Introduction
Acute promyelocytic leukemia (APL) was once
considered to be highly fatal because of its potential bleeding
tendency. Relapse occurred in about 10-30% of patients with the
treatment of all-trans retinoic acid (ATRA) and chemotherapy (CHT)
(1-5).
With the inclusion of arsenic trioxide (ATO), the relapse rate has
decreased to <10%, and the cure rate has exceeded 90% (4-7).
Lo-Coco reported that, compared with ATRA-CHT, the ATRA-ATO
combination significantly improved survival and relapse risk in
patients with newly diagnosed, low- or intermediate-risk APL
(5,8). In AML17 trial, the researchers found
similar results in high-risk APL patients (4).
In the ATRA and CHT era, the relapse rate was
related to white blood cell (WBC) count at diagnosis,
differentiation syndrome (DS), the expression of CD56 and body mass
index (BMI) (9-12).
In the ATRA and ATO era, it is unknown whether the above factors
still affect the relapse rate. In addition, the relationship
between the dosage of ATO and relapse rate needs to be
elucidated.
Many studies have reported the cumulative side
effects, long-term toxicity, and secondary carcinogenesis of ATO.
In a 5-year follow up study, Hu et al performed systemic
physical examination and laboratory studies to screen for possible
toxic effects in 33 eligible patients treated with ATRA/ATO. The
tests performed were as follows: Blood counts with peripheral
smears, electrolyte panels with blood urea nitrogen and creatinine,
urinalysis, liver function test, evaluation of serum tumor markers
(CEA, AFP, CA199, CA125), electrocardiograms (ECGs) and
echocardiograms, chest X-ray, dermatologic consultations,
neurologic consultations, and nerve conduction velocity to test
peripheral neurologic symptoms as necessary. All of these patients
were in generally good condition and presented no skin lesions
(13). In another study with 235
APL patients, Zhu et al reported that no arsenic
accumulation or delayed toxicity was observed with over 10 years of
follow-up (14). However, it was
also reported that an increased risk of solid cancers and
myelodysplasia were related to ATO (15,16).
The actual dosage of ATO was extremely inconsistent
in clinical pratice. In Shanghai Institute of Hematology, ATO was
used at 0.16 mg/kg/d for 30 days for 6 cycles, reached an
accumulative dosage of 28.8 mg/kg in post-remission therapy
(17). In M.D. Anderson Cancer
Center, once in complete remission (CR), ATO was used at 0.15 mg/kg
daily on Monday through Friday of weeks 1 to 4, 9 to 12, 17 to 20,
and 25 to 28, reached an accumulative dosage of 12 mg/kg (18). In C9710 study, ATO was used at 0.15
mg/kg/d for 5 days each week for 5 weeks for 2 courses after CR,
reached an accumulative dosage of 7.5 mg/kg (19). According to the 2019 National
Comprehensive Cancer Network (NCCN) guideline, the accumulative
dosage of ATO in post-remission treatment ranges from 7.95-12
mg/kg. Therefore, the optimal dosage of ATO needs to be further
study. In this retrospective study, the relapse factors of APL and
the appropriate dosage of ATO were to explored.
Patients and methods
Patients
This study retrospectively analyzed 112 patients
diagnosed with APL between July 2008 and December 2015, from First
Affiliated Hospital of Xi'an Jiaotong University and The First
People's Hospital of Yulin. Eligibility criteria included:
Morphologic diagnosis of de novo APL according to
French-American-British criteria; demonstration of PML-RARα fusion
transcripts by reverse-transcriptase-polymerase-chain-reaction
(RT-PCR) or the t(15;17) translocation by means of conventional
karyotyping or fluorescence in situ hybridization (FISH);
Obtaining CR after induction therapy; age older than 1 year with no
upper limit; normal left ventricular ejection fraction and Q-Tc
interval less than 500 ms; a negative pregnancy test in females of
child-bearing potential; absence of serious comorbidity.
In this retrospective study, the difference of the
accumulative ATO dosage in post-remission therapy was due to age
and side effects from treatment. The recommended accumulative ATO
dosage in post-remission therapy on 2019 NCCN guideline was 7.95-12
mg/kg. 12 mg/kg ATO was the most widely used in clinical practice.
