Introduction
The outlook for patients with metastatic melanoma
has been transformed in the last few years, with >40% of
patients treated with combination checkpoint inhibitor therapy or
combination targeted therapy, in case of BRAF-mutated melanoma, in
clinical trials remaining alive after 5 years (1). Novel therapies may hold promise in
terms of durable remissions, but the majority of the patients with
melanoma experience progression, and some require chemotherapy.
Dacarbazine chemotherapy has been the mainstay of
melanoma treatment for >30 years. However, the objective
response (OR) rate was in the order of 10-20%, and median survival
was not prolonged (2,3), although some patients experienced
symptomatic relief, albeit at the cost of limited toxicity. There
was no standard second-line therapy, and the recommended approach
was clinical trials. Patients who were unable or unwilling to enter
a trial, were considered as possible candidates for chemotherapy.
In the early 2000s, carboplatin (with or without other agents, such
as paclitaxel) was the most commonly used second-line agent in the
UK (4). The aim of the present
study was to report the response rate to second-line carboplatin in
patients with melanoma from three UK institutions who were
previously treated and failed to respond to dacarbazine, and
examine whether sequential therapy may be more effective compared
with combination therapy. This may apply to the incorporation of
the newer targeted and immunotherapy treatments available since
this study was commenced.
Patients and methods
Patients and approval
Lists of patients treated with carboplatin for
metastatic melanoma were obtained from chemotherapy electronic
databases at three tertiary referral cancer centres, namely St.
George's Hospital (London), St. James's University Hospital (Leeds)
and Weston Park Hospital (Sheffield). The periods covered by the
analysis were October 2005-January 2011 for Leeds and Sheffield,
and November 2009-September 2015 for St. George's Hospital in
London. Permission to perform the analysis was granted by the local
committees of all three hospitals.
Statistical analysis
Demographic disease-related and treatment data were
extracted from electronic patient records and entered into an Excel
spreadsheet. Data were analysed using Graph Pad Prism software,
version 8.0 (GraphPad Software, Inc.).
Results
Patients and treatment
A total of 104 patients were identified (49 from St.
George's Hospital, 35 from St. James's Hospital and 20 from Weston
Park Hospital). The patient characteristics are summarised in
Table I. The majority of the
patients were treated with carboplatin (area under the curve 5-6)
every 3 weeks for a maximum of 6 cycles. Carboplatin was
administered as second-line treatment after documented disease
progression (no planned switch).
 | Table IPatient demographics prior to
commencing treatment with carboplatin (n=104). |
Table I
Patient demographics prior to
commencing treatment with carboplatin (n=104).
| Variables | No. |
|---|
| Median age, years
(range) | 61 (23-89) |
| Eastern Cooperative
Oncology | |
| Group performance
status | |
|
0 | 48 |
|
1 | 28 |
|
2 | 13 |
|
3 | 3 |
|
Unknown | 12 |
| Primary melanoma
location | |
|
Skin | 91 |
|
Mucosal | 2 |
|
Ocular | 9 |
|
Unknown | 2 |
| American Joint
Committee on Cancer stage (version 7) | |
|
III | 16 |
|
IV | 88 |
| Baseline lactate
dehydrogenase | |
|
Normal | 48 |
|
Elevated | 52 |
|
Not
recorded | 4 |
| First-line
regimen | |
|
Single-agent
dacarbazine | 85 |
|
Temozolomide | 4 |
|
Dacarbazine
in combination with other agents | 15 |
| Best response to
first-line treatment | |
|
Complete
response | 0 |
|
Partial
response | 12 |
|
Stable
disease | 14 |
|
Progressive
disease | 76 |
|
Not
recorded | 2 |
Response to treatment
A total of 102 patients were evaluable for response;
11 patients had an OR [complete response (CR), n=1; partial
response (PR), n=10], and 15 had stable disease (SD), with an OR of
11% and disease control rate (CR + PR + SD) of 26%. Treatment was
generally well-tolerated, with 31 patients requiring at least one
dose reduction, and 8 patients discontinuing treatment due to side
effects.
