Introduction
Oral squamous cell carcinoma (OSCC) is responsible
for 300,000 tumor cases per year (2012; 2.1% of all cancer
worldwide) and is a tumor entity that is one of the 10 most common
types of cancers worldwide (1). The
five-year survival rate has stagnated at around 40-50% (2). For this reason, new molecular
prognostic markers are urgently required to better estimate the
outcome of OSCC patients.
The epidermal growth factor receptor (EGFR) is a
transmembrane receptor with tyrosine kinase activity and regulates
cellular processes such as proliferation, metastasis, radio- and
chemoresistance (3).
EGFR is known to be overexpressed in a large number
of human tumors of epithelial origin (3) and is associated with the outcome of
tumor patients (4). The role of
EGFR in head and neck squamous cell carcinoma (HNSCC) was
extensively studied and the negative prognostic impact of EGFR
overexpression on local control and patient survival has been
described. Therefore, various therapies against EGFR are routinely
used for HNSCC patients (5).
However, the impact of the subcellular
EGFR-distribution on the prognosis is controversially discussed,
especially when data were derived from immunohistochemical analysis
in different OSCC patient cohorts (5-7).
A possible explanation for the contradictory data could be the
different function of e.g. membrane or cytoplasmatic/nuclear
localized EGFR.
Some authors have described that
cytoplasmatic/nuclear localized EGFR might be associated with a
higher proliferation rate in cancer cells (8,9).
In addition, it was found that especially an
increased level of nuclear EGFR was associated with a poor
prognosis of cancer patients (10).
A study examined the prognostic value of nuclear expression of EGFR
in tumor samples of OSCC patients. The authors found a nuclear EGFR
staining in 23 patients (28%) but no correlation with
clinicopathological factors or patient outcome (11).
Another possible reason for the ambiguous prognostic
influence of EGFR for OSCC patients could be the occurrence of
alternative EGFR isoforms, which could induce different signal
transduction pathways compared to full length EGFR (12,13).
Therefore, we investigated the level and the
localization (membranous/cytoplasmatic) of EGFR protein in OSCC
samples and their effects on patient's outcome.
Materials and methods
Tissue samples and histopathologic
data
We examined 45 paraffin-embedded tumor samples from
OSCC patients. All patients underwent surgery at the Department of
Oral and Maxillofacial Plastic Surgery at the University of
Halle-Wittenberg, Germany. The clinical and histomorphological
parameters of the cohort of OSCC patients are listed in Table I. The mean age of the patients was
57 years. Twenty eight patients (62%) died after an average of 16.1
months, and 17 OSCC patients (38%) were still alive after an
average of 60 months. The study was conducted in accordance with
the Helsinki Declaration and approved by the Ethics Committee of
the Medical Faculty of the University Halle-Wittenberg (ethic