Based on the actual dosage of ATO in post-remission therapy, a
total of 112 APL patients were divided into the high-dose group
(ATO dosage ≥12 mg/kg) and low-dose group (<12 mg/kg). This
study was approved by the Ethical Committee of the First Affiliated
Hospital of Xi'an Jiaotong University in Xi'an, China (approval
document no. 2015-012).
Treatment
In this retrospective study, 112 patients with newly
diagnosed APL received the combination of ATRA, anthracycline-based
chemotherapy and different dose of ATO for variable cycles. The
induction comprised 25 mg/m2/d ATRA in two divided doses
until CR, 0.15 mg/kg/d intravenous ATO until CR, anthracyclines on
days 1-3, and cytarabine on days 1-7. The post-remission treatment
included 25 mg/m2/d ATRA for 2 weeks every 4 weeks for a
total of 7-12 courses, anthracycline-based chemotherapy for more
than 2 courses. ATO was used at 0.15 mg/kg/d for different
courses.
In the whole courses of treatment, the dose of ATRA,
ATO, and chemotherapy were adjusted according to age and side
effects.
Supportive measures, and management of
complications
To prevent and treat the APL associated
coagulopathy, platelet concentrates were used to maintain the
platelet count >20x109/l, and fresh-frozen plasma was
transfused to maintain fibrinogen >1.5 g/l. To improve the
anemia, packed red cells were transfused to maintain hemoglobin
levels >60 g/l (20). All
patients received hydroxyurea based on WBC counts to control
hyperleukocytosis. When DS was suspected clinically, ATRA, ATO, or
both were temporarily discontinued, and 10 mg/12 h dexamethasones
were given for a minimum of 3 days until the disappearance of
symptoms and signs. Prophylactic heparin and Granulocyte Colony
Stimulating Factor (G-CSF) were not recommended.
Definitions and endpoints
CR and relapse were defined using conventional
criteria (21). Hematologic CR
required that patients achieved the morphologic leukemia-free state
of bone marrow (BM) and had the absolute neutrophils
>1.0x109/l, platelets >100x109/l in the
peripheral blood. Molecular CR assessed by RT-PCR was defined as
undetectable PML-RARα transcript. Hematologic relapse was diagnosed
based on the presence of >20% blasts or abnormal promyelocytes
in the BM. Molecular relapse was defined as the reappearance of the
PML-RARα-specific band in two consecutive BM samples collected at
any time after molecular CR (22).
RT-PCR analysis
Bone-marrow quantitative RT-PCR for PML-RARα was
performed in all patients at diagnosis, after induction, each
consolidation cycle, and then every 3 months for 2 years.
Statistical analysis
Statistical analysis was performed by SPSS Version
18.0 statistical software. Comparisons were made using Student's
t-test or the Mann-Whitney test for comparison of continuous
variables and Pearson's χ2 test for dichotomous
variables. Chi-square test or Fisher's exact test will be used for
comparison of categorical variables between the two groups. The
survival functions were estimated by using the Kaplan-Meier method
and were compared by using the log-rank test. Multiple factor
analysis and OR were determined by the the binary logistic
regression analysis. All tests were two-sided, accepting P<0.05
as indicating a statistically significant difference.
Results
Patient characteristics at
presentation
A total of 112 patients were enrolled from July 2008
to December 2015. These included five children, age range from 4 to
13 years, three in the high-dose group, and two in the low-dose
group. All patients' main clinical data at presentation were shown
in Table I. Based on the
accumulative actual ATO dosage in post-remission therapy, 72
(64.3%) patients were in the low-dose group, whereas 40 (35.7%)
patients were in the high-dose group. The median ATO dosage was
4.83 and 15.59 mg/kg in the low-dose group and high-dose group
respectively. DS occurred in 14 (19.44%) patients in the low-dose
group and 8 (20.0%) patients in the high-dose group (P=0.94). There
was no significant difference in the baseline characteristics
between the two groups, including age, sex, WBC counts, PLT counts,
Sanz risk and PML-RARα transcript type. All of 112 enrolled
patients obtained CR, and the median time to achieve CR is 33 days
(range, 25-46 days). There was no death during the follow-up
period.
 | Table IPatient characteristics at
diagnosis. |
Table I
Patient characteristics at
diagnosis.
| Characteristics | Low-dose group
(n=72) | High-dose group
(n=40) | P-value |
|---|
| Median age, years
(range) | 38 (8-65) | 36.5 (4-76) | 0.86 |
| Sex, no. (%) | | | 0.20 |
|
Male | 36 (50.0) | 25 (62.5) | |
|
Female | 36 (50.0) | 15 (37.5) | |
| Median WBC,
x109/l (range) | 1.24 (0.5-157.3) | 1.42
(0.71-91.19) | 0.74 |
| Median PLT,
x109/l (range) | 45.5 (2-118) | 34 (8-115) | 0.29 |
| Sanz risk, no.