Survival
Progression-free-survival (PFS) data were available
for all the patients. Overall survival (OS) data were available for
102 patients. All data were censored at 36 months. A total of 3
patients remained alive at the time of analysis. The median PFS was
1.8 months (range, 0.2-36+ months) and the median OS was 4.6 months
(range, 0.2-36+ months). Patients with an Eastern Cooperative
Oncology Group (ECOG) performance status of 0-1 had a longer PFS
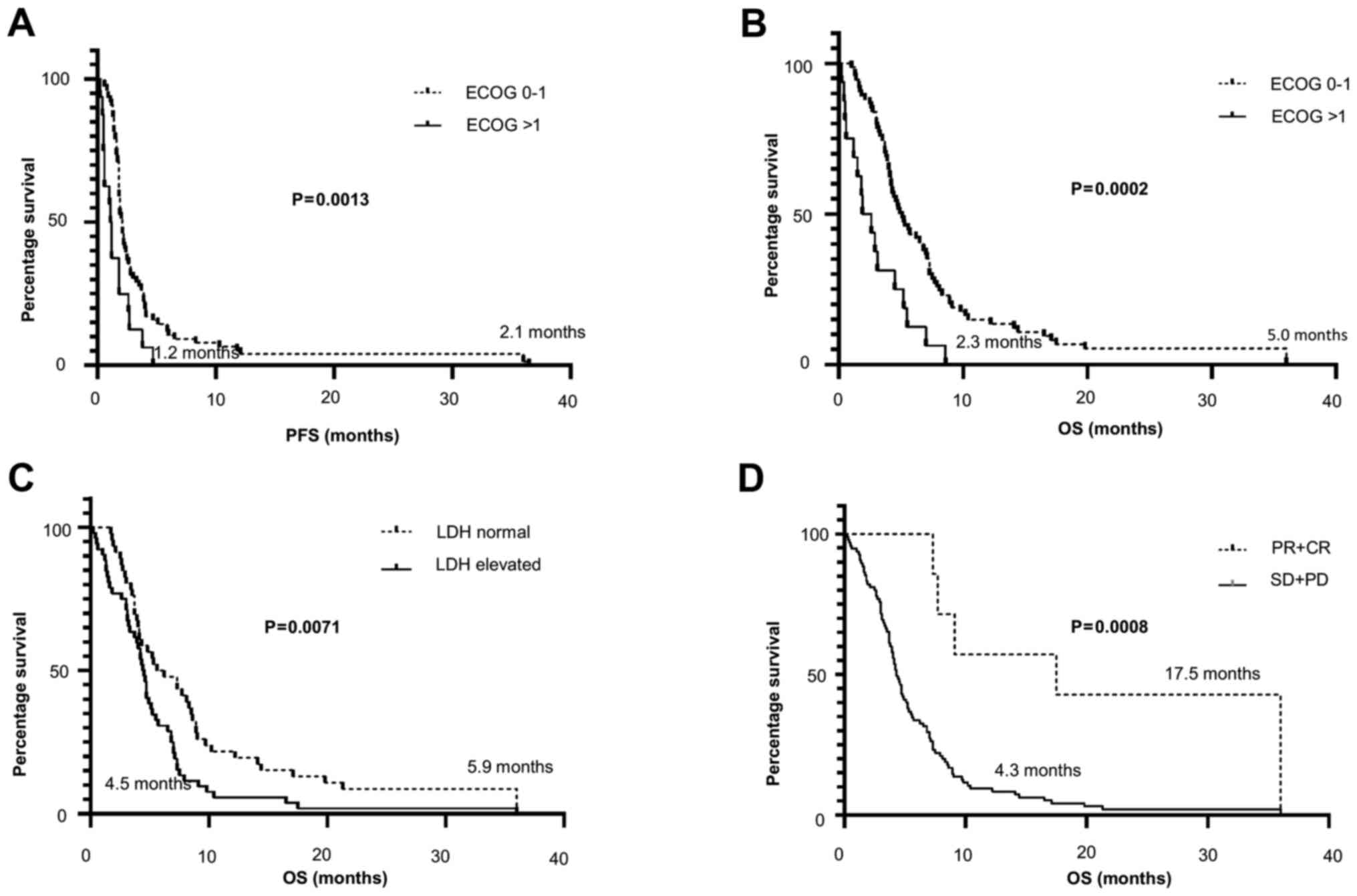
(2.1 vs. 1.2 months; P=0.0013 log-rank test; Fig. 1A) and extended median OS (5.0 vs.
