Introduction
Precision medicine enables patients with cancer with
genetic alterations of driver oncogenes to receive effective
treatment. Patients with non-small cell lung cancer (NSCLC)
harboring mutations in the gene encoding epidermal growth factor
receptor (EGFR) dramatically respond to the initial administration
of EGFR tyrosine kinase inhibitors (TKIs). Unfortunately, tumors
become drug-resistant after approximately 1 year. The most
frequently encountered mechanism of resistance is associated with
the presence of the secondary mutation EGFR T790M. Another
major mechanism involves amplification of MET or HER2
and activation of bypass signaling by MET (1). Furthermore, adenocarcinoma (ADC) cells
may undergo transformation to a different phenotype. Most published
cases involve transformation from ADC to small cell lung cancer
(SCLC), and few cases convert from ADC to squamous cell carcinoma
(SqCC) (2,3). Moreover, histological transformation
during long-term treatment with EGFR-TKIs rarely occurs. Here we
report a patient who underwent surgery for an ADC with a deletion
of EGFR exon 19. The ADC underwent transformation to SqCC,
which was associated with long-term administration of two
EGFR-TKIs.
Case report
A 56-year-old female who never smoked underwent left
upper lobectomy and mediastinal lymph node dissection. She was
diagnosed with pathological stage IIIA (p-T1N2M0), invasive ADC
(acinar predominant) with an EGFR mutation (deletion of exon
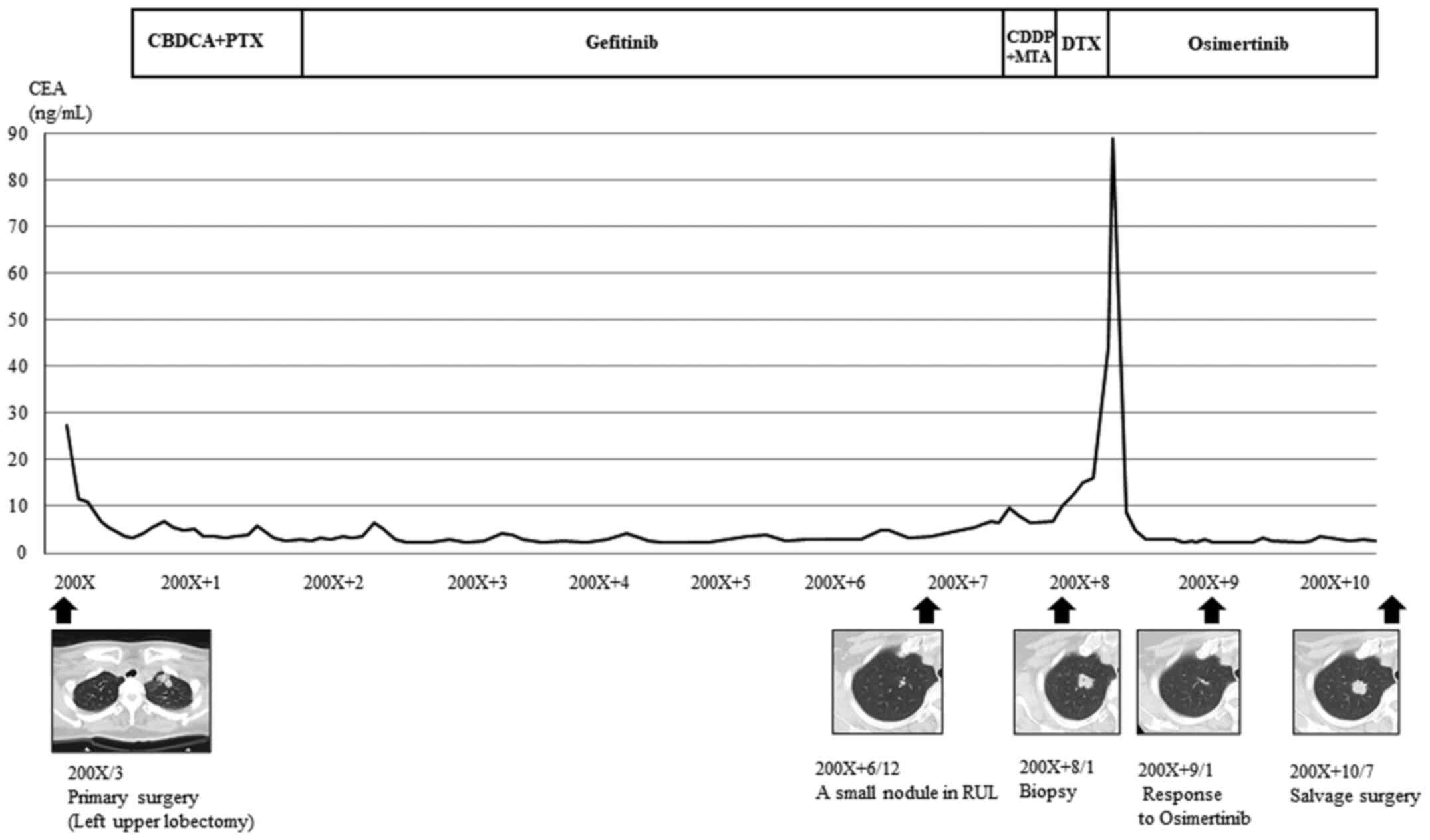
19). She subsequently received four cycles of platinum doublet
adjuvant chemotherapy, followed by gefitinib, for approximately 6
