of atypical
small acinar proliferation.
| Factor | Cancer (n=16) | No cancer (n=18) | P-value |
|---|
| Age, years median
(range) | 73.0 | (65-82) | 71.5 | (56-76) | 0.1359a |
| PSA, ng/ml median
(range) | 9.2 | (4.5-29) | 9.5 | (1.7-30.2) | 0.6662a |
| Volume, ml median
(range) | 33.2 | (20.3-64.7) | 47.9 | (25.8-72.1) | 0.0473a |
| PSAD, ng/ml/ml median
(range) | 0.28 | (0.11-0.99) | 0.17 | (0.03-1.02) | 0.0324a |
| Time to repeat
biopsy, days median (range) | 413.5 | (50-1994) | 269.5 | (19-1231) | 0.1205a |
| Type of biopsy | | | | | |
|
Systematic | 5 | | 11 | | 0.0817b |
|
Systematic +
target | 11 | | 7 | | |
| PSA velocity,
ng/ml/year median (range) | 1.4 | (-2.4-7.8) | 1.1 | (-9.4-20.6) | 0.9781a |
| PSA doubling time,
months median (range) | 34.9 | (13.9-105.5) | 37.2 | (6.4-100.0) | 0.5000a |
 | Table IIIMultivariate logistic regression
analysis for the identification of factors associated with cancer
detected on repeat biopsy following a diagnosis of atypical small
acinar proliferation. |
Table III
Multivariate logistic regression
analysis for the identification of factors associated with cancer
detected on repeat biopsy following a diagnosis of atypical small
acinar proliferation.
| | Multivariate | |
|---|
| Variable | Univariate
P-value | OR | (95% CI) | P-value |
|---|
| Age, years | 0.0741 | 1.20 | (1.02-1.50) | 0.0297 |
| Volume, ml | 0.0437 | 0.93 | (0.86-0.99) | 0.0250 |
| PSA, ng/ml | 0.5244 | | | |
| PSAD, ng/ml/ml | 0.2181 | 0.70 | (0.01-105.01) | 0.8657 |
| PSA velocity,
ng/ml/year | 0.2500 | | | |
| PSA doubling time,
months | 0.6600 | | | |
Table IV summarizes
the pathological features of the 16 cases in which cancer was
detected on repeat biopsy. Of these 16 patients, 3 (18.7), 6
(37.5), 2 (12.5), and 5 (31.3%) had Gleason scores of 3+3, 3+4,
4+3, and 4+4, respectively. The number of positive cores was 1, 2,
and 3 in 7 (43.8), 3 (18.7), and 6 (37.5%) patients, respectively.
The maximal involvement of cancer in a positive core was <50 in
11 (68.7) and ≥50% in 5 (31.3%) patients, respectively.
 | Table IVPathological features of 16 cases in
which cancer was detected on repeat biopsy following a diagnosis of
atypical small acinar proliferation. |
Table IV
Pathological features of 16 cases in
which cancer was detected on repeat biopsy following a diagnosis of
atypical small acinar proliferation.
| Variable | n (%) |
|---|
| Gleason score |
|
3+3 | 3 (18.7) |
|
3+4 | 6 (37.5) |
|
4+3 | 2 (12.5) |
|
4+4 | 5 (31.3) |
| Number of positive
cores |
|
1 | 7 (43.8) |
|
2 | 3 (18.7) |
|
3 | 6 (37.5) |
| PSAD, n (%) |
|
≤0.15 | 3 (18.9) |
|
>0.15 | 13 (81.1) |
| Maximal involvement
of cancer in a positive core |
|
<50 | 11 (68.7) |
|
≥50 | 5 (31.3) |
Discussion
In this study, we found that patients with higher
PSA velocity and shorter PSA doubling time were more susceptible to
repeat biopsy among patients who were found to have ASAP on initial
biopsy and followed by urologists in a real-world practice. In this
cohort, 47.1% were found to have prostate adenocarcinoma on repeat
biopsy. Multivariate analysis found that small prostate volume and
increasing age were significantly associated with cancer detection
on repeat biopsy. Of the cancers found on repeat biopsy, 81.3% were
diagnosed as Gleason score greater than 6.