2.3 months; P=0.0002; Fig. 1B).
Patients with normal lactate dehydrogenase (LDH) levels prior to
commencing treatment with carboplatin also had a longer median OS
(5.9 vs. 4.5 months; P<0.0071; Fig.
1C). Patients who achieved an OR to treatment had a longer
median OS (4.3 vs. 17.5 months; P=0.0008; Fig. 1D) compared with non-responders.
Discussion
The outlook for patients with metastatic melanoma
has improved greatly in the last decade. The anti-CTLA-4 monoclonal
antibody ipilimumab was the first drug to achieve prolongation of
survival in patients with metastatic melanoma (5). Further improvements have been observed
with the introduction of the anti-programmed cell death protein 1
(PD1) antibodies pembrolizumab and nivolumab, which are more
effective and less toxic compared with ipilimumab, and with the
combination of ipilimumab and nivolumab, which achieved an OR rate
of 58% (6). Patients whose
melanomas harbour BRAF mutations also have the option of treatment
with BRAF inhibitors (vemurafenib or dabrafenib), or with
combinations of BRAF and MEK inhibitors (dabrafenib with
trametinib, or encorafenib with binimetinib). The OR with
single-agent BRAF inhibitor was ~55%, compared with 68% in patients
on combination therapy (7). Over
half of the patients treated with combination therapies remained
alive at 3 years (6,7).
Despite these advances, it is likely that most
patients with metastatic melanoma will relapse. A number of
patients are not suitable for combination therapy, others are
unable to receive immunotherapy due to pre-existing autoimmune
conditions, and 60% of melanomas are wild-type for BRAF (8). These patients may still require
treatment, and it is therefore important to assess pre-existing
therapies and evaluate previous experience. To the best of our
knowledge, the present review of 104 consecutive patients in three
centres who were treated with carboplatin for metastatic melanoma
is the largest such series to date. In our cohorts of patients,
response and disease stabilisation were observed, with no
unexpected complications. Patients with an ECOG performance status
of 0-1, normal levels of LDH, and those who achieved an OR,
exhibited a significantly longer OS, in keeping with the data in
the literature (9,10). Surprisingly, the majority of the
patients who benefited from second-line carboplatin therapy were
those with visceral distant metastases, the survival of whom would
not be expected to exceed 6 months after first-line treatment.
Dacarbazine plus cisplatin or carboplatin, when given together,
appear to have no synergistic benefit (11), and it is unclear whether dacarbazine
exerts any significant effect on the susceptibility of tumour cells
to carboplatin, or whether there is an indirect effect on the
microenvironment or the immune response. It is therefore of great
interest that a small but true benefit was observed with the
sequential use of these agents, where the overall clinical benefit
of 26% is notably higher compared with that reported by several
first-line studies (2,3). It is particularly important, given
that there appears to be a concern that multiple drug combinations
may achieve better response rates, but do not affect the overall
outcome or survival. Of the 3 patients who remain alive and who had
an OR to treatment, 2 also received a course of low-dose
interleukin-2 post-carboplatin, which may also suggest a type of
synergy of the sequential treatment (12).
In conclusion, the observations of the present study
may provide a rationale for exploring the potential of carboplatin
in patients with failure of targeted treatment and immunotherapy,
and for other treatments to be used sequentially rather than
concurrently, where the only definitive outcome that can be
expected is enhanced toxicity.
Acknowledgements
Not applicable.