number 2017-81 issued on June 27th, 2017). The human tissue samples
were collected between 1998 and 2002. All patients underwent
surgery according standardized regimens and gave their written
consent (14,15).
 | Table IClinicopathological data of patients
with oral squamous cell carcinoma. |
Table I
Clinicopathological data of patients
with oral squamous cell carcinoma.
| | | membEGFR protein
level | cytoEGFR protein
level | | |
|---|
| Category | Number of cases | Negative (IRS0-2),
n | Positive (IRS3-12),
n | P-value | Negative (IRS0-2),
n | Positive (IRS3-12),
n | P-value |
|---|
| Total | 45 | 36 | 9 | | 20 | 25 | |
| Sex | | | | | | | |
|
Male | 31 | 24 | 7 | | 13 | 18 | |
|
Female | 14 | 12 | 2 | 0.524 | 7 | 7 | 0.618 |
| Age, years | | | | | | | |
|
<50 | 11 | 10 | 1 | | 6 | 5 | |
|
≥50 | 34 | 26 | 8 | 0.303 | 14 | 20 | 0.443 |
| T-stage | | | | | | | |
|
I | 6 | 5 | 1 | | 2 | 4 | |
|
II | 17 | 13 | 4 | | 9 | 8 | |
|
III | 11 | 9 | 2 | | 2 | 9 | |
|
IV | 11 | 9 | 2 | 0.870 | 7 | 4 | 0.633 |
| N-stage | | | | | | | |
|
N0 | 19 | 14 | 5 | | 10 | 9 | |
|
N1-3 | 26 | 22 | 4 | 0.371 | 10 | 16 | 0.350 |
| Grading | | | | | | | |
|
1 | 3 | 2 | 1 | | 3 | 0 | |
|
2 | 18 | 16 | 2 | | 9 | 9 | |
|
3 | 24 | 18 | 6 | 0.501 | 8 | 16 | 0.058 |
Immunohistochemistry
The IHC staining procedure was applied as previously
described (15). Sections (4 µm) of
paraffin-embedded tissues were heated to 56˚C. Briefly, the tumor
slides were deparaffinized with xylol and transferred into a series
of ethanol dilution. The EGFR antibody (D38B1) (Cell Signaling
Technology Inc.), tested by us in a previous work (13,16),
and routinely established for IHC staining in the Institute of
Pathology (DRK Kliniken Berlin Westend), was used. The antibody was
diluted according to the manufactory specifications (1:50). The
staining (labeled streptavidin-biotin method) was performed by
using a standard protocol on a semiautomatic staining facility
(Ventana BenchMark; Roche Diagnostics). After staining, the
sections were counterstained with Mayer's hematoxylin. The staining
protocol followed a standard protocol of the Institute of Pathology
(DRK Kliniken Berlin Westend) which always include necessary
controls (omitting of the primary antibody). The stained slides
were evaluated by an experienced pathologist (MS) and reevaluated
by C.W. and D.B. using the Remmele Score system and taking into
account the location of staining (membranous or cytoplasmic)
(17). In detail: First the
percentage of positive cells was assigned as: 1-10% positive cells
as a score 1, 11-50% positive cells as a score 2; 51-80% positive
cells as a score 3 or >80% positive cells as a score 4.
Secondly, the staining intensity was scored as negative (1), moderate (2) or intense (3). Scores for the percentage of positive
cells and scores for the expression intensities were multiplied to
calculate the immunoreactive score [IRS according to Remmele and
Stegner (17)]: 0-2, no staining;