years because of chronic elevated concentrations of serum
carcinoembryonic antigen (CEA). Seven years after initial surgery,
a small nodule was detected in her right upper lobe that gradually
enlarged. Histopathology of a transbronchial tumor biopsy revealed
ADC and SqCC. The tumor harbored a secondary EGFR mutation
(T790M) as well as inherent sensitive mutation. After some
treatments, osimertinib was administered, and a partial response
was achieved without adverse effects.
After 2 years, computed tomography (CT) detected
growth of the tumor previously identified in the right upper lobe,
which we suspected had acquired resistance to osimertinib. Positron
emission tomography/CT showed active uptake of 2-deoxy-2-[18F]
fluoroglucose into the tumor. However, lesions in the mediastinal
and hilar lymph nodes indicating oligoprogression were not
detected. The patient underwent salvage surgery involving a right
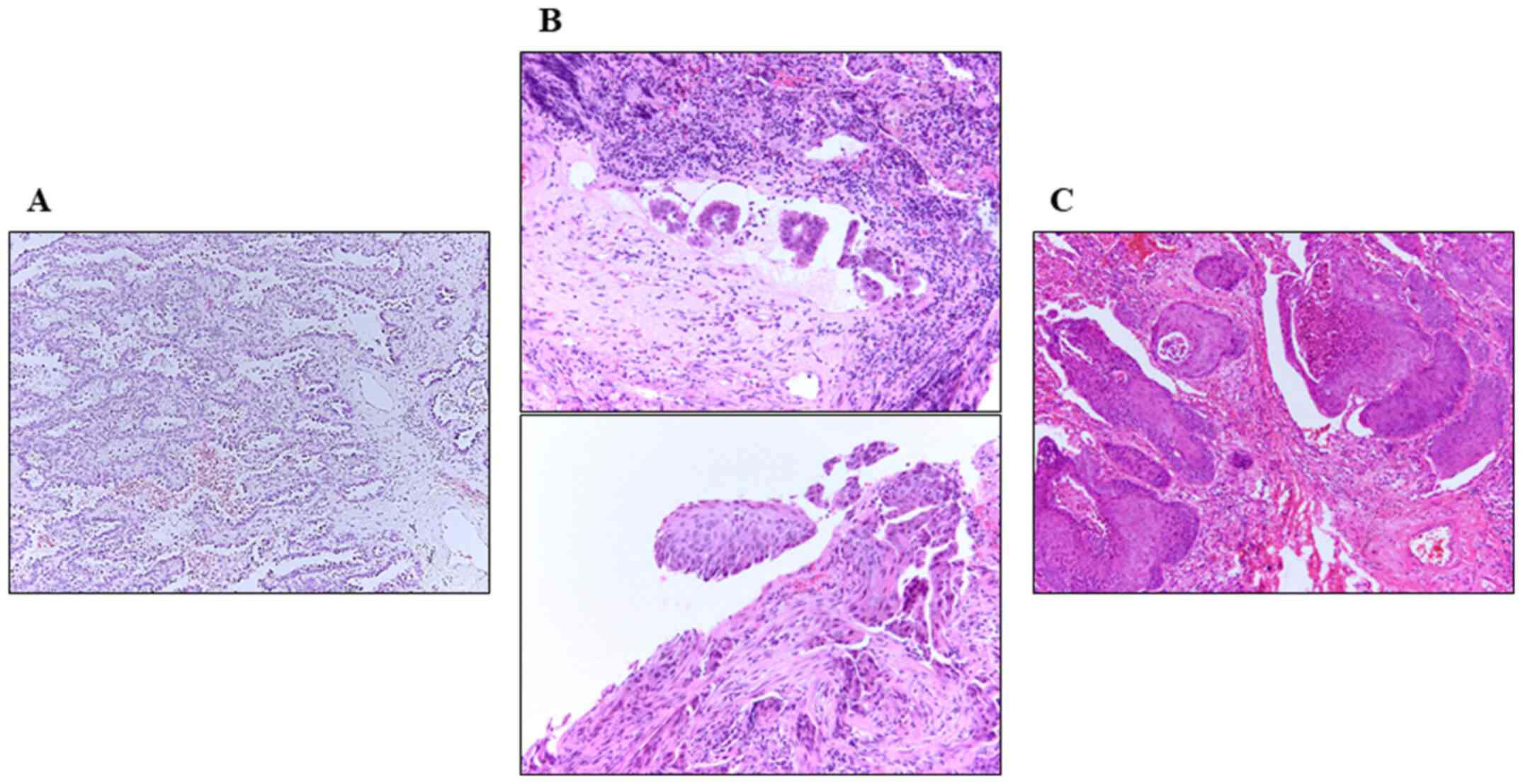
upper lobectomy and mediastinal lymph node dissection (Fig. 1). Histological examination detected
a keratinizing SqCC with mediastinal lymph node metastasis. There
was no evidence of an ADC cells in the tumors or metastatic lymph
nodes.
The serum levels of the tumor markers CEA and the
cytokeratin 19 fragment (SqCC marker) were not elevated.
Immunohistochemical analysis detected p63-positive tumor cells but
not TTF-1. The tumor was diagnosed as an SqCC harboring EGFR
T790M, indicating that it arose from the recurred ADC (Fig. 2). Moreover, MET amplification
was detected. The patient developed distant metastases in the
kidneys and para-aortic lymph nodes 6 months after salvage