Funding
The work of AF is funded by the Institute for Cancer
Vaccines and Immunotherapy-ICVI (Registered Charity no.
1080343).
Availability of data and materials
The dataset used and analysed during the present
study is available from the corresponding author on reasonable
request.
Authors' contributions
MM and AGD conceived the original idea for the
manuscript. DH, LC, JD, EM, FT, SD and IV collected the data. AF
analysed the data. AGD and AF wrote the manuscript. All the authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Permission to perform the analysis was granted by
the Hospitals' Local Ethics Committees.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mario S: Long-term survival outcomes with
new treatments for advanced melanoma: Questions still in need of
answers (ASCO post) Available from: https://ascopost.com/issues/october-25-2019/long-term-survival-outcomes-with-new-treatments-for-advanced-melanoma/.
Accessed June 30, 2020.
|
|
2
|
Chapman PB, Einhorn LH, Meyers ML, Saxman
S, Destro AN, Panageas KS, Begg CB, Agarwala SS, Schuchter LM,
Ernstoff MS, et al: Phase III multicenter randomized trial of the
Dartmouth regimen versus dacarbazine in patients with metastatic
melanoma. J Clin Oncol. 17:2745–2751. 1999.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Middleton MR, Grob JJ, Aaronson N,
Fierlbeck G, Tilgen W, Seiter S, Gore M, Aamdal S, Cebon J, Coates
A, et al: Randomized phase III study of temozolomide versus
dacarbazine in the treatment of patients with advanced metastatic
malignant melanoma. J Clin Oncol. 18:158–166. 2000.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lorigan P, Marples M, Harries M, Wagstaff
J, Dalgleish AG, Osborne R, Maraveyas A, Nicholson S, Davidson N,
Wang Q, et al: Treatment patterns, outcomes, and resource
utilization of patients with metastatic melanoma in the U.K: The
MELODY study. Br J Dermatol. 170:87–95. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wolchok JD, Chiarion-Sileni V, Gonzalez R,
Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D,
Ferrucci PF, et al: Overall survival with combined nivolumab and
ipilimumab in advanced melanoma. N Engl J Med. 377:1345–1356.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Long GV, Stroyakovskiy D, Gogas H,
Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A,
Grob JJ, et al: Dabrafenib and trametinib versus dabrafenib and
placebo for Val600 BRAF-mutant melanoma: A multicentre,
double-blind, phase 3 randomised controlled trial. Lancet.
386:444–451. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kong BY, Carlino MS and Menzies AM:
Biology and treatment of BRAF mutant metastatic melanoma. Melanoma
Manag. 3:33–45. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Minor DR, Moore D, Kim C, Kashani Sabet M,
Venna SS, Wang W, Boasberg P and O'Day S: Prognostic factors in
metastatic melanoma patients treated with biochemotherapy and
maintenance immunotherapy. Oncologist. 14:995–1002. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hauschild A, Larkin J, Ribas A, Dréno B,
Flaherty KT, Ascierto PA, Lewis KD, McKenna E, Zhu Q, Mun Y and
McArthur GA: Modelled prognostic subgroups for survival and
treatment outcomes in BRAF V600-Mutated metastatic melanoma: Pooled
analysis of 4 randomized clinical trials. JAMA Oncol. 4:1382–1388.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jungnelius U, Ringborg U, Aamdal S,
Mattsson J, Stierner U, Ingvar C, Malmström P, Andersson R,
Karlsson M, Willman K, et al: Dacarbazine-vindesine versus
dacarbazine-vindesine-cisplatin in disseminated malignant melanoma.
A randomised phase III trial. Eur J Cancer. 34:1368–1374.
1998.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Legha SS, Ring S, Bedikian A, Plager C,
Eton O, Buzaid AC and Papadopulos N: Treatment of metastatic
melanoma with combined chemotherapy containing cisplatin,
vinblastine and dacarbazine (CVD) and biotherapy using
interleukin-2 and interferon-alpha. Ann Oncol. 7:827–835.
1996.PubMed/NCBI View Article : Google Scholar
|