3-4, weak staining; 6-8, moderate staining; 9-12, strong staining
(15). The EGFR staining was
classified as i) membEGFR (20% positive 9/45, in detail n=36
negative, n=2 weak, n=7 moderate and n=0 strong staining), ii)
cytoEGFR (55.5% positive 25/45, in detail n=20 negative, n=12 weak,
n=12 moderate and n=1 strong staining). For survival analysis, the
cohort of OSCC patients was separated into two groups according to
the expression level of membranous or cytoplasmic EGFR as negative
(IRS 0-2) vs. positive staining (IRS 3-12).
Statistical analysis
The Cox's regression hazard model and Kaplan-Meier
analysis was used to estimate a correlation between EGFR protein
level and overall survival or relapse-free survival of OSCC
patients. The model was adjusted for the prognostic effects of
covariates (clinical T-stage, N-stage and grading of the tumor and
the sex and age of the patients). Correlation analysis was
performed using the Kruskal-Wallis test or the Spearman rank
correlation test. A probability (P) of <0.05 was defined as
significant and the relative risk (RR) was calculated as well as
the confidence interval (CI). The statistical analysis was
performed using SPSS software version 25.0 (SPSS Inc.).
Results
Prognostic effect of membranous EGFR
and cytoplasmatic EGFR on overall and relapse free survival
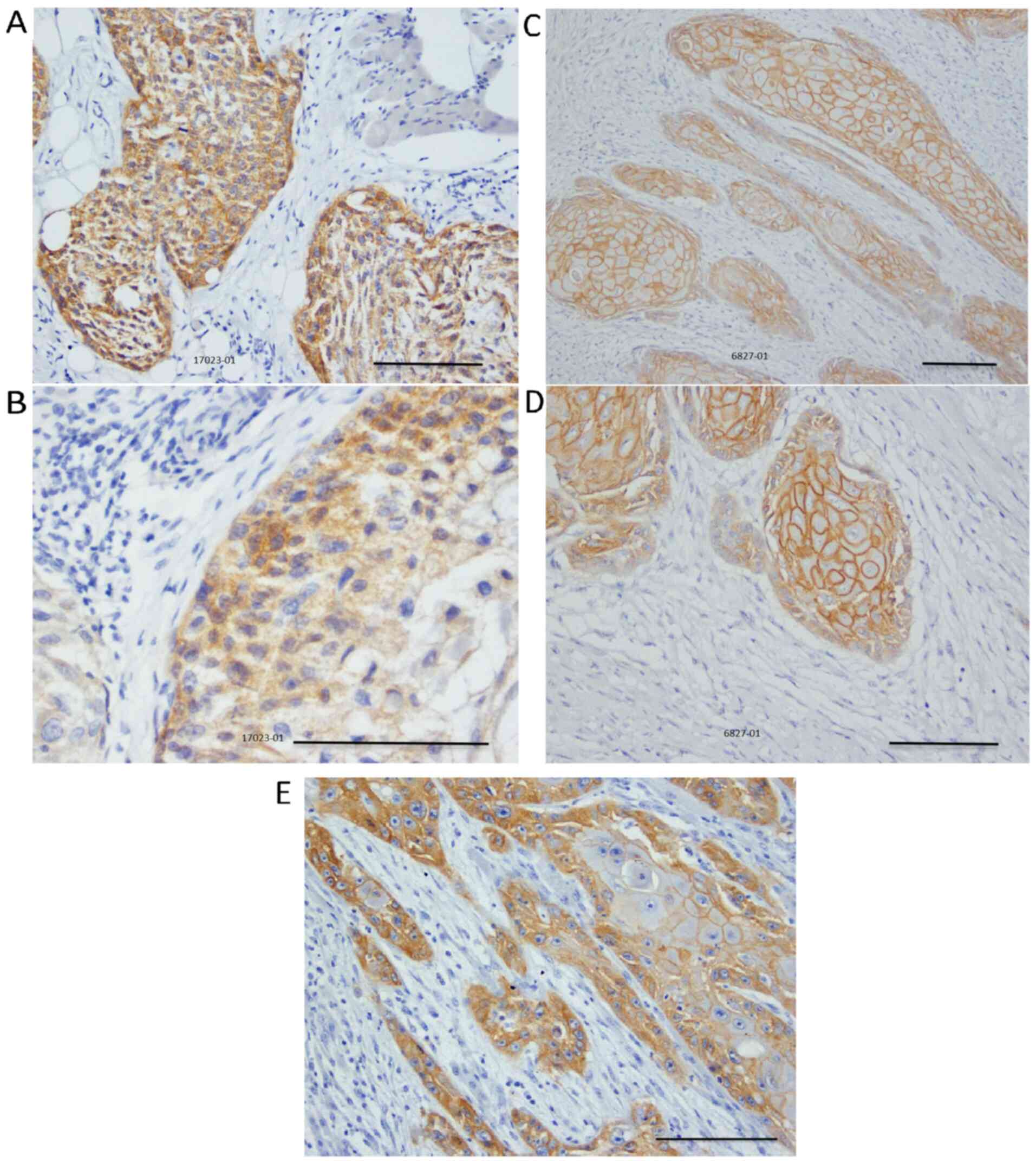
The EGFR staining i) membEGFR ii) cytoEGFR (Fig. 1) was separated into two groups [low
vs. median/high level (please see Materials and methods)].
By Kaplan-Meier analysis, we found that patients
with a positive cytoEGFR level died on average 13.5 months (P=0.03)
earlier than patients with a negative cytoEGFR protein localization
(Table I). The membEGFR level had
no significant correlation with the prognosis in the Kaplan-Meier
analysis (Table II).
 | Table IISurvival data of patients (n=45) with
oral squamous cell carcinoma. |
Table II
Survival data of patients (n=45) with
oral squamous cell carcinoma.
| | membEGFR protein
level | | | cytoEGFR protein
level | | |
|---|
| Category | Negative
(IRS0-2) | Positive
(IRS3-12) | P-value | CI | Negative
(IRS0-2) | Positive
(IRS3-12) | P-value | CI |
|---|
| Total, n | 36 | 9 | | | 20 | 25 | | |
| Kaplan-Meier
analysis | | | | | | | | |
|
Mean
survival time, months | 33.2±7.6 | 30.9±17.7 | 0.971
(log-rank) | | 40.2±10.0 | 26.7±9.2 | 0.031
(log-rank) | |
| Overall
survival | | | | | | | | |
|
Univariate
Cox | Ref. | RR=1.02 | 0.972 | 0.39-2.7 | Ref. | RR=2.31 | 0.039 | 1.04-5.1 |
|
Multivariate
Cox | Ref. | RR=1.31 | 0.61 | 0.47-3.6 | Ref. | RR=3.03 | 0.035 | 1.08-8.5 |
| Relapse-free
survival | | | | | | | | |
|
Univariate
Cox | Ref. | RR=1.17 | 0.78 | 0.39-3.5 | Ref. | RR=1.55 | 0.33 | 0.64-3.8 |
|
Multivariate
Cox | Ref. | RR=1.53 | 0.49 | 0.46-5.1 | Ref. | RR=2.57 | 0.13 | 0.76-8.6 |
In the univariate Cox's proportional hazard
regression analysis a positive cytoEGFR staining showed a
significant correlation with a worse prognosis of the patients
(RR=2.3; P=0.039), while membEGFR do not correlate with the
prognosis.