Describing the characteristics of patients both with
and without a repeat biopsy is important to proper interpretation
of the analysis results of repeat biopsy because the implementation
of a repeat biopsy should be biased in a retrospectively collected
cohort. However, only a few studies have mentioned possible factors
biasing patient selection for repeat biopsy after a diagnosis of
ASAP (12,14). One study reported that patients with
a repeat biopsy were younger than those without a repeat biopsy
(14), and the other reported that
patients with a repeat biopsy had a higher Charlson Comorbidity
Index score than patients without a repeat biopsy (12). In the present study, patients who
received a repeat biopsy had higher PSA velocity and shorter PSA
doubling time than those who did not. These data indicate that the
implementation of a repeat biopsy for patients with ASAP could be
affected by the patient's characteristics in a real-world setting
despite the current recommendation of the guidelines endorsing
immediate repeat biopsy within 3-6 months after an initial
diagnosis of ASAP (10,11). We need to take these biases into
consideration when interpreting the results in retrospective
studies on repeat biopsy after a diagnosis of ASAP.
In the current cohort, small prostate volume and
increasing age were statistically significant predictors for cancer
detection on repeat biopsy after a diagnosis of ASAP. This finding
is consistent with those in some of the preceding studies (16,18).
In general, increasing age is a well-established risk factor for
prostate cancer (11). As for
prostate volume, the standard systemic biopsy with a fixed number
of biopsy cores failed to detect cancer in large prostates
(24,27,28).
This could be one reason, therefore, that small prostate volume was
found to be a predictor for cancer detection in the present
analysis. Actually, the rate of cancer detection was reported to
rise as the number of biopsy cores was increased on repeat biopsy
for large prostates in patients with a previous diagnosis of ASAP
(21). The increasing number of
cores would contribute to the detection of cancer on repeat biopsy
after a diagnosis of ASAP, especially for patients with large
prostates.
The probability of Gleason score greater than 6,
which is considered high-grade cancer, on repeat biopsy after a
diagnosis of ASAP substantially varies from 8.0 to 66.7% among
different studies (12,14-17,19,21,22).
In the present cohort, 13 (81.3%) of the 16 cancers were diagnosed
as Gleason score greater than 6 on repeat biopsy after a diagnosis
of ASAP, which is higher than the rates in the preceding reports.
The high incidence of Gleason score greater than 6 in the current
cohort may partly be explained by interobserver variability
(7). Nakai et al reported
the issue of interobserver reproducibility of lesions in biopsy
specimens of prostate glands including ASAP (29). The concordance rate of Gleason
grading between general pathologists and urologic pathologists was
only 47.5%. Moreover, of the 23 biopsy specimens signed out as ASAP
by general pathologists, 4 (17.4) and 4 (17.4%) cancers were
diagnosed as Gleason score 6 and 7, respectively, on the review of
urologic pathologists. ASAP might have been underdiagnosed on
initial biopsy in the present cohort, leading to the high incidence
of Gleason grade greater than 6 on repeat biopsy after a diagnosis
of ASAP.
Limitations of the present study include its
retrospective and single-institutional nature and the small cohort,
which potentially would cause a degree of bias. The criteria for
the implementation of repeat biopsy, the methodology of biopsy, and
the follow-up protocol were not consistent throughout the study
period. Patients who received pre-biopsy magnetic resonance imaging
followed by the addition of targeted biopsy cores might have a
better chance to be diagnosed as having cancer, which would affect
the rate of cancer detection. Nonetheless, the present study
reflects clinical practice after a diagnosis of ASAP in a
real-world setting, in which physicians' discretion and patients'
preference play a crucial role in the decision-making process.
Considering that no prospective study on ASAP has been available so
far, well-defined prospective studies need to be conducted to
establish the optimal management strategies for patients with a
diagnosis of ASAP.
In conclusion, patients with repeat biopsy had
higher PSA velocity and shorter PSA doubling time than patients
without repeat biopsy after a diagnosis of ASAP on initial biopsy
in a real-world setting. Small prostate volume and increasing age
were identified as statistically significant predictors of cancer
detection on repeat biopsy after a diagnosis of ASAP. Consideration
of prostate volume and age would aid in the decision-making process
to perform repeat biopsy in patients with high PSA velocity and
short PSA doubling time after a diagnosis of ASAP.