surgery.
Discussion
Transformation from EGFR-mutated ADC to SCLC
occurs in 3 to 14% of patients with acquired resistance to initial
EGFR-TKI treatment (4).
Transformation of ADCs to SqCC is less common. For example, a
recent study (3) reported for the
first time, the transition from EGFR-mutated lung ADC to
SqCC after osimertinib treatment. This same study (3) reviewed reports describing the
development of an SqCC phenotype in 16 patients with
EGFR-mutated NSCLC who were treated with TKIs (3). According to their report, most of the
previous cases were diagnosed with affected lesions by limited
biopsy because of the advanced staged diseases, so there were some
possibilities of mixed tumor of ADC and SqCC in primary or
recurrent sites. In our case, the possibility could be denied
because the whole resected primary and recurrent tumors were
completely evaluated by two surgeries, and our comprehensive
analyses of these tumors clearly distinguish our studies from the
others.
Although a recent study presents in vitro and
in vivo evidence supporting the transdifferentiation of ADC
to SqCC associated with EGFR-mutated lung cancers treated
with TKIs (5), the underlying
mechanism is unknown and therefore requires further study. In
addition, the correlation between the duration of TKI treatment and
the histological transformation also remains unclear. Roca reported
that the TKI treatment times were from 4 to 69 months in the ADC
patients with SqCC transformation, suggesting that there was a poor
correlation between the treatment duration and the phenomenon
(3). Further accumulation of
similar cases is needed to clarify this point.