Furthermore, we also performed a multivariate Cox's
proportional hazard regression analysis adjusted to the T-stage,
N-stage, grading, sex and age of the patients (Table III). The Cox's analysis
demonstrated that the cytoEGFR protein level is an independent
prognostic biomarker for the overall survival of OSCC patients
(RR=3.0; P=0.035) (Fig. 2; Table II), while the membEGFR level has no
significant influence on survival in the same OSCC cohort (Table II).
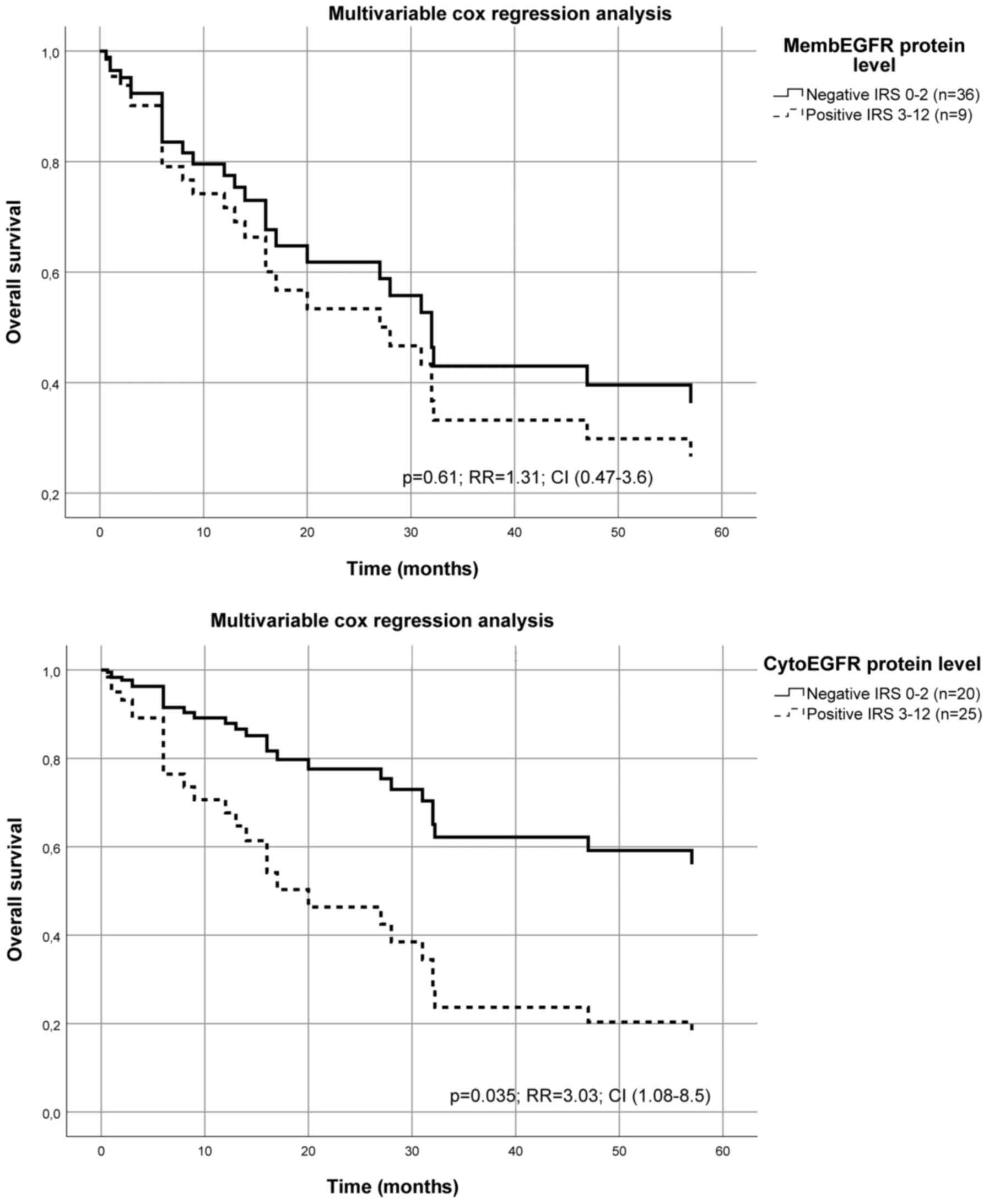 | Figure 2Multivariate Cox's hazard regression
model for cytoplasmatic EGFR protein level or membranous EGFR
protein level and survival in patients with OSCC. The protein
expression levels of cytoEGFR or membEGFR for 45 patients with OSCC
were associated with survival. The model was adjusted for tumor
stage, n-stage and grading of the tumor, as well the age and sex of
the patients. The relative risk of death was significantly
increased for patients with a higher cytoEGFR protein level
calculated using a multivariate Cox's hazard regression model
(P=0.035; RR=3.03; CI, 1.08-8.5). For membEGFR the calculated
multivariate Cox's hazard regression model missed the significant
level (P=0.61; RR=1.31; CI, 0.47-3.6). The immunohistochemical
staining was analyzed using the IRS of Remmele and Stegner
(17) described in detail (15). CI, confidence interval; RR, relative
risk; cyto, cytoplasmatic; EGFR, epidermal growth factor-receptor;
memb, membranous; OSCC, oral squamous cell carcinoma; IRS,
immunoreactive score. |
 | Table IIIClinicopathological data of patients
with oral squamous cell carcinoma. |
Table III
Clinicopathological data of patients
with oral squamous cell carcinoma.