Acknowledgements
The authors would like to thank Dr Kazumasa Oka
(Department of Pathology, Hyogo Prefectural Nishinomiya Hospital)
for the pathological evaluation of specimens.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TI, TY, and KN conceived and designed the current
study. TI and TY performed the statistical analysis and wrote the
manuscript. TI, TY, AT, KY and HK acquired, analyzed and
interpreted the data, and wrote and critically revised the
manuscript for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Board of the Hyogo Prefectural Nishinomiya Hospital
(approval no. H30-37). Due to the retrospective nature of the
study, informed consent was waived per protocol.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kimura T and Egawa S: Epidemiology of
prostate cancer in Asian countries. Int J Urol. 25:524–531.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Saishin Gan Toukei (The Latest Cancer
Statistics in Japan). https://ganjoho.jp/reg_stat/statistics/stat/summary.html.
Accessed Jul 1, 2019.
|
|
4
|
Litwin MS and Tan HJ: The diagnosis and
treatment of prostate cancer: A review. JAMA. 317:2532–2542.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Romero-Otero J, García-Gómez B,
Duarte-Ojeda JM, Rodríguez-Antolín A, Vilaseca A, Carlsson SV and
Touijer KA: Active surveillance for prostate cancer. Int J Urol.
23:211–218. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Montironi R, Scattoni V, Mazzucchelli R,
Lopez-Beltran A, Bostwick DG and Montorsi F: Atypical foci
suspicious but not diagnostic of malignancy in prostate needle
biopsies (also referred to as ‘atypical small acinar proliferation
suspicious for but not diagnostic of malignancy’). Eur Urol.
50:666–674. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Epstein JI and Herawi M: Prostate needle
biopsies containing prostatic intraepithelial neoplasia or atypical
foci suspicious for carcinoma: Implications for patient care. J
Urol. 175:820–834. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Scattoni V, Roscigno M, Freschi M, Dehò F,
Raber M, Briganti A, Fantini G, Nava L, Montorsi F and Rigatti P:
Atypical small acinar proliferation (ASAP) on extended prostatic
biopsies: Predictive factors of cancer detection on repeat
biopsies. Arch Ital Urol Androl. 77:31–36. 2005.PubMed/NCBI
|
|
9
|
Borboroglu PG, Sur RL, Roberts JL and
Amling CL: Repeat biopsy strategy in patients with atypical small
acinar proliferation or high grade prostatic intraepithelial
neoplasia on initial prostate needle biopsy. J Urol. 166:866–870.
2001.PubMed/NCBI
|
|
10
|
Carroll PR, Parsons JK, Andriole G,
Bahnson RR, Barocas DA, Catalona WJ, Dahl DM, Davis JW, Epstein JI,
Etzioni RB, et al: Prostate cancer early detection, version 1.2014
Featured updates to the NCCN guidelines. J Natl Compr Canc Netw.
12:1211–1219. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Heidenreich A, Bastian PJ, Bellmunt J,
Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T,
Zattoni F, et al: EAU guidelines on prostate cancer. Part 1:
Screening, diagnosis, and local treatment with curative
intent-update 2013. Eur Urol. 65:124–137. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ynalvez LA, Kosarek CD, Kerr PS, Mahmoud
AM, Eyzaguirre EJ, Orihuela E, Sonstein JN and Williams SB:
Atypical small acinar proliferation at index prostate biopsy:
Rethinking the re-biopsy paradigm. Int Urol Nephrol. 50:1–6.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Leone L, Lacetera V, Montironi R, Cantoro
U, Conti A, Sbrollini G, Quaresima L, Mariani L, Muzzonigro G and
Galosi AB: Biopsy follow-up in patients with isolated atypical
small acinar proliferation (ASAP) in prostate biopsy. Arch Ital
Urol Androl. 86:332–335. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Leone A, Rotker K, Butler C, Mega A, Li J,
Amin A, Schiff SF, Pareek G, Golijanin D and Renzulli JF II:
Atypical small acinar proliferation: Repeat biopsy and detection of
high grade prostate cancer. Prostate Cancer.