(%) | | | 0.52 |
|
Low- or
intermediate | 61 (84.7) | 32 (80.0) | |
|
High | 11 (15.3) | 8 (30.0) | |
| PML/RARA, no.
(%) | | | 0.42 |
|
BCR1-2 | 43 (59.7) | 27 (67.5) | |
|
BCR3 | 29 (40.3) | 13 (32.5) | |
| DS, no. (%) | | | 0.94 |
|
Yes | 14 (19.4) | 8 (20.0) | |
|
No | 58 (25.0) | 32 (80.0) | |
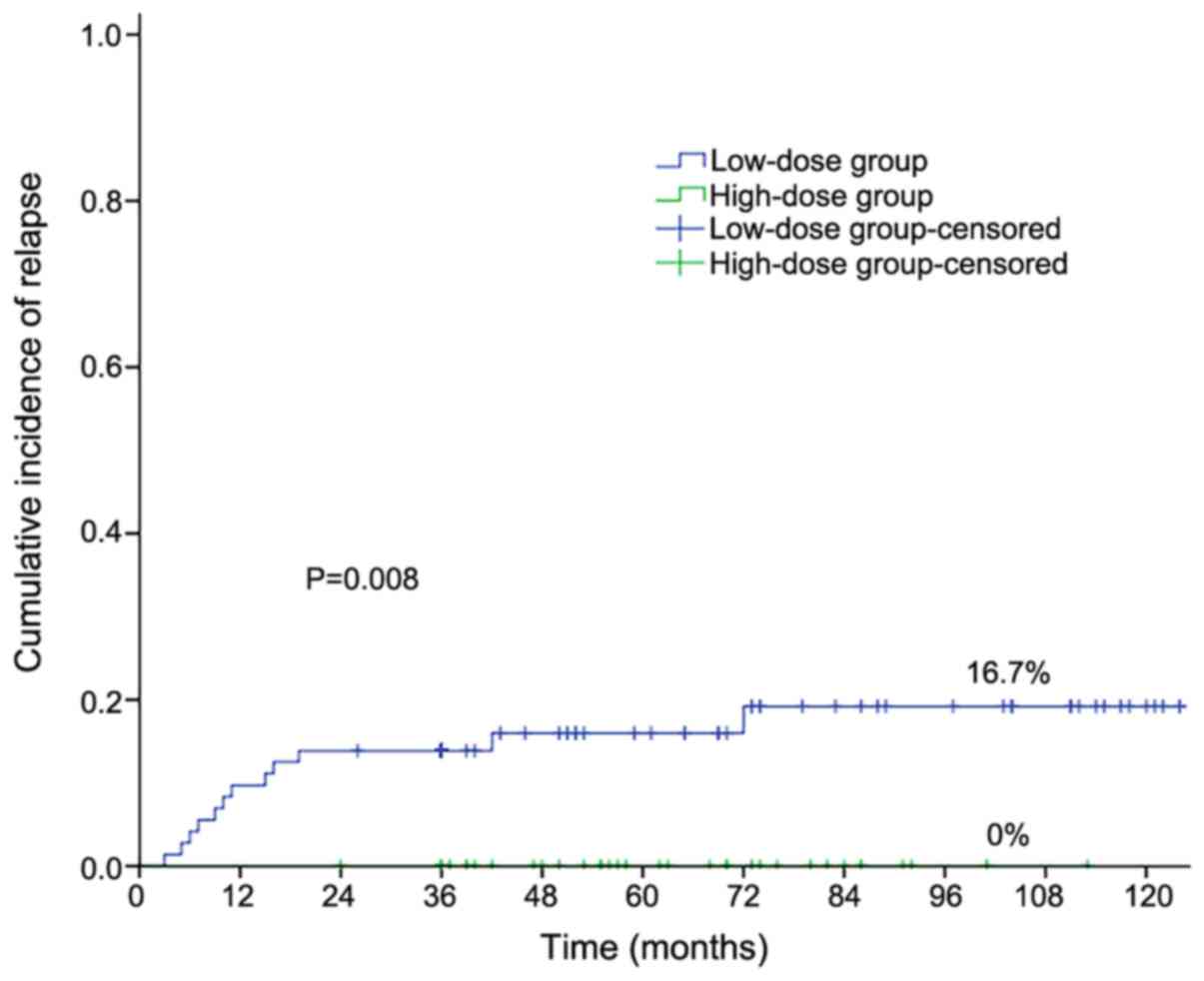
Cumulative incidence of relapse
The median follow-up of 112 patients considered for
the CIR estimate was 53 months (range, 3 to 125 months). 12
(10.71%) out of 112 patients relapsed during the follow-up.