| | | Univariate Cox
analysis | Multivariate Cox
analysis |
|---|
| Category | Number of
cases | RR | P-value | RR | P-value |
|---|
| Total | 45 | | | | |
| Sex | | | | | |
|
Male | 31 | Ref. | | Ref. | |
|
Female | 14 | 0.63 | 0.30 | 0.61 | 0.32 |
| Age, years | | | | | |
|
<50 | 11 | Ref. | | Ref. | |
|
≥50 | 34 | 2.35 | 0.09 | 4.55 | 0.01 |
| T-stage | | | | | |
|
I | 6 | Ref. | | Ref. | |
|
II | 17 | 0.48 | 0.23 | 0.18 | 0.12 |
|
III | 11 | 0.64 | 0.47 | 0.25 | 0.05 |
|
IV | 11 | 1.05 | 0.93 | 0.36 | 0.12 |
| N-stage | | | | | |
|
N0 | 19 | Ref. | | Ref. | |
|
N1-3 | 26 | 3.16 | 0.01 | 3.09 | 0.02 |
| Grading | | | | | |
|
1 | 3 | Ref. | | Ref. | |
|
2 | 18 | 0.52 | 0.41 | 0.61 | 0.55 |
|
3 | 24 | 1.10 | 0.90 | 1.75 | 0.49 |
Interestingly, in multivariate Cox's proportional
hazard regression analysis (RR=2.57; P=0.13) we found that even a
slight increase of cytoEGFR protein level was associated with a
higher risk of disease relapse. Again, membEGFR did not correlate
with the probability of relapse in this cohort of OSCC patients
(Table II).
Correlation of membranous EGFR and
cytoplasmatic EGFR with other parameters
In bivariate two-sided Spearman correlation
analysis, we calculated a significant correlation between membEGFR
level and cytoEGFR level (correlation coefficient: 0.57;
P<0.001). No correlation was observed between the membEGFR level
or cytoEGFR level and T-stage, N-stage, grading, sex and age of the
patients (Table I).
Discussion
In this immunohistochemical analysis, we could show
that a cytoEGFR localization is an independent prognostic marker
for overall survival (OS) in a cohort of 45 OSCC patients (RR=3.0,
P=0.035). Moreover, the cytoEGFR was detectable in 56% of all
cases, whereas high membEGFR level was detectable in only 20% of
all cases. No evidence of nuclear localized EGFR was found in our
cohort.
The prognostic effect of subcellular localization of
EGFR protein (membranous/cytoplasmatic/nuclear) on overall and
disease free survival (DFS) is assessed heterogeneously in the
literature. Bossi et al (6)
examined the prognostic effects of EGFR IHC data extracted from
nine articles dealing with OSCC patients data. While three of these
studies found a positive association of EGFR expression on survival
(OS or DFS) of OSCC patients, no such correlation was found in the
six other studies (6).
It is noteworthy that even a loss of EGFR expression
may be related to invasiveness and epithelial-mesenchymal
transition in oral squamous cell carcinoma (18). This could be explained by the
observation that the detection of a receptor not always correlates
with the activity of the induced pathway, but to the opposite.
Therefore it might be possible that the tumorous EGFR-protein level
is very low, but the signaling pathway is highly activated. This is
what we found in a cohort of STS patients, where EGFR levels were
low but pAKT S473 protein levels were elevated (19,20).