2015(810159)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Leone A, Gershman B, Rotker K, Butler C,
Fantasia J, Miller A, Afiadata A, Amin A, Zhou A, Jiang Z, et al:
Atypical small acinar proliferation (ASAP): Is a repeat biopsy
necessary ASAP? A multi-institutional review. Prostate Cancer
Prostatic Dis. 19:68–71. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Warlick C, Feia K, Tomasini J, Iwamoto C,
Lindgren B and Risk M: Rate of Gleason 7 or higher prostate cancer
on repeat biopsy after a diagnosis of atypical small acinar
proliferation. Prostate Cancer Prostatic Dis. 18:255–259.
2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Koca O, Calışkan S, Oztürk Mİ, Güneş M and
Karaman MI: Significance of atypical small acinar proliferation and
high-grade prostatic intraepithelial neoplasia in prostate biopsy.
Korean J Urol. 52:736–740. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ryu JH, Kim YB, Lee JK, Kim YJ and Jung
TY: Predictive factors of prostate cancer at repeat biopsy in
patients with an initial diagnosis of atypical small acinar
proliferation of the prostate. Korean J Urol. 51:752–756.
2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Srirangam V, Rai BP, Abroaf A, Agarwal S,
Tadtayev S, Foley C, Lane T, Adshead J and Vasdev N: Atypical small
acinar proliferation and high grade prostatic intraepithelial
neoplasia: Should we be concerned? An observational cohort study
with a minimum follow-up of 3 years. Curr Urol. 10:199–205.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cool DW, Romagnoli C, Izawa JI, Chin J,
Gardi L, Tessier D, Mercado A, Mandel J, Ward AD and Fenster A:
Comparison of prostate MRI-3D transrectal ultrasound fusion biopsy
for first-time and repeat biopsy patients with previous atypical
small acinar proliferation. Can Urol Assoc J. 10:342–348.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Aglamis E, Kocaarslan R, Yucetas U, Toktas
G, Ceylan C, Doluoglu OG and Unluer E: How many cores should be
taken in a repeat biopsy on patients in whom atypical small acinar
proliferation has been identified in an initial transrectal
prostate biopsy? Int Braz J Urol. 40:605–612. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Merrick GS, Galbreath RW, Bennett A,
Butler WM and Amamovich E: Incidence, grade and distribution of
prostate cancer following transperineal template-guided mapping
biopsy in patients with atypical small acinar proliferation. World
J Urol. 35:1009–1013. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Scattoni V, Roscigno M, Freschi M,
Briganti A, Fantini GV, Bertini R, Salonia A, Montorsi F and
Rigatti P: Predictors of prostate cancer after initial diagnosis of
atypical small acinar proliferation at 10 to 12 core biopsies.
Urology. 66:1043–1047. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ficarra V, Novella G, Novara G, Galfano A,
Pea M, Martignoni G and Artibani W: The potential impact of
prostate volume in the planning of optimal number of cores in the
systematic transperineal prostate biopsy. Eur Urol. 48:932–937.
2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Memorial Sloan Kettering Cancer Center,
PSA Doubling Time. https://www.mskcc.org/nomograms/prostate/psa_doubling_time.
Accessed Aug 1, 2019.
|
|
26
|
Paller CJ, Olatoye D, Xie S, Zhou X,
Denmeade SR, Eisenberger MA, Antonarakis ES, Carducci MA and Rosner
GL: The effect of the frequency and duration of PSA measurement on
PSA doubling time calculations in men with biochemically recurrent
prostate cancer. Prostate Cancer Prostatic Dis. 17:28–33.
2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Letran JL, Meyer GE, Loberiza FR and
Brawer MK: The effect of prostate volume on the yield of needle
biopsy. J Urol. 160:1718–1721. 1998.PubMed/NCBI
|
|
28
|
Leibovici D, Shilo Y, Raz O, Stav K,
Sandbank J, Segal M and Zisman A: Is the diagnostic yield of
prostate needle biopsies affected by prostate volume? Urol Oncol.
31:1003–1005. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nakai Y, Tanaka N, Shimada K, Konishi N,
Miyake M, Anai S and Fujimoto K: Review by urological pathologists
improves the accuracy of Gleason grading by general pathologists.
BMC Urol. 15(70)2015.PubMed/NCBI View Article : Google Scholar
|