Table II shows the 4-year CIR
values according to ATO dosage in post-remission treatment, WBC
counts, PML/RARA transcript, and DS in single factor
analysis. No statistically significant associations were detected
between WBC counts, PML-RARα transcript type, the occurrence
of DS and CIR. The accumulative ATO dosage in post-remission
treatment was the only one relative factor of relapse.
 | Table IICIR by patient characteristics. |
Table II
CIR by patient characteristics.
| Characteristic | CIR (%) | P-value |
|---|
| All patients | 10.7 | |
| ATO dosage in
post-remission therapy | | <0.01 |
|
Low-dose
group | 16.7 | |
|
High-dose
group | 0.0 | |
| WBC at diagnosis | | 0.23 |
|
≤10x109/l | 8.6 | |
|
>10x109/l | 21.1 | |
| PML/RARA | | 0.99 |
|
BCR1-2 | 11.4 | |
|
BCR3 | 5.7 | |
| Differentiation
syndrome | | 0.91 |
|
Yes | 13.6 | |
|
No | 10.0 | |
The Binary logistic regression analysis was
performed to define the risk factors of relapse for a total of 112
patients in Table III. An
increased ATO dosage has an independent protective factor in terms
of probability of relapse (odds ratio=0.67, 95% CI, 0.51-0.88,
P=0.004). Age, WBC count, and the occurrence of DS had no
relationship with the relapse rate (P>0.05). This result was
consistent with the single factor analysis.
 | Table IIIMultivariate analysis of the risk
factors of relapse in acute promyelocytic leukemia. |
Table III
Multivariate analysis of the risk
factors of relapse in acute promyelocytic leukemia.
| Variable | P-value | Odds ratio (95%
CI) |
|---|
| Age | 0.53 | 0.98 (0.93,
1.04) |
| WBC count | 0.85 | 1.00 (0.98,
1.03) |
| The occurrence of
DS | 0.84 | 0.85 (0.17,
4.34) |
| ATO dosage | <0.01 | 0.67 (0.51,
0.88) |
As shown in Fig. 1,
the 4-year cumulative incidence of relapse (CIR) was 16.7% in the
low-dose group and 0% in the high-dose group respectively
(P=0.008). The median relapse time was 13 months (range, 3-42
months). Low-dose ATO was associated with higher CIR.
Among the 112 patients, we observed no one relapsed
when the ATO dosage in post-remission more than 6 mg/kg. The 4-year
CIR was 23.5% in the patients received ATO less than 6 mg/kg.
Toxicity
In previous studies, ATO rarely caused hematologic
toxicixities, including neutropenia and thrombocytopenia.
Therefore, we only discussed nonhematologic toxicities in this
study. As is shown in Table IV,
the incidence of hepatic toxicity was significantly higher in
high-dose group than that of the low-dose group. No one has cardiac
function (grade 3-4) in two groups. Only one patient developed
chronic renal insufficiency in the high-dose group. There was no
significant difference between the two groups in nephrotoxicity and
skin reaction. One patient was diagnosed with breast cancer after
finishing the treatment of ATO for 3 years.
 | Table IVToxicity. |
Table IV
Toxicity.