An explanation for such a situation could be that the interaction
of high levels of EGFR ligands (e.g. EGF) can lead to a higher
turnover of EGFR proteins. This ligand-receptor complex is normally
internalized into the cytoplasm and only 50% of the internalized
EGFR is recycled and transported back to the cell surface. When
this cycle starts again, the level of membranous EGFR decreases and
the level of cytoplasmic EGFR increases.
However, at the end of such a strongly induced
EGFR-pathway the EGFR protein is no longer detectable, although the
pathway is highly active (13).
This indicates that very low levels of EGFR protein might have two
opposite reasons i) very low expression of EGFR or II) a very high
active EGFR-pathway associated with high level of internalization
and degradation of the receptor.
Therefore point II) could be one reason why patients
without detectable EGFR levels in their tumors could benefit from
EGFR- specific therapies. The EGFR level could be low, because the
turnover and the activity of the EGFR-pathway is very high. It is
also possible, that high levels of HER2 binds activated EGFR, forms
a heterodimer and the internalization of this complex caused a
reduced level of EGFR (21). In
particular, the endocytosis, traffic, recycling and the degradation
of EGFR is very complex and influenced by various parameters and
this could be important for therapeutic options (21).
Therefore, it is difficult to derive reliable
prognostic information only from the EGFR content in the tumor, as
has been done in some studies in OSCC patients.
For example, Ryott et al (22) (investigated 78 OSCC), Diniz-Freitas
et al (23) (investigated 44
OSCC), Christensen et al (5)
(investigated 192 OSCC) and Shah et al (24) (investigated 89 OSCC) but found no
prognostic effects of EGFR protein levels. However, an indication
of the activity of the EGFR pathway could be a better prognostic
marker (which could be the level of pAKT473 protein) or the level
of internalization of EGFR. The internalization could be estimated
from the level of membranous vs. cytoplasmatic EGFR.
This is possible because Monteiro et al
(25) published the prognostic
effect of the combination of membEGFR and cytoEGFR protein level on
OS in a cohort of 67 OSCC (RR=4.92, P=0.039). Nevertheless, this
study does not assess the different prognostic effects of membEGFR
compared to cytoEGFR protein levels. In a multivariate Cox's
regression analysis, Huang and colleagues described a prognostic
effect of membEGFR for 160 OSCC (OS HR: 1.775 (95% CI, 1.136-2.772)
(26), whereby the cytoEGFR protein
level was not examined. In another cohort of 100 OSCC, only
membEGFR was found to have a significant prognostic effect
(P=0.02). The authors could not demonstrate such a correlation for
the combination of membEGFR and/or cytoEGFR protein level (27).
In Fig. 1E, it is
shown that the membEGFR and cytoEGFR could be found simultaneously
in different regions of the same tumor. Here membEGFR is localised
in the center whereas cytoEGFR is localised at the periphery of the
tumor bulk. Such a picture can be explained by a higher content of
functional EGFR ligand in the periphery, which is able to bind the
receptor (followed by internalization and activation of the
pathway). While lower ligand levels in the center of the tumor are
unable to activate EGFR and, therefore, the level of membEGFR is
high and the level of cytoEGFR is low. Our interpretation of higher
cytoEGFR levels is that an activated EGF-receptor is more likely to
be internalized and thus a higher proportion of cytoEGFR could
represent a more activate EGFR pathway.
Taguchi (11)
focused on a study of nuclear EGFR in a cohort of 82 OSCC patients.
The authors found a positive staining reaction for nuclear EGFR in
28% of the tumor samples, but no significant correlation with
patient survival. The nuclear localization of EGFR is of great
interest, e.g. Yang et al (28) found that nuclear EGFR protein level
was a better prognostic factor than the cytoplasmic EGFR level in
rectal cancer patients.
In our opinion, it is important to consider the
different localization of biomarkers, since the known biological
properties of these markers highly depend on the different cell
compartments. It was shown that the membEGFR can be internalized in
a ligand dependent manner (29),
while internalized cytoEGFR could be partially recycled and return
to the cell surface. There is evidence that some cytoEGFR molecules
can be translocated into the nucleus of tumor cells (9,29). In
addition, different isoforms of EGFR could even have different
targets/induce different pathways which could have different
biological and therapeutic effects (13,19).
Nuclear EGFR functioned as a transcription factor
and induced proliferation-associated genes and increase the chemo-
and radioresistance of tumor cells (3). The therapeutic options should take
into account the different turnover and traffic of such a receptor
(21,30).