The few patients in which EGFR-mutated ADC
converts to SqCC during the administration of EGFR-TKIs hinders
development of a specific optimal treatment. For example, an
EGFR-mutated ADC undergoing transformation to SqCC with
undetectable EGFR T790M exhibited a durable response to
afatinib (6), suggesting the
possibility that the conversion to SqCC does not directly
contribute to acquired resistance to EGFR-TKIs. Thus, further
studies are required to determine if the conversion to SqCC is the
‘cause’ or ‘outcome’ of the development of resistance to
EGFR-TKIs.
MET amplification, which was newly detected
here in the recurrent tumor, may serve as a potential target of
therapy. MET amplification enhances the proliferation of
EGFR-mutated cultured NSCLC cells and increases the growth
of tumors and their metastasis in vivo (7). Furthermore, a MET inhibitor achieves
significant antitumor activity in patients with NSCLC with
MET amplification (8,9). These
findings suggest a strategy for developing effective
therapeutics.
Acknowledgements
The authors would like to thank Professor Yukihisa
Umekita for performing pathological diagnoses.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
TH treated the patient, acquired the data, performed
the literature review, and wrote the manuscript. YoK analyzed the
pathological findings. AN, SM, YaK, YTak, YTan and HN evaluated the
patient and participated in the therapy. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Tottori University, Faculty of Medicine (Tottori,
Japan) (grant no. 19A143).
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of data and materials.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nagano T, Tachihara M and Nishimura Y:
Mechanism of resistance to epidermal growth factor
receptor-tyrosine kinase inhibitors and a potential treatment
strategy. Cells. 7(212)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Oser MG, Niederst MJ, Sequist LV and
Engelman JA: Transformation from non-small-cell lung cancer to
small-cell lung cancer: Molecular drivers and cells of origin.
Lancet Oncol. 16:e165–e172. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Roca E, Pozzari M, Vermi W, Tovazzi V,
Baggi A, Amoroso V, Nonnis D, Intagliata S and Berruti A: Outcome
of EGFR-mutated adenocarcinoma NSCLC patients with changed
phenotype to squamous cell carcinoma after tyrosine kinase
inhibitors: A pooled analysis with an additional case. Lung Cancer.
127:12–18. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sequist LV, Waltman BA, Dias-Santagata D,
Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger
S, Cosper AK, et al: Genotypic and histological evolution of lung
cancers acquiring resistance to EGFR inhibitors. Sci Transl Med.
3(75ra26)2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hou S, Zhou S, Qin Z, Yang L, Han X, Yao S
and Ji H: Evidence, mechanism, and clinical relevance of the
transdifferentiation from lung adenocarcinoma to squamous cell
carcinoma. Am J Pathol. 187:954–962. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sato M, Matsui A, Shimoyama Y, Omote N,
Morise M, Hase T, Tanaka I, Suzuki K and Hasegawa Y: An
EGFR-mutated lung adenocarcinoma undergoing squamous cell carcinoma
transformation exhibited a durable response to Afatinib. Intern
Med. 57:3429–3432. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Baldacci S, Kherrouche Z, Cockenpot V,
Stoven L, Copin MC, Werkmeister E, Marchand N, Kyheng M, Tulasne D
and Cortot AB: MET amplification increases the metastatic spread of
EGFR-mutated NSCLC. Lung Cancer. 125:57–67. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Angevin E, Spitaleri G, Rodon J, Dotti K,
Isambert N, Salvagni S, Moreno V, Assadourian S, Gomez C, Harnois
M, et al: A first-in-human phase I study of SAR125844, a selective
MET tyrosine kinase inhibitor, in patients with advanced solid
tumours with MET amplification. Eur J Cancer. 87:131–139.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Drilon A, Cappuzzo F, Ou SI and Camidge
DR: Targeting MET in lung cancer: Will expectations finally be MET?
J Thorac Oncol. 12:15–26. 2017.PubMed/NCBI View Article : Google Scholar
|