| Toxicity | Low-dose group
(n=72) | High-dose group
(n=40) | P-value |
|---|
| Hepatic toxicity
(grade 3-4) | 7 | 11 | 0.01 |
| Cardiac function
(grade 3-4) | 0 | 0 | |
| Nephrotoxicity
(grade 3-4) | 0 | 1 | 0.36 |
| Skin reaction | 2 | 3 | 0.35 |
| Secondary
tumor | 0 | 1 | 0.36 |
Discussion
In APML3 trial, ATRA/idarubicin-based protocol
produced durable remissions in most patients, but the relapse rate
was up to 28.1% (3). Comparing with
APML3, APML4 patients were treated with ATO in both induction and
consolidation (6), and the relapse
rate decreased to 5%. APL0406 and AML17 trial showed similar
results of the reduced relapse rate with ATO-based combination
therapy (4,5). The addition of ATO was greatly related
to the relapse of APL. In this retrospective study, the
accumulative ATO dosage in post-remission treatment was the only
relative factor of relapse based on the single and multiple factor
analysis. The relapse rate was significantly lower in the high-dose
group (0%) than it in the low-dose group (16.7%, P=0.004).
High-dose ATO really reduced the relapse rate statistically. The
relapse rate was not related to age, WBC counts and DS.
In previous studies, the ATO was effective at the
total dosage from 7.5 to 28 mg/kg in post-remission therapy
(17-19).
Currently, the dose of ATO was significantly different in different
trials. In APL0406 trial, the prescribed dose was 0.15 mg/kg/d, and
the total dosage in post-remission was 12 mg/kg (5). The same daily dose was used in APML4
trial, but the total dosage was 7.95 mg/kg (6). Besides, 0.25 or 0.3 mg/kg/d ATO was
given to participants in AML17 trial, and the total dosage was at
the same as APL0406 trial (4). All
of three trials resulted in ≤5% of 4-year CIR. These showed that
the ATO was active at a wide range of dosage. Although ATO is
highly effective in the treatment of APL, it is a toxic agent known
for centuries. The toxicity should be taken into account, including
QT interval prolongation, severe ventricular arrhythmia,
hyperlipidemia, liver and kidney dysfunction, skin reaction. An
increased risk of solid cancers was recognized after long-term
exposure to arsenic compounds (16), and myelodysplasia was also reported
(15). It has been demonstrated
that the occurrence of some toxic events could be reduced in the
lower-dose group than those in the higher-dose group (23). We also found the ATO-related side
effects were relatively low in the low-dose group. It is necessary
to explore the optimal ATO dosage to minimize the side effects
while obtaining high antileukemic efficacy.
In our study, when the ATO dosage in post-remission
treatment was more than 6 mg/kg, no one among the 112 patients
relapsed. That was, the CIR is 0% regardless of the ATO dosage
greater than 12 mg/kg or 6 mg/kg. While the CIR was 23.5% with
patients received ATO less than 6 mg/kg. Therefore, the total
dosage of ATO could be reduced appropriately. This could decrease
drug-related adverse events and achieve therapeutic effects as
well.
In this study, the patients with ATO dosage ≥6 mg/kg
had significantly lower relapse rate than those in previous APL
trials. The reason for the lower relapse rate may be the different
schedule of ATRA and ATO. In other studies, the cycles and courses
of ATRA and ATO were different, which resulted in that patients did
not start and stop taking above two medicines at the same time. For
example, patients received ATRA for 2 weeks every 4 weeks, and ATO
for 4 weeks every 8 weeks in APL0406 trial. In this study, most
patients received ATRA and ATO simultaneously for 2 weeks every 4
weeks. It has been reported that receiving ATRA and ATO
simultaneously could accelerate the degradation of PML-RARα fusing
protein (13). We started a trial
about the simultaneous application of ATRA and ATO in
post-remission therapy. It was registered with the Chinese Clinical
Trial Registry (ChiCTR-IPR-15006821) (24). To date, none of the participants
relapsed. Therefore, the lower relapse rate may be related to the
simultaneous application of ATRA and ATO.
In conclusion, our real-world results support that
increasing dosage of ATO could reduce the relapse risk of APL, and
the ATO dosage could be decreased appropriately in the future. The
number of patients evaluated in this study is not enough, the
prospective, randomized, controlled trials are needed to explore
the optimal ATO dosage in the future.
Acknowledgements
The authors would like to thank Dr Xiaorui Wang
(Xian No. 1 Hospital) for assisting with language editing. The
abstract was presented at the 24rth congress of the European
Hematology Association June 13-16 2019 in Amsterdam and published
as abstract no. PF258 in HemaSphere 3 (Suppl 1) p81, 2019.