In conclusion, this study shows that EGFR located in
the cytoplasm is an independent prognostic biomarker of OSCC
overall survival that may be important for individualized
therapeutic approaches.
Acknowledgements
The authors would like to thank Dr Marcus Bauer
(Institute of Pathology, Halle, Germany) for the technical support
concerning the photodocumentation of the histological slides.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KD, MK and AWE contributed to conception, design,
data collection and manuscript writing. MS performed IHC analysis
and interpretation of data. CW and DB supervised, received the
ethics vote, reevaluated the IHC results and drafted the
manuscript. Furthermore, DB was involved in the acquisition of data
and the analysis of these data. WR and SR were involved in the data
collection, and revised and drafted the manuscript. BAN supervised,
funded the work, was involved in drafting the manuscript, and made
substantial contributions to conception and gave final approval of
the version to be published. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Medical Faculty of the University Halle (ethic number 2017-81
issued on June 27, 2017). All procedures were in accordance with
the Declaration of Helsinki. All patients provided written
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Eckert AW, Wickenhauser C, Salins PC,
Kappler M, Bukur J and Seliger B: Clinical relevance of the tumor
microenvironment and immune escape of oral squamous cell carcinoma.
J Transl Med. 14(85)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lo HW and Hung MC: Nuclear EGFR signalling
network in cancers: Linking EGFR pathway to cell cycle progression,
nitric oxide pathway and patient survival. Br J Cancer. 94:184–188.
2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yarden Y: Grundlagen der
Signaltransduktion. Onkologie. 28 (Suppl 4):S14–S17. 2005.(In
German). PubMed/NCBI View Article : Google Scholar
|
|
5
|
Christensen A, Kiss K, Lelkaitis G, Juhl
K, Persson M, Charabi BW, Mortensen J, Forman JL, Sørensen AL,
Jensen DH, et al: Urokinase-type plasminogen activator receptor
(uPAR), tissue factor (TF) and epidermal growth factor receptor
(EGFR): Tumor expression patterns and prognostic value in oral
cancer. BMC Cancer. 17(572)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bossi P, Resteghini C, Paielli N, Licitra
L, Pilotti S and Perrone F: Prognostic and predictive value of EGFR
in head and neck squamous cell carcinoma. Oncotarget.
7:74362–74379. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Søland TM and Brusevold IJ: Prognostic
molecular markers in cancer-quo vadis? Histopathology. 63:297–308.
2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lo HW, Hsu SC, Ali-Seyed M, Gunduz M, Xia
W, Wei Y, Bartholomeusz G, Shih JY and Hung MC: Nuclear interaction
of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer
Cell. 7:575–589. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Marti U, Burwen SJ, Wells A, Barker ME,
Huling S, Feren AM and Jones AL: Localization of epidermal growth
factor receptor in hepatocyte nuclei. Hepatology. 13:15–20.
1991.PubMed/NCBI
|
|
10
|
Lo HW, Xia W, Wei Y, Ali-Seyed M, Huang SF
and Hung MC: Novel prognostic value of nuclear epidermal growth
factor receptor in breast cancer. Cancer Res. 65:338–348.
2005.PubMed/NCBI
|
|
11
|
Taguchi T: Nuclear translocation of
epidermal growth factor receptor and its relation to
clinicopathological factors in oral squamous cell carcinomas.
Kokubyo Gakkai Zasshi. 81:45–52. 2014.(In Japanese). PubMed/NCBI
|
|
12
|
Maramotti S, Paci M, Manzotti G, Rapicetta
C, Gugnoni M, Galeone C, Cesario A and Lococo F: Soluble epidermal
growth factor receptors (sEGFRs) in cancer: Biological aspects and
clinical relevance. Int J Mol Sci. 17(593)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Weinholdt C, Wichmann H, Kotrba J, Ardell
DH, Kappler M, Eckert AW, Vordermark D and Grosse I: Prediction of
regulatory targets of alternative isoforms of the epidermal growth
factor receptor in a glioblastoma cell line. BMC Bioinformatics.
20(434)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Eckert AW, Lautner MH, Schütze A, Taubert
H, Schubert J and Bilkenroth U: Coexpression of hypoxia-inducible
factor-1α and glucose transporter-1 is associated with poor
prognosis in oral squamous cell carcinoma patients. Histopathology.
58:1136–1147. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Eckert AW, Schutze A, Lautner MH, Taubert
H, Schubert J and Bilkenroth U: HIF-1alpha is a prognostic marker
in oral squamous cell carcinomas. Int J Biol Markers. 25:87–92.
2010.PubMed/NCBI
|
|
16
|
Wichmann H, Güttler A, Bache M, Taubert H,
Rot S, Kessler J, Eckert AW, Kappler M and Vordermark D: Targeting
of EGFR and HER2 with therapeutic antibodies and siRNA: A
comparative study in glioblastoma cells. Strahlenther Onkol.
191:180–191. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Remmele W and Stegner HE: Vorschlag zur
einheitlichen definition eines immunreaktiven score (IRS) für den
immunhistochemischen ostrogenrezeptor-nachweis (ER-ICA) im
mammakarzinomgewebe. Pathologe. 8:138–140. 1987.
|
|
18
|
Kimura I, Kitahara H, Ooi K, Kato K,
Noguchi N, Yoshizawa K, Nakamura H and Kawashiri S: Loss of
epidermal growth factor receptor expression in oral squamous cell
carcinoma is associated with invasiveness and
epithelial-mesenchymal transition. Oncol Lett. 11:201–207.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rot S, Taubert H, Bache M, Greither T,
Würl P, Holzhausen HJ, Eckert AW, Vordermark D and Kappler M: Low
HIF-1α and low EGFR mRNA expression significantly associate with
poor survival in soft tissue sarcoma patients; the proteins react
differently. Int J Mol Sci. 19(3842)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Greither T, Koser F, Holzhausen HJ,
Güttler A, Würl P, Kappler M, Wach S and Taubert H: MiR-155-5p and
MiR-203a-3p are prognostic factors in soft tissue sarcoma. Cancers
(Basel). 12(2254)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sigismund S, Avanzato D and Lanzetti L:
Emerging functions of the EGFR in cancer. Mol Oncol. 12:3–20.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ryott M, Wangsa D, Heselmeyer-Haddad K,
Lindholm J, Elmberger G, Auer G, Avall Lundqvist E, Ried T and
Munck-Wikland E: EGFR protein overexpression and gene copy number
increases in oral tongue squamous cell carcinoma. Eur J Cancer.
45:1700–1708. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Diniz-Freitas M, García-Caballero T,
Antúnez-López J, Gándara-Rey JM and García-García A:
Pharmacodiagnostic evaluation of EGFR expression in oral squamous
cell carcinoma. Oral Dis. 13:285–290. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shah NG, Trivedi TI, Tankshali RA, Goswami
JV, Jetly DH, Shukla SN, Shah PM and Verma RJ: Prognostic
significance of molecular markers in oral squamous cell carcinoma:
A multivariate analysis. Head Neck. 31:1544–1556. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Monteiro LS, Diniz-Freitas M,
Garcia-Caballero T, Warnakulasuriya S, Forteza J and Fraga M:
Combined cytoplasmic and membranous EGFR and p53 overexpression is
a poor prognostic marker in early stage oral squamous cell
carcinoma. J Oral Pathol Med. 41:559–567. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Huang SF, Cheng SD, Chien HT, Liao CT,
Chen IH, Wang HM, Chuang WY, Wang CY and Hsieh LL: Relationship
between epidermal growth factor receptor gene copy number and
protein expression in oral cavity squamous cell carcinoma. Oral
Oncol. 48:67–72. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Maiorano E, Favia G, Maisonneuve P and
Viale G: Prognostic implications of epidermal growth factor
receptor immunoreactivity in squamous cell carcinoma of the oral
mucosa. J Pathol. 185:167–174. 1998.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yang CC, Lin LC, Lin YW, Tian YF, Lin CY,
Sheu MJ, Li CF and Tai MH: Higher nuclear EGFR expression is a
better predictor of survival in rectal cancer patients following
neoadjuvant chemoradiotherapy than cytoplasmic EGFR expression.
Oncol Lett. 17:1551–1558. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lo HW, Hsu SC and Hung MC: EGFR signaling
pathway in breast cancers: From traditional signal transduction to
direct nuclear translocalization. Breast Cancer Res Treat.
95:211–218. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Takei J, Kaneko MK, Ohishi T, Kawada M,
Harada H and Kato Y: A novel anti-EGFR monoclonal antibody
(EMab-17) exerts antitumor activity against oral squamous cell
carcinomas via antibody-dependent cellular cytotoxicity and
complement-dependent cytotoxicity. Oncol Lett. 19:2809–2816.
2020.PubMed/NCBI View Article : Google Scholar
|