Funding
This work was supported by the Clinical Research
Award Fund of First Affiliated Hospital of Xi'an Jiaotong
University (grant no. XJTU1AF2016LSL-017) and the Shaanxi National
Science Foundation (grant no. 2016JM8113).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SG analyzed and interpreted the data, performed
statistical analysis and wrote the manuscript. HZ helped with the
data analysis. WL, XZ and CZ acquired the data. HW conceived and
designed the research. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethical
Committee of the First Affiliated Hospital of Xi'an Jiaotong
University in Xi'an, China (Approval no. 2015-012) and was
conducted in accordance with the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sanz MA, Montesinos P, Vellenga E, Rayón
C, de la Serna J, Parody R, Bergua JM, León A, Negri S, González M,
et al: Risk-adapted treatment of acute promyelocytic leukemia with
all-trans retinoic acid and anthracycline monochemotherapy:
Long-term outcome of the LPA 99 multicenter study by the PETHEMA
group. Blood. 112:3130–3134. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lo-Coco F, Avvisati G, Vignetti M, Breccia
M, Gallo E, Rambaldi A, Paoloni F, Fioritoni G, Ferrara F, Specchia
G, et al: Front-line treatment of acute promyelocytic leukemia with
AIDA induction followed by risk-adapted consolidation for adults
younger than 61 years: Results of the AIDA-2000 trial of the GIMEMA
group. Blood. 116:3171–3179. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Iland H, Bradstock K, Seymour J, Hertzberg
M, Grigg A, Taylor K, Catalano J, Cannell P, Horvath N, Deveridge
S, et al: Results of the APML3 trial incorporating
all-trans-retinoic acid and idarubicin in both induction and
consolidation as initial therapy for patients with acute
promyelocytic leukemia. Haematologica. 97:227–234. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Burnett AK, Russell NH, Hills RK, Bowen D,
Kell J, Knapper S, Morgan YG, Lok J, Grech A, Jones G, et al:
Arsenic trioxide and all-trans retinoic acid treatment for acute
promyelocytic leukaemia in all risk groups (AML17): Results of a
randomised, controlled, phase 3 trial. Lancet Oncol. 16:1295–1305.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Platzbecker U, Avvisati G, Cicconi L,
Thiede C, Paoloni F, Vignetti M, Ferrara F, Divona M, Albano F,
Efficace F, et al: Improved outcomes with retinoic acid and arsenic
trioxide compared with retinoic acid and chemotherapy in
non-high-risk acute promyelocytic leukemia: Final results of the
Randomized Italian-German APL0406 trial. J Clin Oncol. 35:605–612.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Iland HJ, Collins M, Bradstock K, Supple
SG, Catalano A, Hertzberg M, Browett P, Grigg A, Firkin F, Campbell
LJ, et al: Use of arsenic trioxide in remission induction and
consolidation therapy for acute promyelocytic leukaemia in the
Australasian Leukaemia and Lymphoma group (ALLG) APML4 study: A
non-randomised phase 2 trial. Lancet Haematol. 2:e357–e366.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Abaza Y, Kantarjian H, Garcia-Manero G,
Estey E, Borthakur G, Jabbour E, Faderl S, O'Brien S, Wierda W,
Pierce S, et al: Long-term outcome of acute promyelocytic leukemia
treated with all-trans-retinoic acid, arsenic trioxide, and
gemtuzumab. Blood. 129:1275–1283. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lo-Coco F, Avvisati G, Vignetti M, Thiede
C, Orlando SM, Iacobelli S, Ferrara F, Fazi P, Cicconi L, Di Bona
E, et al: Retinoic acid and arsenic trioxide for acute
promyelocytic leukemia. N Engl J Med. 369:111–121. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sanz MA, Lo Coco F, Martin G, Avvisati G,
Rayón C, Barbui T, Díaz-Mediavilla J, Fioritoni G, González JD,
Liso V, et al: Definition of relapse risk and role of
nonanthracycline drugs for consolidation in patients with acute
promyelocytic leukemia: A joint study of the PETHEMA and GIMEMA
cooperative groups. Blood. 96:1247–1253. 2000.PubMed/NCBI
|
|
10
|
De Botton S, Chevret S, Coiteux V, Dombret
H, Sanz M, San Miguel J, Caillot D, Vekhoff A, Gardembas M,
Stamatoulas A, et al: Early onset of chemotherapy can reduce the
incidence of ATRA syndrome in newly diagnosed acute promyelocytic
leukemia (APL) with low white blood cell counts: Results from APL
93 trial. Leukemia. 17:339–342. 2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Montesinos P, Rayón C, Vellenga E, Brunet
S, González J, González M, Holowiecka A, Esteve J, Bergua J,
González JD, et al: Clinical significance of CD56 expression in
patients with acute promyelocytic leukemia treated with all-trans
retinoic acid and anthracycline-based regimens. Blood.
117:1799–1805. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Breccia M, Mazzarella L, Bagnardi V,
Disalvatore D, Loglisci G, Cimino G, Testi AM, Avvisati G, Petti
MC, Minotti C, et al: Increased BMI correlates with higher risk of
disease relapse and differentiation syndrome in patients with acute
promyelocytic leukemia treated with the AIDA protocols. Blood.
119:49–54. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hu J, Liu YF, Wu CF, Xu F, Shen ZX, Zhu
YM, Li JM, Tang W, Zhao WL, Wu W, et al: Long-term efficacy and
safety of all-trans retinoic acid/arsenic trioxide-based therapy in
newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci
USA. 106:3342–3347. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhu HH, Wu DP, Jin J, Li JY, Ma J, Wang
JX, Chen SJ and Huang XJ: Long-term survival of acute promyelocytic
leukaemia patients treated with arsenic and retinoic acid. Br J
Haematol. 174:820–822. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rezuke WN, Anderson C, Pastuszak WT,
Conway SR and Firshein SI: Arsenic intoxication presenting as a
myelodysplastic syndrome: A case report. Am J Hematol. 36:291–293.
1991.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Firkin F: Carcinogenic risk of retained
arsenic after successful treatment of acute promyelocytic leukemia
with arsenic trioxide: A cause for concern? Leuk Lymphoma.
55:977–978. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shen ZX, Shi ZZ, Fang J, Gu BW, Li JM, Zhu
YM, Shi JY, Zheng PZ, Yan H, Liu YF, et al: All-trans retinoic
acid/As2O3 combination yields a high quality remission and survival
in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci
USA. 101:5328–5335. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Estey E, Garcia-Manero G, Ferrajoli A,
Faderl S, Verstovsek S, Jones D and Kantarjian H: Use of all-trans
retinoic acid plus arsenic trioxide as an alternative to
chemotherapy in untreated acute promyelocytic leukemia. Blood.
107:3469–3473. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Powell BL, Moser B, Stock W, Gallagher RE,
Willman CL, Stone RM, Rowe JM, Coutre S, Feusner JH, Gregory J, et
al: Arsenic trioxide improves event-free and overall survival for
adults with acute promyelocytic leukemia: North American leukemia
intergroup study C9710. Blood. 116:3751–3757. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Junbo Ge and Yongjian Xu: Internal
Medicine. 8th Edition. People's Medical Publishing house, Beijing,
pp928-932, 2014.
|
|
21
|
Ye Y, Gaugler B, Mohty M and Malard F: Old
dog, new trick: Trivalent arsenic as an immunomodulatory drug. Br J
Pharmacol. 177:2199–2214. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cheson BD, Bennett JM, Kopecky KJ, Büchner
T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister
TA, et al: Revised recommendations of the international working
group for diagnosis, standardization of response criteria,
treatment outcomes, and reporting standards for therapeutic trials
in acute myeloid leukemia. J Clin Oncol. 21:4642–4649.
2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lo Coco F, Diverio D, Avvisati G, Petti
MC, Meloni G, Pogliani EM, Biondi A, Rossi G, Carlo-Stella C,
Selleri C, et al: Therapy of molecular relapse in acute
promyelocytic leukemia. Blood. 94:2225–2229. 1999.PubMed/NCBI
|
|
24
|
Zhang X, Zhang H, Chen L, Wang M, Xi J,
Liu X, Xie M, Li D, Gulati ES, Gong S and Wang H: Arsenic trioxide
and all-trans retinoic acid (ATRA) treatment for acute
promyelocytic leukemia in all risk groups: Study protocol for a
randomized controlled trial. Trials. 19(476)2018.PubMed/NCBI View Article : Google Scholar
|